Abstract
1. The effect of β-adrenergic and dopaminergic agonists and antagonists on the chemoreceptor response to graded hypoxia and hypercapnia was tested in nineteen cats and ten rabbits anaesthetized either with chloralose—urethane or pentobarbitone sodium, paralysed with pancuronium bromide and artificially ventilated.
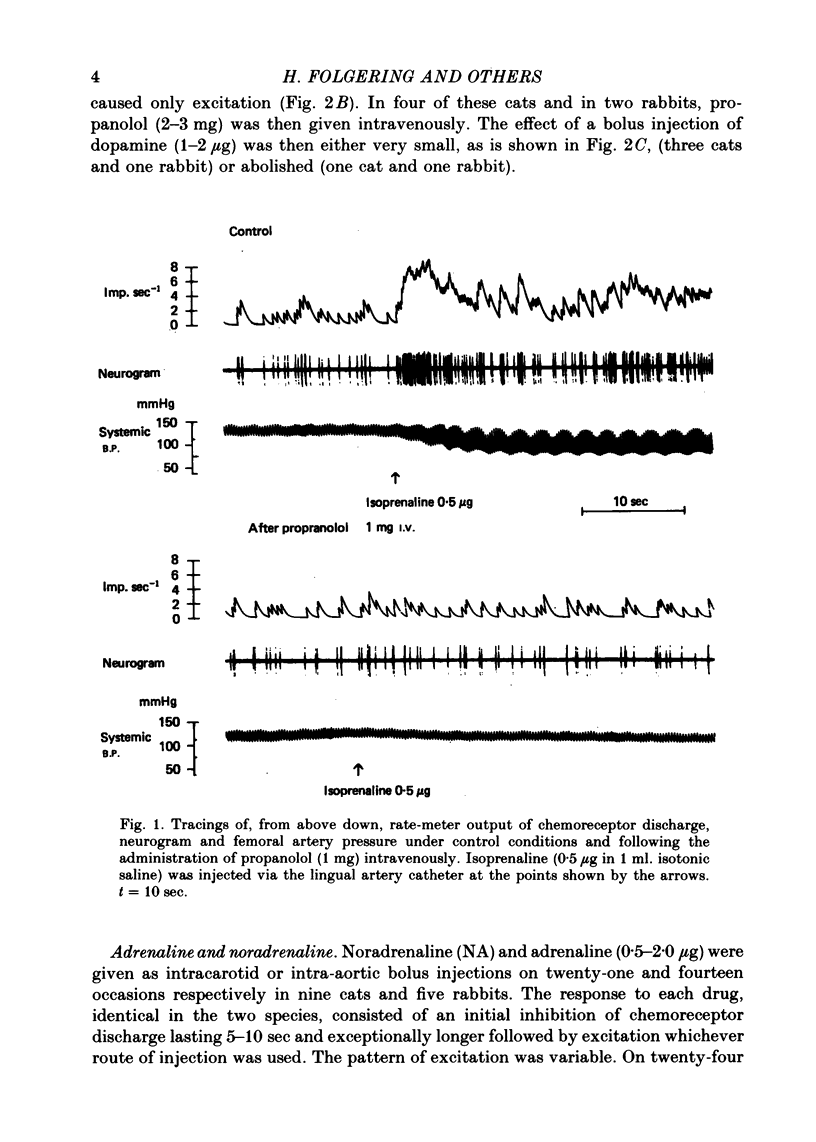
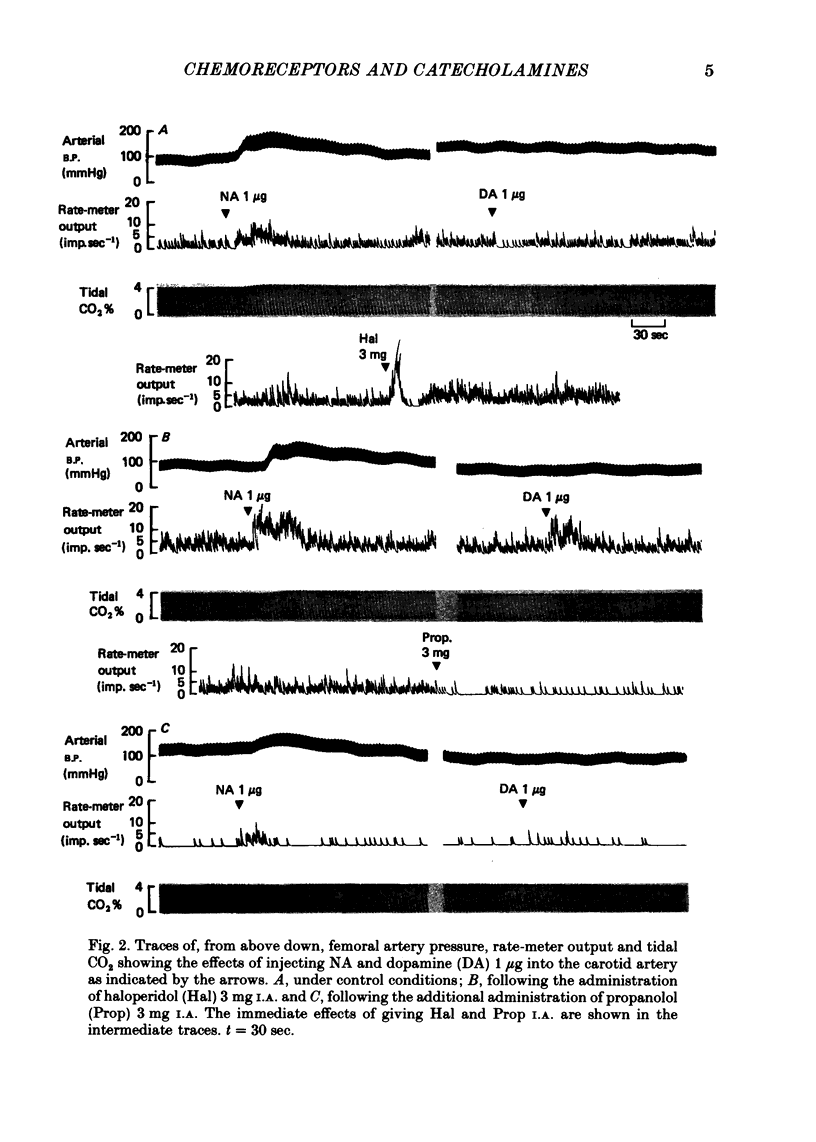
