Abstract
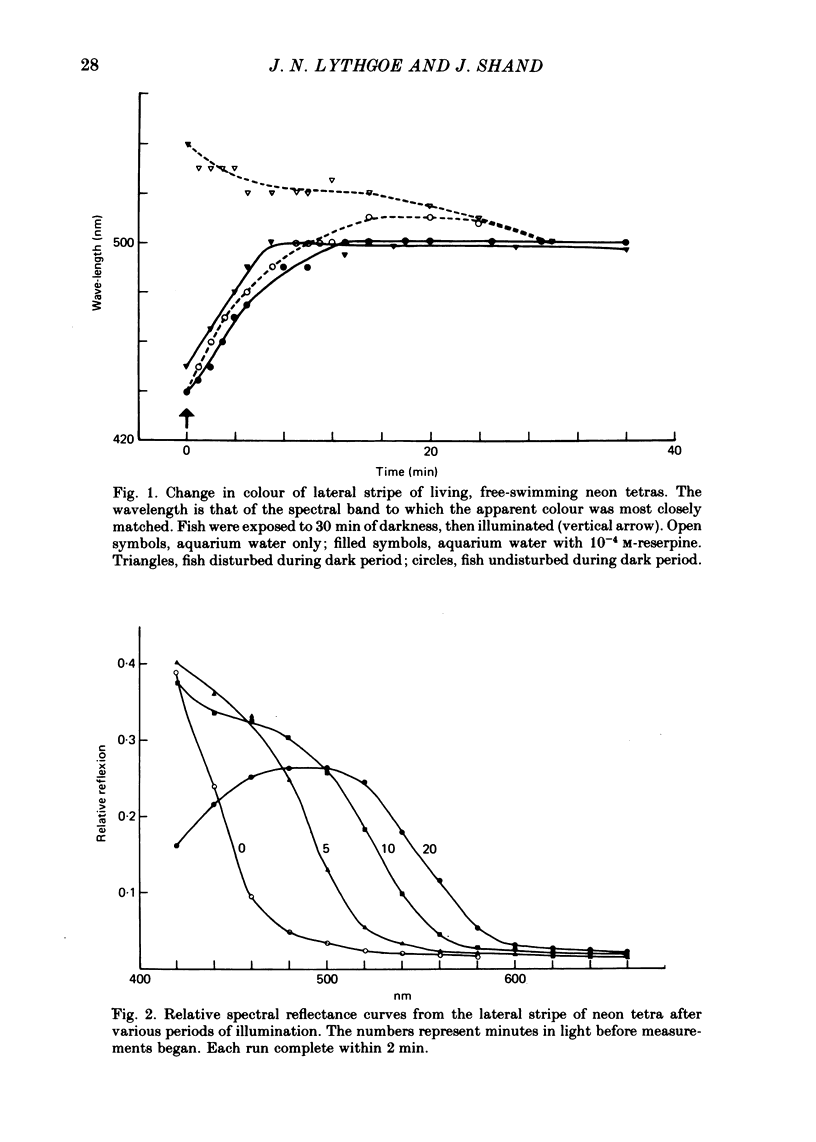
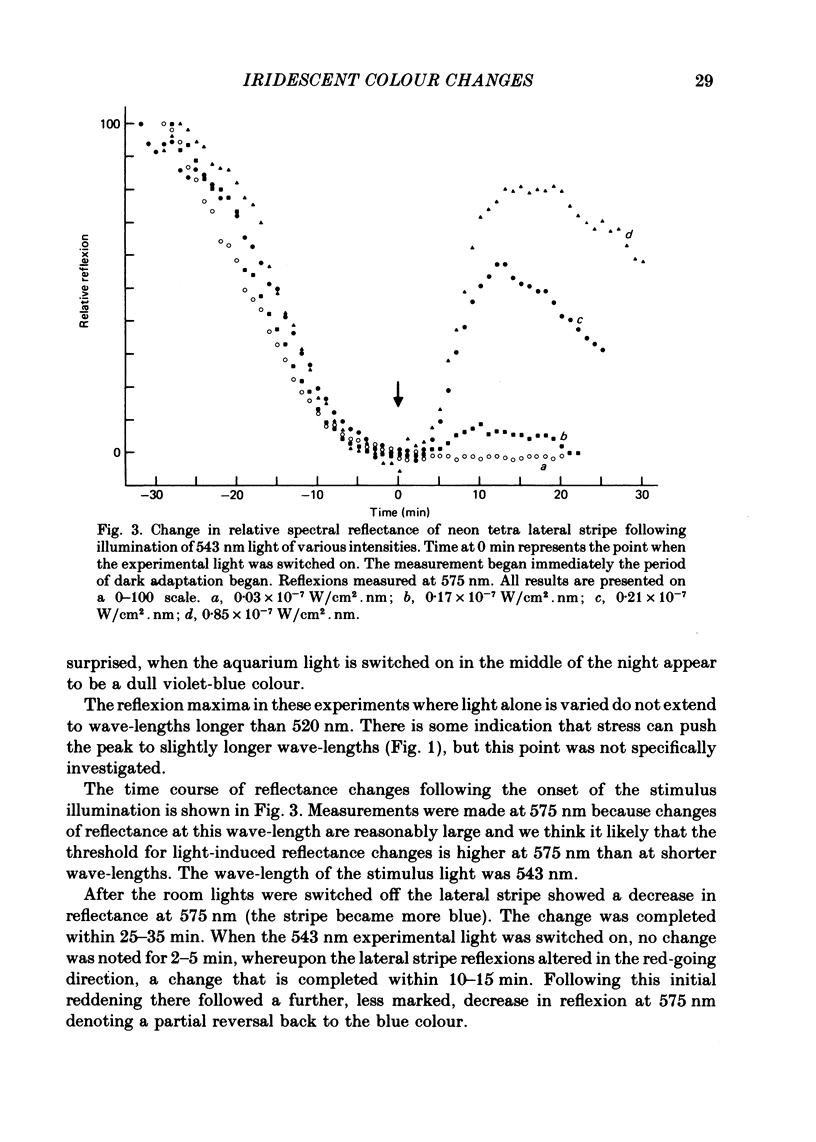
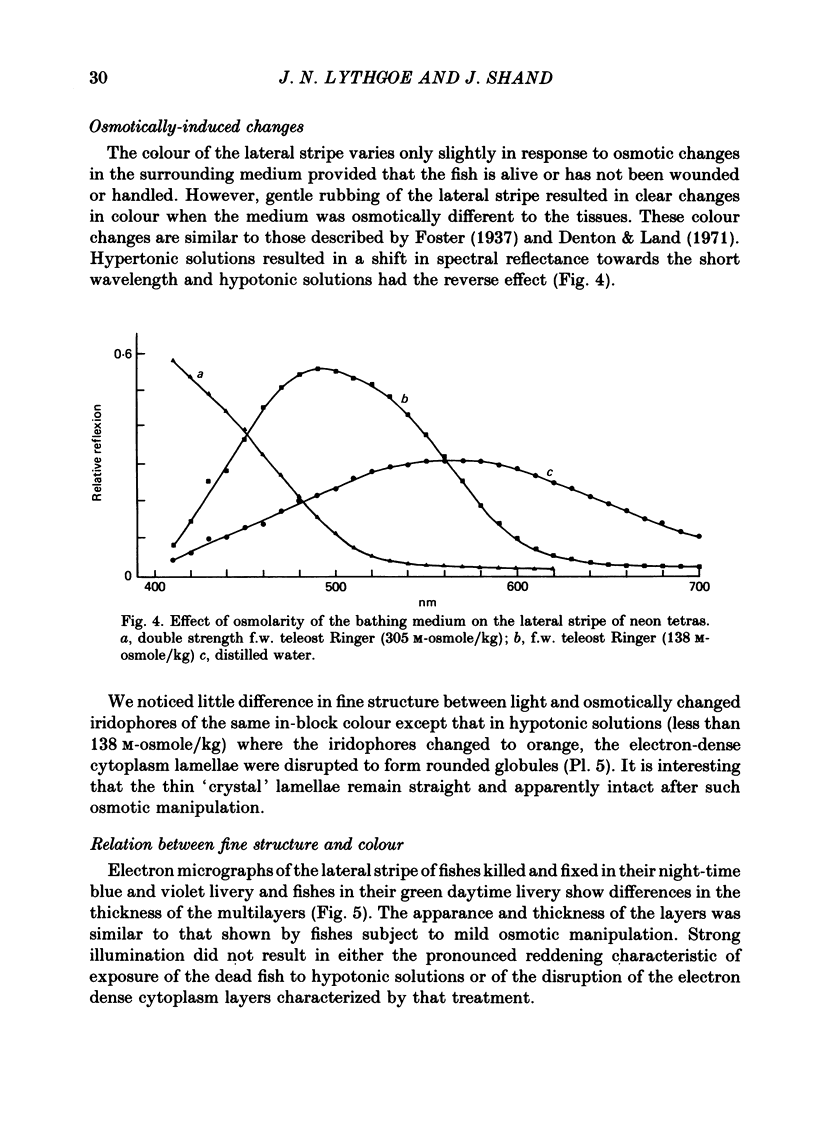
1. The iridescent stripe of the freshwater teleost, the neon tetra, changes from green in the daytime to violet-blue at night. 2. Spectral reflectance measurements were used to follow these colour changes. 3. Light causes a shift in reflectance to longer wavelengths in living fish and in isolated tissue from the lateral stripe. The change is reversed in darkness. 4. The spectral reflectance shifts to longer wavelengths when the fish is disturbed in darkness. No such colour changes were seen in fishes kept alive in 10(-4) M-reserpine. 5. Hypotonic Ringer solution causes a reflectance shift to longer wavelengths and hypertonic solution causes a shift to shorter wavelengths. 6. The iridescent reflexions from the lateral stripe which is continued across the iris originate from iridophores in the dermis. These iridophores contain regular stacks of