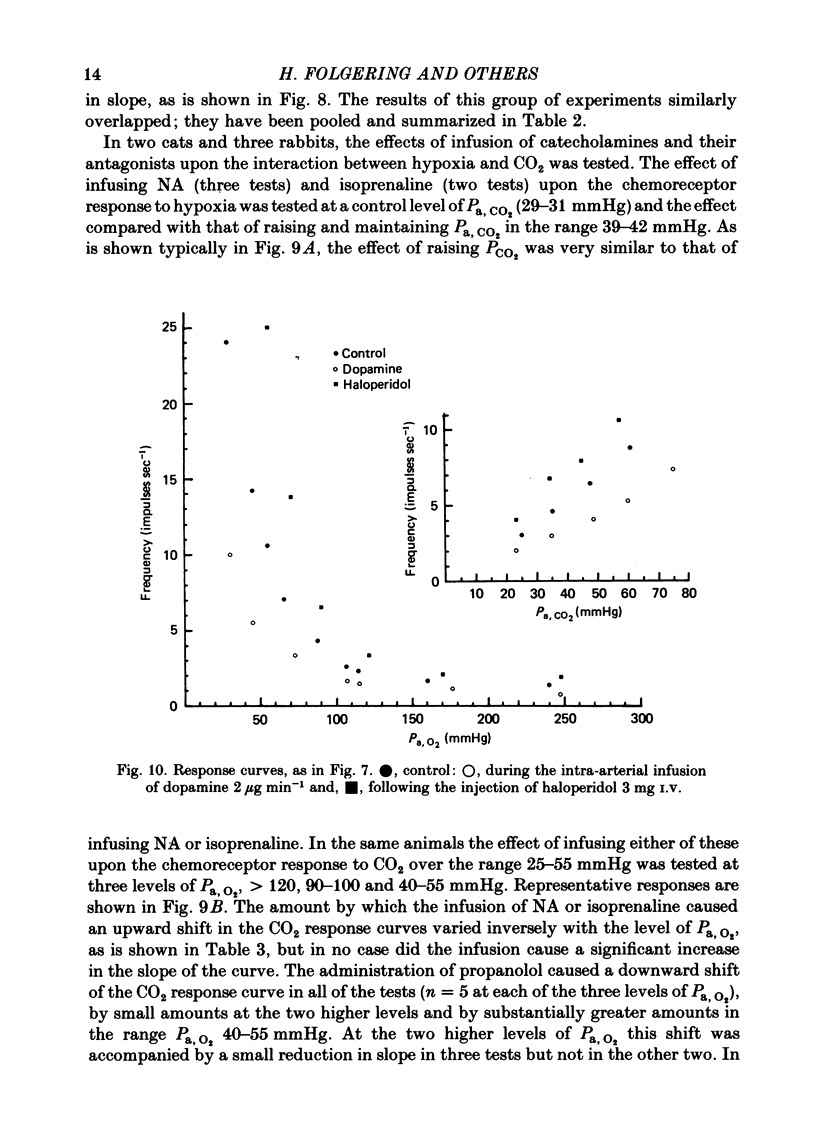
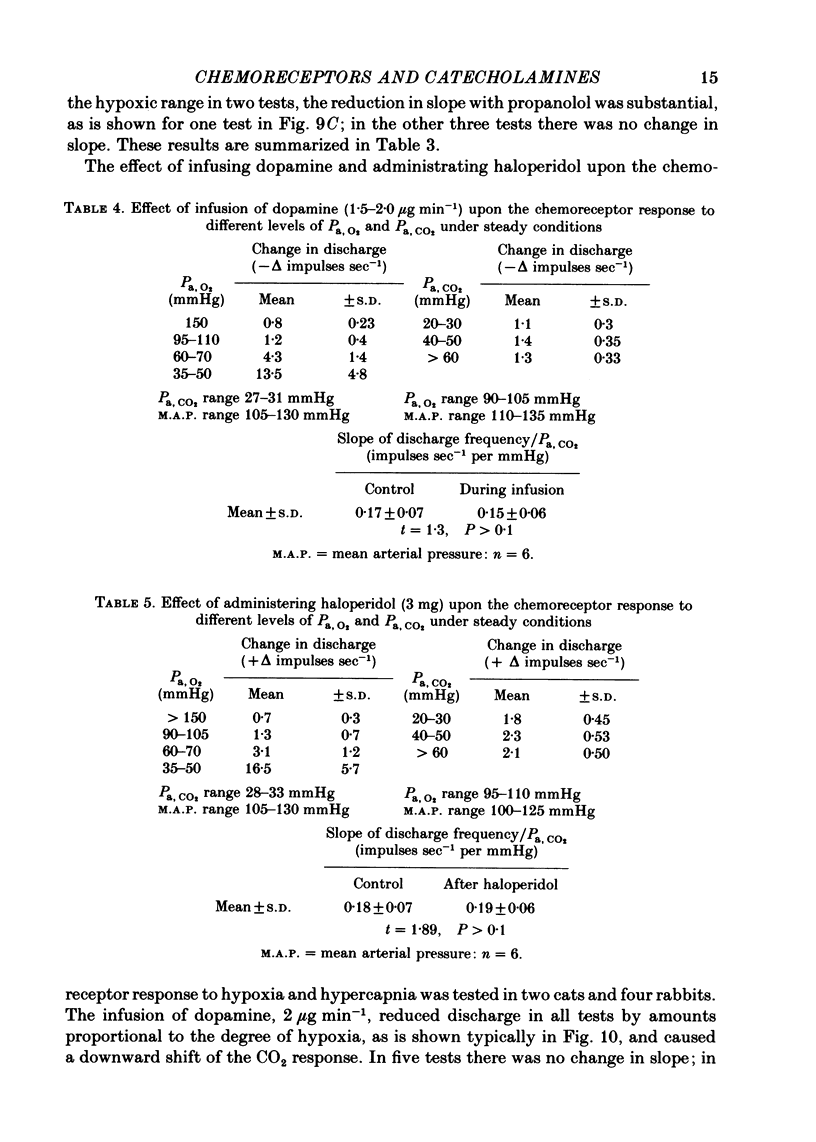
2. The inhibitory action of dopamine was confirmed. The inhibition following intra-arterial bolus injection was blocked by haloperidol; dopamine then excited and this excitation was blocked with propranolol. Adrenaline or noradrenaline caused a transient inhibition followed by a marked excitation. The inhibition was blocked with haloperidol and the excitation blocked with propranolol or metoprolol. Isoprenaline excited without inhibition and this was blocked with propranolol or metoprolol.
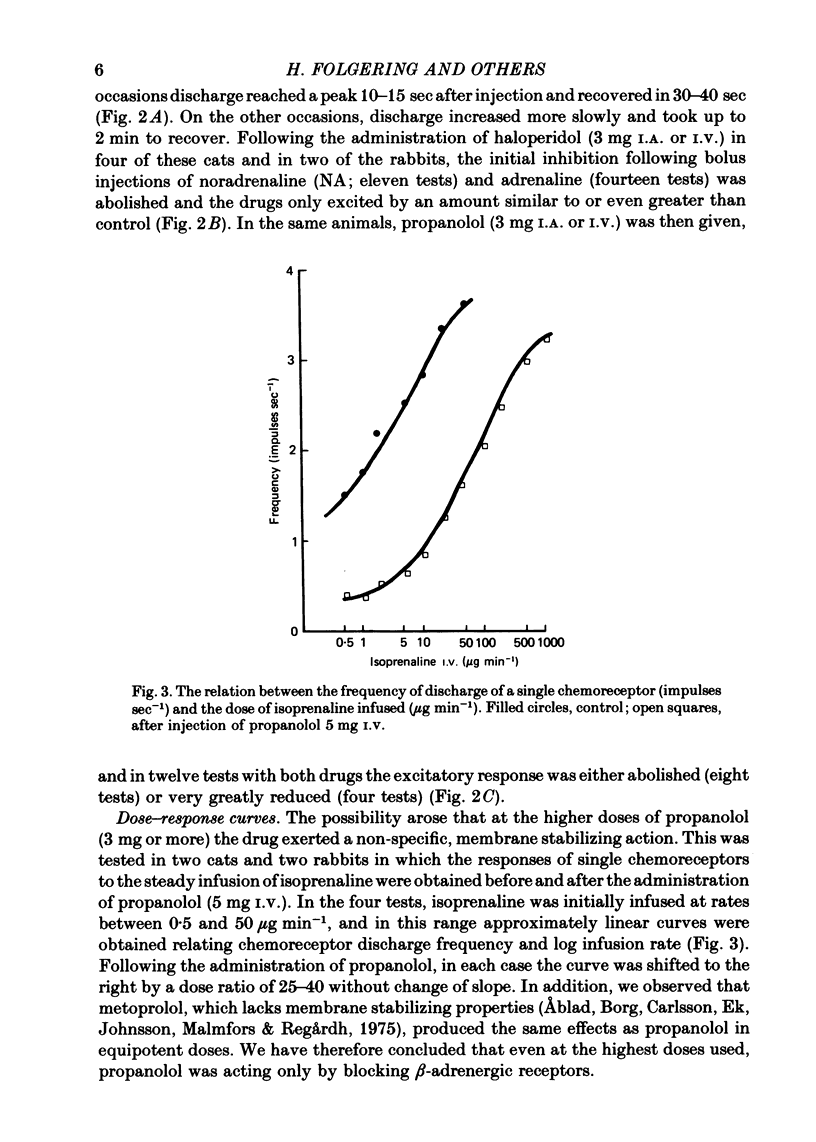
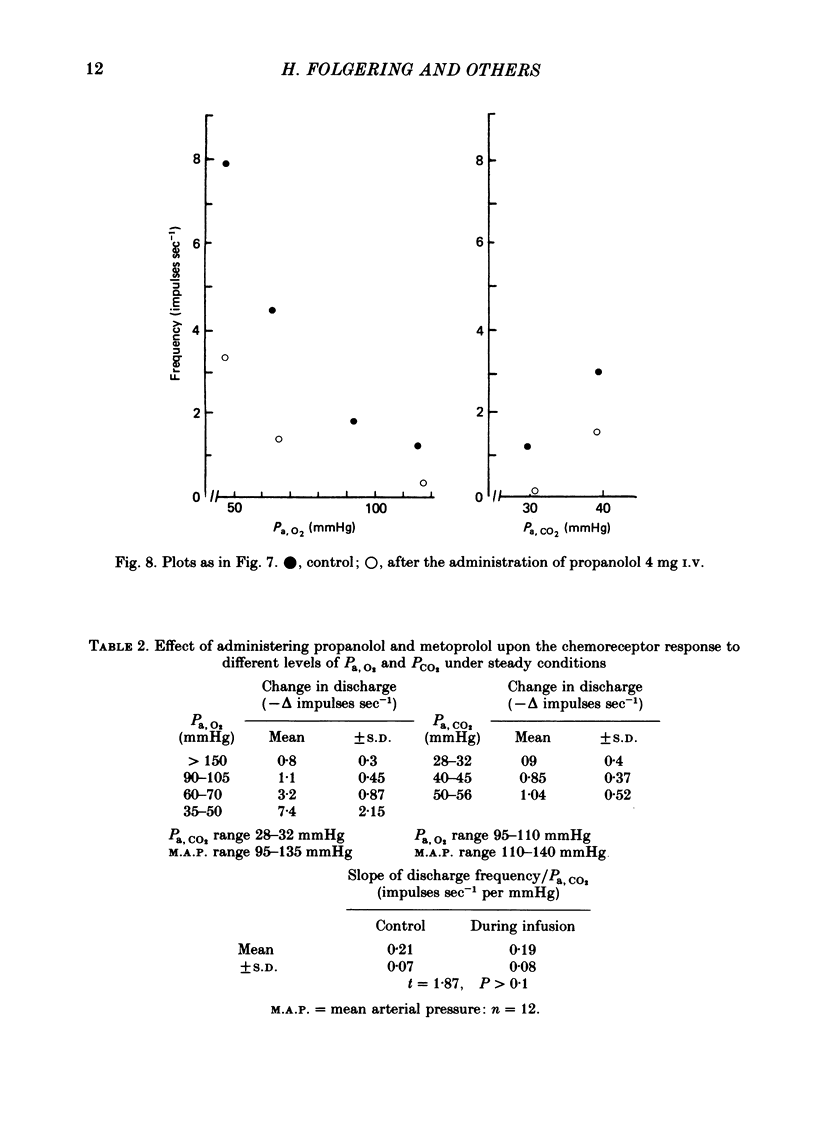
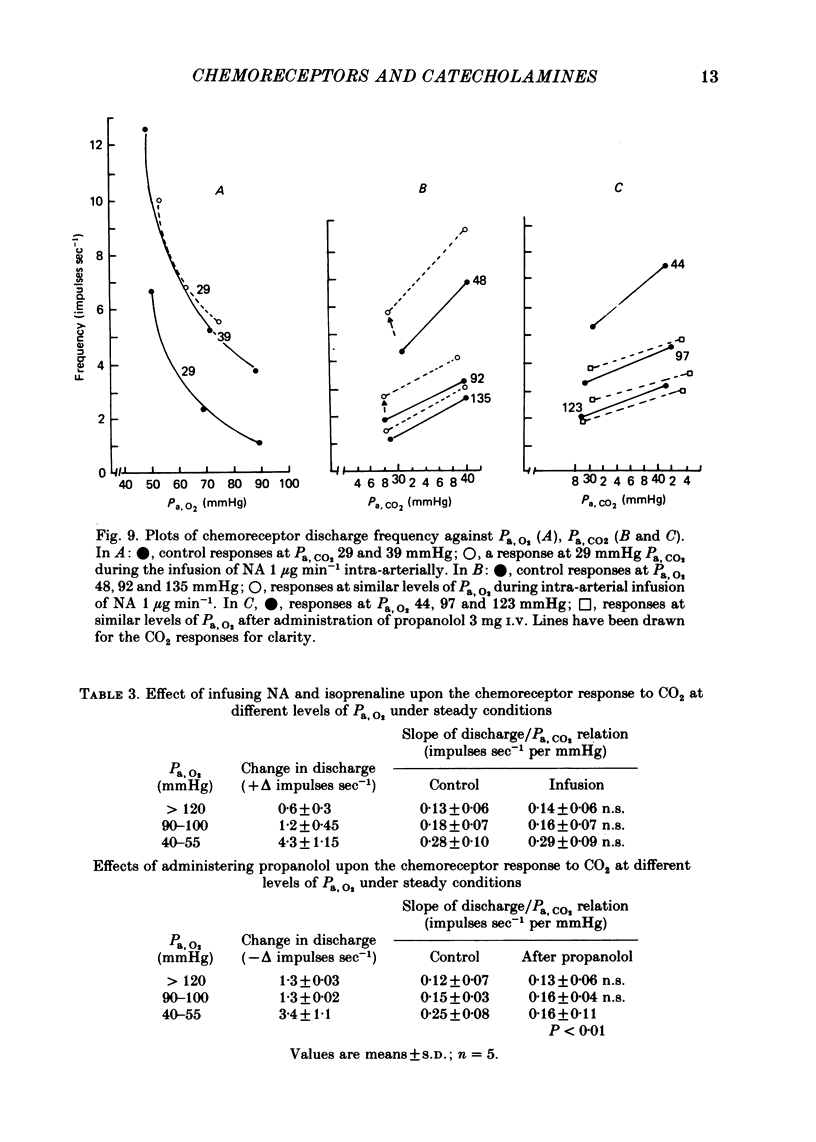
3. A novel finding was that the chemoreceptor response to hypoxia was markedly reduced or even abolished with propranolol or metoprolol. The response was enhanced with a constant infusion of isoprenaline, adrenaline or noradrenaline in proportion to the degree of hypoxia, an effect mimicked by raising CO2. The chemoreceptor response to hypoxia was similarly enhanced by haloperidol and depressed by a constant infusion of dopamine in proportion to the degree of hypoxia.