broad, double-sided hexagonal plates that are about 10 nm thick. Each plate is contained within a pouch in the cytoplasm and is separated from its neighbour by approximately one quarter the wavelength of light. 7. A distinction is drawn between the physiologically active iridophores in the lateral stripe and iris that have broad hexagonal crystal plates which are very thin and the physiologically inactive iridophores that are also found in the iris, but in addition are found on the flanks below the lateral stripe, and on the head. These iridophores contain hexagonal crystals that are usually narrower than the active type, but are about 60-100 nm thick.
Full text
PDF




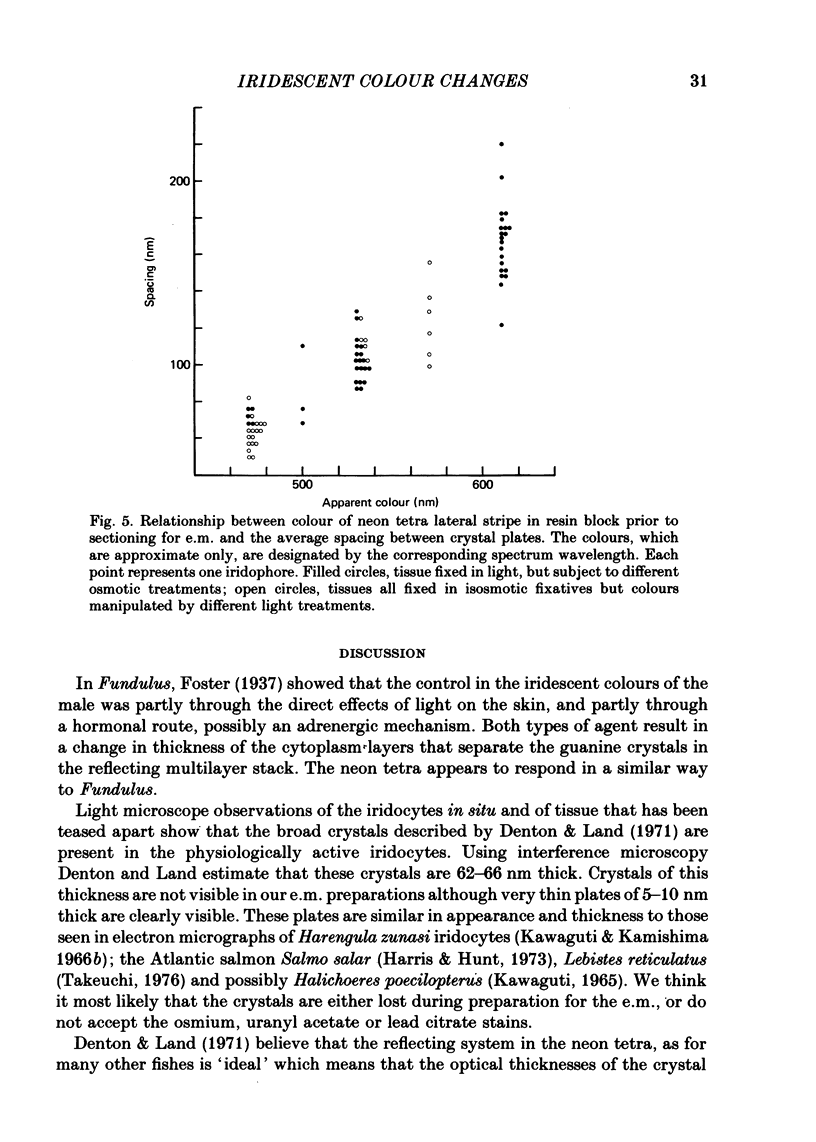







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker H. E., Cone R. A. Light-stimulated electrical responses from skin. Science. 1966 Nov 25;154(3752):1051–1053. doi: 10.1126/science.154.3752.1051. [DOI] [PubMed] [Google Scholar]