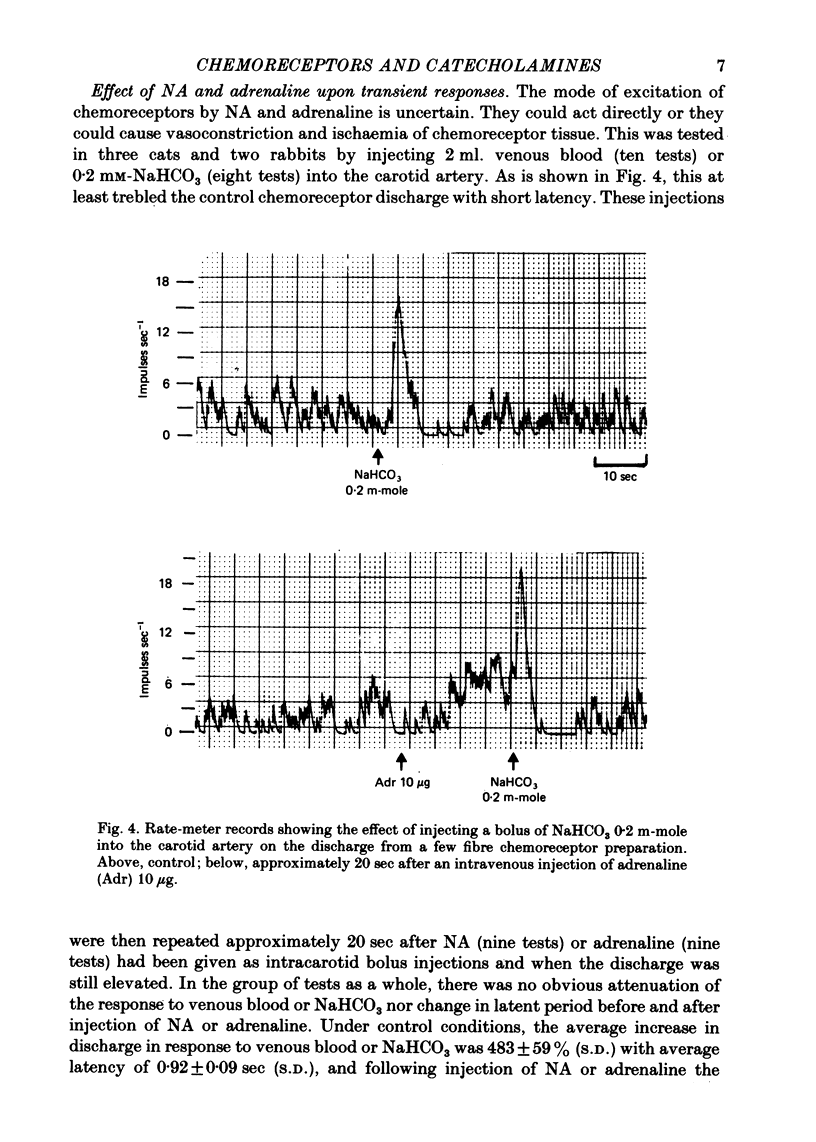
4. The effect of these drugs on the chemoreceptor response to hypercapnia was less constant. In the majority of tests the aminergic agonists and antagonists caused a parallel shift of the CO2 response curves in the same direction as the O2 response curves and by amounts proportional to the degree of hypoxia. In some tests these drugs caused a change in the slope of the CO2 response curves but only if Pa, O2 was less than 60 mmHg.
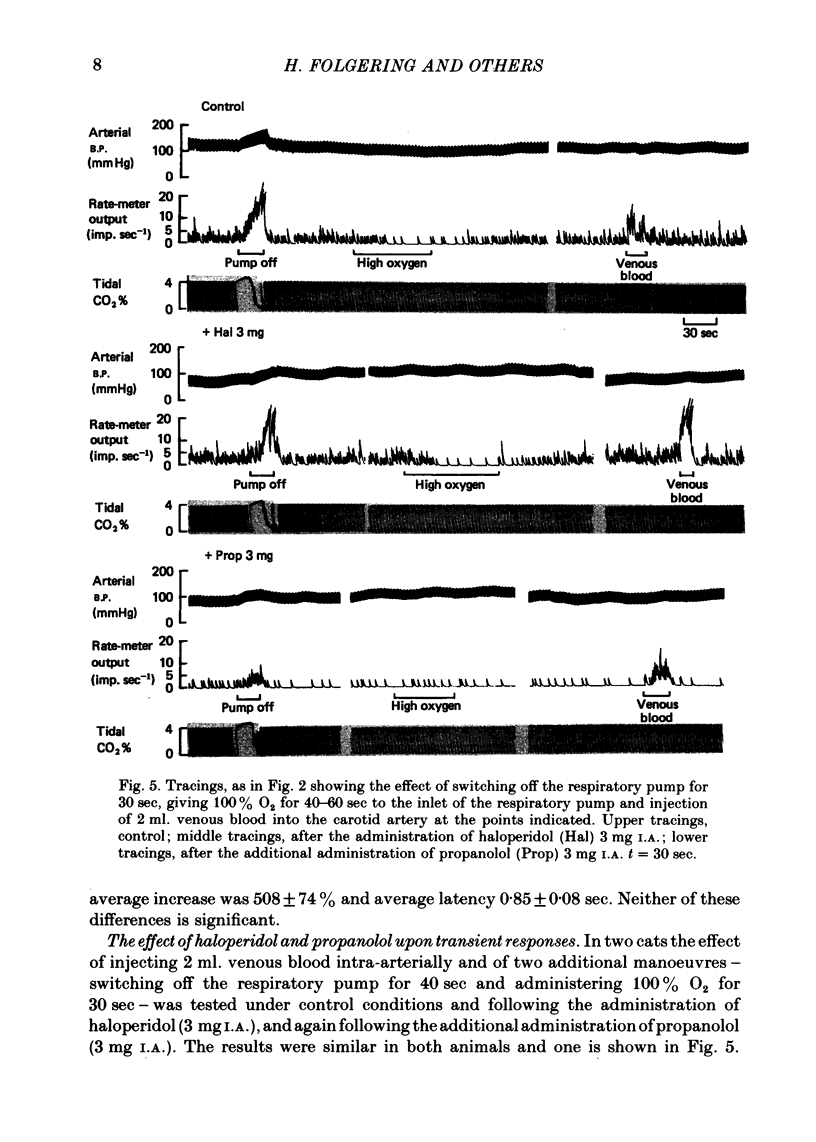
5. One interpretation of these results is that hypoxia exerts a presynaptic action, causing the release of noradrenaline and dopamine from Type I cells, and that these substances act upon aminergic receptors on the sensory fibre, causing a change in potential and discharge frequency proportional to the rates of dopamine and noradrenaline release.
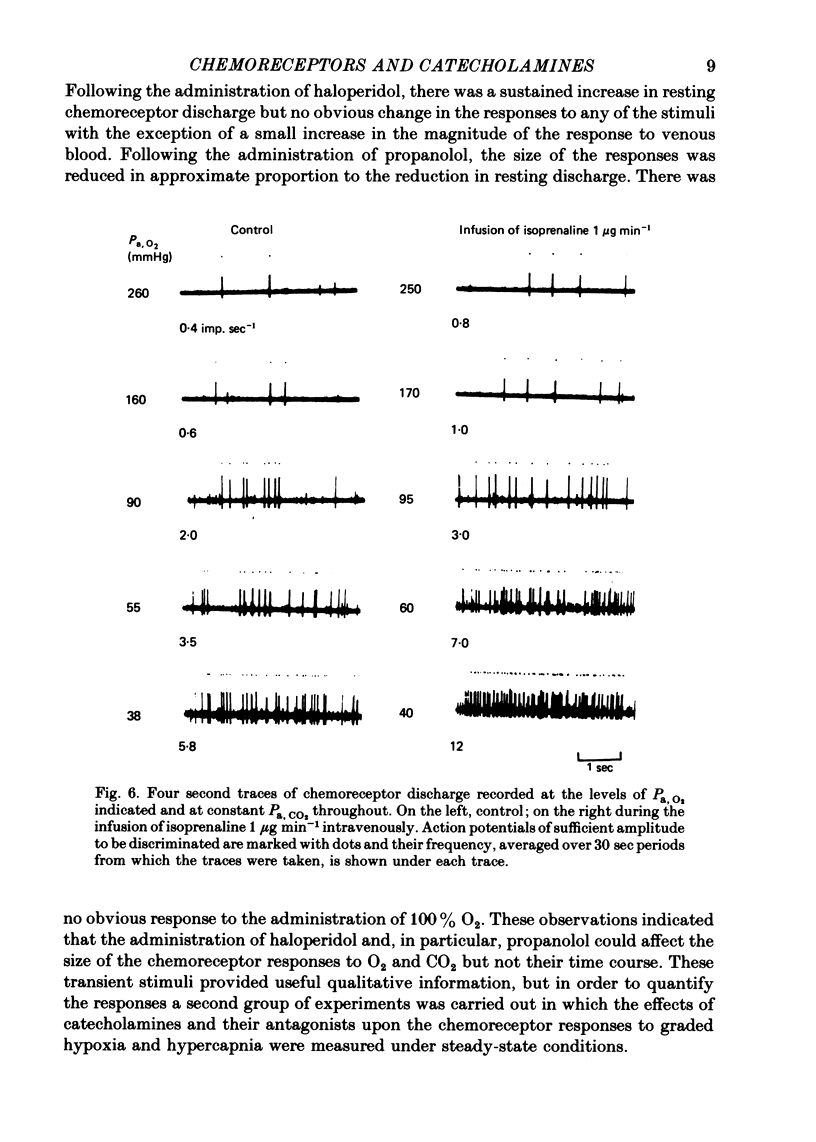
6. An additional or alternative interpretation is that O2 and CO2 (the latter most probably acting on intracellular pH) alter the sensitivity of the aminergic receptors to their agonists.