- Bone Q., Denton E. J. The osmotic effects of electron microscope fixatives. J Cell Biol. 1971 Jun;49(3):571–581. doi: 10.1083/jcb.49.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton E. J., Land M. F. Mechanism of reflexion in silvery layers of fish and cephalopods. Proc R Soc Lond B Biol Sci. 1971 Jun 15;178(1050):43–61. doi: 10.1098/rspb.1971.0051. [DOI] [PubMed] [Google Scholar]
- Foster K. W. Color Changes in Fundulus with Special Reference to the Color Changes of the Iridosomes. Proc Natl Acad Sci U S A. 1933 May;19(5):535–540. doi: 10.1073/pnas.19.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. E., Hunt S. The fine structure of iridosphores in the skin of the Atlantic salmon (Salmo salar L.). Tissue Cell. 1973;5(3):479–488. doi: 10.1016/s0040-8166(73)80039-4. [DOI] [PubMed] [Google Scholar]
- Harth M. S., Heaton M. B. Nonvisual photic responsiveness in newly hatched pigeons (Columba livia). Science. 1973 May 18;180(4087):753–755. doi: 10.1126/science.180.4087.753. [DOI] [PubMed] [Google Scholar]
- Land M. F. The physics and biology of animal reflectors. Prog Biophys Mol Biol. 1972;24:75–106. doi: 10.1016/0079-6107(72)90004-1. [DOI] [PubMed] [Google Scholar]
- Rohrlich S. T. Fine structural demonstration of ordered arrays of cytoplasmic filaments in vertebrate iridophores. A comparative survey. J Cell Biol. 1974 Aug;62(2):295–304. doi: 10.1083/jcb.62.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguti G. Ultrastructure of guanophores. J Ultrastruct Res. 1967 May;18(3):324–332. doi: 10.1016/s0022-5320(67)80121-7. [DOI] [PubMed] [Google Scholar]
- Steven D. M. The dermal light sense. Biol Rev Camb Philos Soc. 1963 May;38(2):204–240. doi: 10.1111/j.1469-185x.1963.tb00783.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi I. K. Electron microscopy of two types of reflecting chromatophores (iridophores and leucophores) in the guppy, Lebistes reticulatus Peters. Cell Tissue Res. 1976 Oct 1;173(1):17–27. doi: 10.1007/BF00219263. [DOI] [PubMed] [Google Scholar]