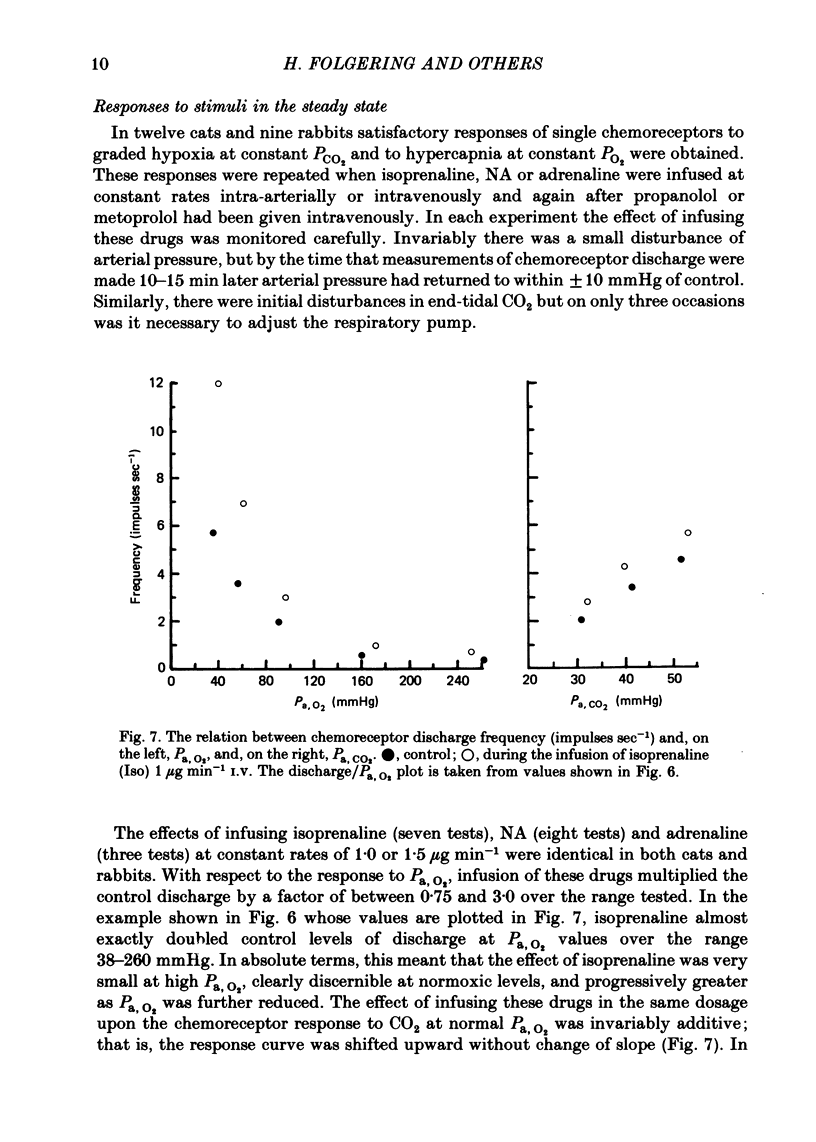
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablad B., Borg K. O., Carlsson E., EK L., Johnson G., Malmfors T., Regårdh C. G. A survey of the pharmacological properties of metoprolol in animals and man. Acta Pharmacol Toxicol (Copenh) 1975;36(Suppl 5):7–23. doi: 10.1111/j.1600-0773.1975.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Acker H., O'Regan R. G. Autonomic nervous influences upon total flow, local flow, tissue PO2 and chemosensory activity of the cat carotid body [proceedings]. J Physiol. 1979 Oct;295:95P–96P. [PubMed] [Google Scholar]
- Biscoe T. J. Carotid body: structure and function. Physiol Rev. 1971 Jul;51(3):437–495. doi: 10.1152/physrev.1971.51.3.437. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Observations on carotid body chemoreceptor activity and cervical sympathetic discharge in the cat. J Physiol. 1967 Jun;190(3):413–424. doi: 10.1113/jphysiol.1967.sp008218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970 May;208(1):121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J. Some effects of drugs on the isolated superfused carotid body. Nature. 1965 Oct 16;208(5007):294–295. doi: 10.1038/208294a0. [DOI] [PubMed] [Google Scholar]
- Bisgard G. E., Forster H. V., Klein J. P., Manohar M., Bullard V. A. Depression of ventilation by dopamine in goats--effects of carotid body excision. Respir Physiol. 1980 Jun;40(3):379–392. doi: 10.1016/0034-5687(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Bisgard G. E., Mitchell R. A., Herbert D. A. Effects of dopamine, norepinephrine and 5-hydroxytryptamine on the carotid body of the dog. Respir Physiol. 1979 May;37(1):61–80. doi: 10.1016/0034-5687(79)90092-6. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM D. J., HEY E. N., PATRICK J. M., LLOYD B. B. The effect of noradrenaline infusion on the relation between pulmonary ventilation and the alveolar PO2 and PCO2 in man. Ann N Y Acad Sci. 1963 Jun 24;109:756–771. doi: 10.1111/j.1749-6632.1963.tb13504.x. [DOI] [PubMed] [Google Scholar]
- DE BURGH DALY M., LAMBERTSEN C. J., SCHWEITZER A. Observations on the volume of blood flow and oxygen utilization of the carotid body in the cat. J Physiol. 1954 Jul 28;125(1):67–89. doi: 10.1113/jphysiol.1954.sp005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CASTRO F. Sur la structure de la synapse dans les chemocepteurs; leur mécanisme d'excitation et rôle dans la circulation sanguine locale. Acta Physiol Scand. 1951 Feb 21;22(1):14–43. doi: 10.1111/j.1748-1716.1951.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Docherty R. J., McQueen D. S. Inhibitory action of dopamine on cat carotid chemoreceptors. J Physiol. 1978 Jun;279:425–436. doi: 10.1113/jphysiol.1978.sp012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty R. J., McQueen D. S. The effects of acetylcholine and dopamine on carotid chemosensory activity in the rabbit. J Physiol. 1979 Mar;288:411–423. [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Monti-Bloch L. Similarities and differences in the physiology and pharmacology of cat and rabbit carotid bodies. Fed Proc. 1980 Jul;39(9):2653–2656. [PubMed] [Google Scholar]
- FLOYD W. F., NEIL E. The influence of the sympathetic innervation of the carotid bifurcation on chemoceptor and baroceptor activity in the cat. Arch Int Pharmacodyn Ther. 1952 Sep 1;91(1-2):230–239. [PubMed] [Google Scholar]
- Joels N., White H. The contribution of the arterial chemoreceptors to the stimulation of respiration by adrenaline and noradrenaline in the cat. J Physiol. 1968 Jul;197(1):1–23. doi: 10.1113/jphysiol.1968.sp008541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienecker E. W., Knoche H., Bingmann D. Functional properties of regenerating sinus nerve fibres in the rabbit. Neuroscience. 1978;3(10):977–988. doi: 10.1016/0306-4522(78)90118-5. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Nishino T., Mokashi A., Mulligan E. Interaction of dopamine and haloperidol with O2 and CO2 chemoreception in carotid body. J Appl Physiol Respir Environ Exerc Physiol. 1980 Jul;49(1):45–51. doi: 10.1152/jappl.1980.49.1.45. [DOI] [PubMed] [Google Scholar]
- Llados F., Zapata P. Effects of dopamine analogues and antagonists on carotid body chemosensors in situ. J Physiol. 1978 Jan;274:487–499. doi: 10.1113/jphysiol.1978.sp012162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E., Smith P. G., Slotkin T. A., Breese G. Role of carotid body catecholamines in chemoreceptor function. Neuroscience. 1978;3(12):1137–1146. doi: 10.1016/0306-4522(78)90134-3. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Sinha A. K., Mcdonald D. M. Chemoreceptive properties of regenerated endings of the carotid sinus nerve. Brain Res. 1972 Aug 25;43(2):681–685. doi: 10.1016/0006-8993(72)90430-1. [DOI] [PubMed] [Google Scholar]
- Purves M. J. The role of the cervical sympathetic nerve in the regulation of oxygen consumption of the carotid body of the cat. J Physiol. 1970 Aug;209(2):417–431. doi: 10.1113/jphysiol.1970.sp009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson S. R., Aminoff M. J., Jaffe R. A., Vidruk E. H. A pharmacological analysis of neurally induced inhibition of carotid body chemoreceptor activity in cats. J Pharmacol Exp Ther. 1976 Apr;197(1):119–125. [PubMed] [Google Scholar]
- Sampson S. R. Mechanism of efferent inhibition of carotid body chemoreceptors in the cat. Brain Res. 1972 Oct 13;45(1):266–270. doi: 10.1016/0006-8993(72)90236-3. [DOI] [PubMed] [Google Scholar]
- Scheen A., Juchmes J., Cession-Fossion A. Diminution de l'hyperventilation d'exercise par le blocage des récepteurs beta-adrénergiques. C R Seances Soc Biol Fil. 1976;170(1):220–223. [PubMed] [Google Scholar]
- Smith P. G., Mills E. Physiological and ultrastructural observations on regenerated carotid sinus nerves after removal of the carotid bodies in cats. Neuroscience. 1979;4(12):2009–2020. doi: 10.1016/0306-4522(79)90072-1. [DOI] [PubMed] [Google Scholar]
- Verna A., Roumy M., Leitner L. M. Loss of chemoreceptive properties of the rabbit carotid body after destruction of the glomus cells. Brain Res. 1975 Dec 12;100(1):13–23. doi: 10.1016/0006-8993(75)90239-5. [DOI] [PubMed] [Google Scholar]
- Verna A. Ulstrastructure of the carotid body in the mammals. Int Rev Cytol. 1979;60:271–330. doi: 10.1016/s0074-7696(08)61265-6. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Mitchell R. A., Berger A. J., Casaburi R., Davis J. A. Mechanism of the isoproterenol hyperpnea in the cat. Respir Physiol. 1979 Dec;38(3):359–376. doi: 10.1016/0034-5687(79)90061-6. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Heistad D. D., Abboud F. M. Depression of ventilation by dopamine in man. Evidence for an effect on the chemoreceptor reflex. J Clin Invest. 1978 Mar;61(3):708–713. doi: 10.1172/JCI108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn R., Hildebrandt J. R., Hildebrandt J. Cardiorespiratory responses following isoproterenol injection in rabbits. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):352–359. doi: 10.1152/jappl.1979.47.2.352. [DOI] [PubMed] [Google Scholar]
- Zapata P., Hess A., Bliss E. L., Eyzaguirre C. Chemical, electron microscopic and physiological observations on the role of catecholamines in the carotid body. Brain Res. 1969 Jul;14(2):473–496. doi: 10.1016/0006-8993(69)90123-1. [DOI] [PubMed] [Google Scholar]
- Zapata P., Hess A., Eyzaguirre C. Reinnervation of carotid body and sinus with superior laryngeal nerve fibers. J Neurophysiol. 1969 Mar;32(2):215–228. doi: 10.1152/jn.1969.32.2.215. [DOI] [PubMed] [Google Scholar]
- Zapata P., Stensaas L. J., Eyzaguirre C. Axon regeneration following a lesion of the carotid nerve: electrophysiological and ultrastructural observations. Brain Res. 1976 Aug 27;113(2):235–253. doi: 10.1016/0006-8993(76)90939-2. [DOI] [PubMed] [Google Scholar]