Abstract
1. The structures of seven γ-motoneurones (axonal conduction velocities of 15-48 m/sec) were compared with those of nine α-motoneurones (axonal conduction velocities of 71-91 m/sec) by using histochemical methods to reveal horseradish peroxidase which had previously been injected intracellularly into indentified motoneurones in the cat lumbosacral spinal cord.
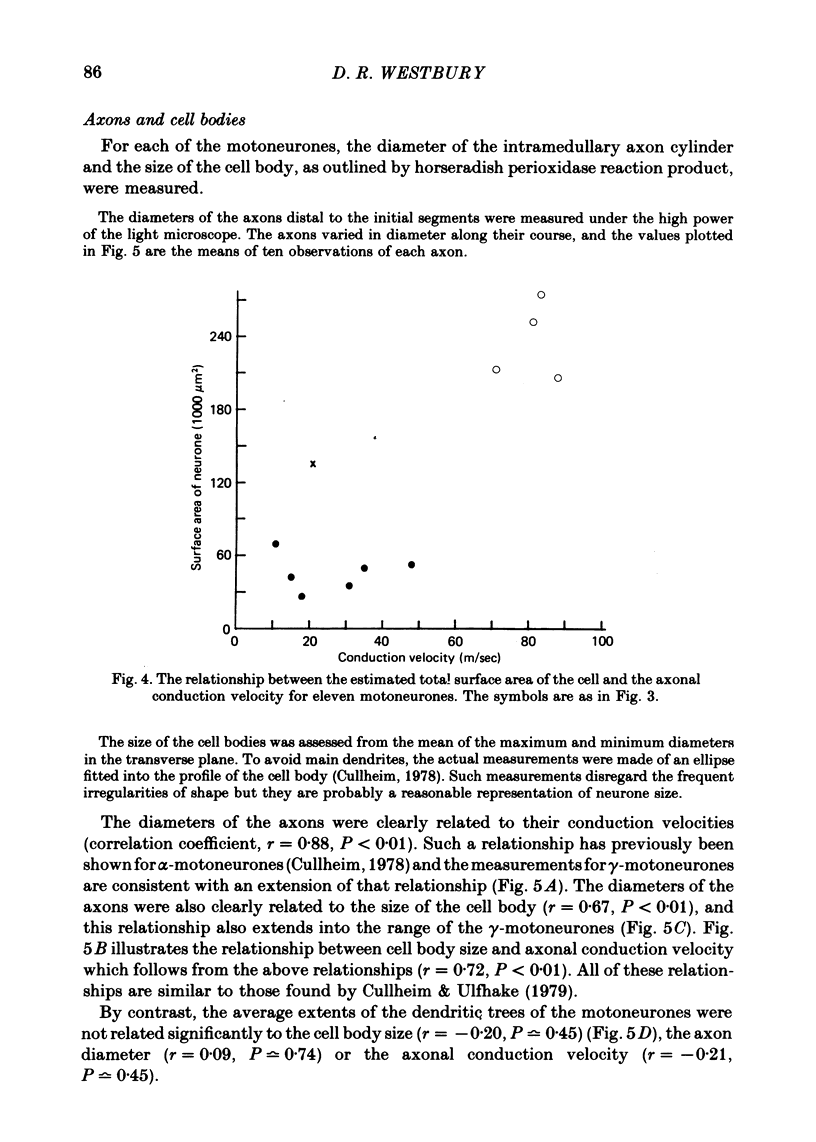
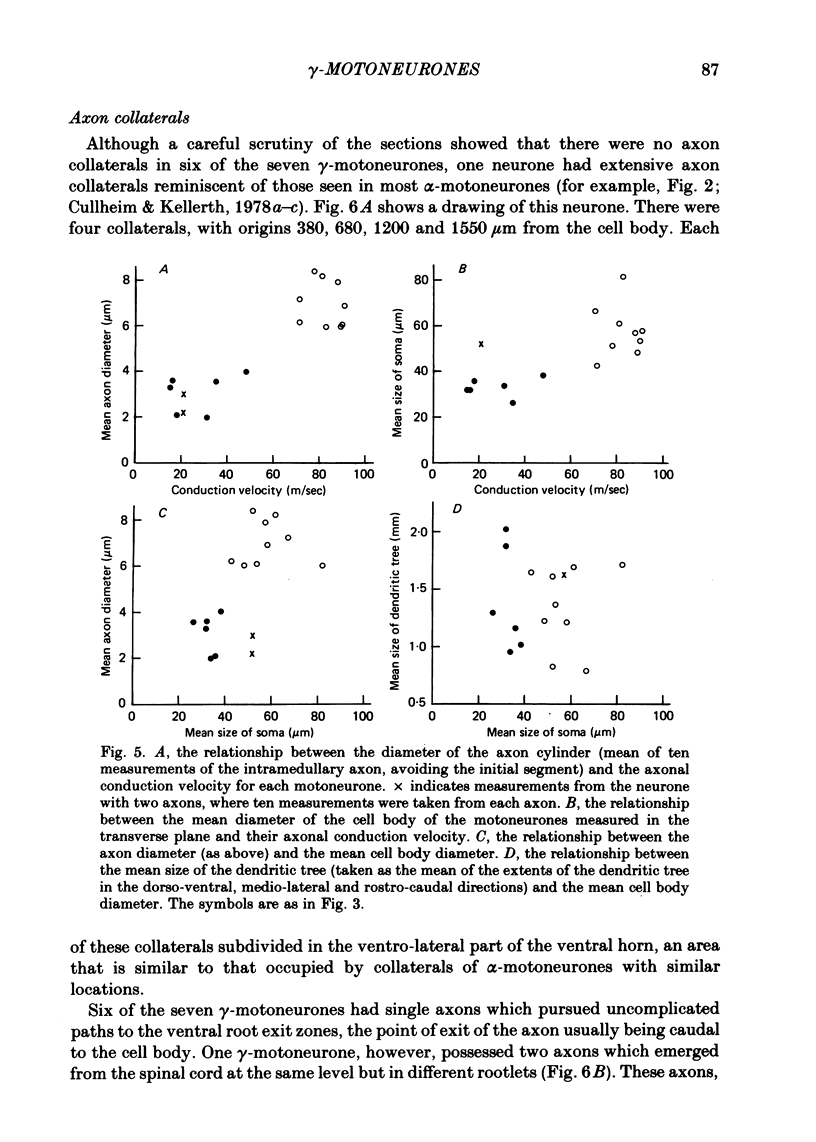
2. The size of the cell bodies of the motoneurones, and the diameters of their intramedullary axons, were related to their axonal conduction velocities over the whole range studied.
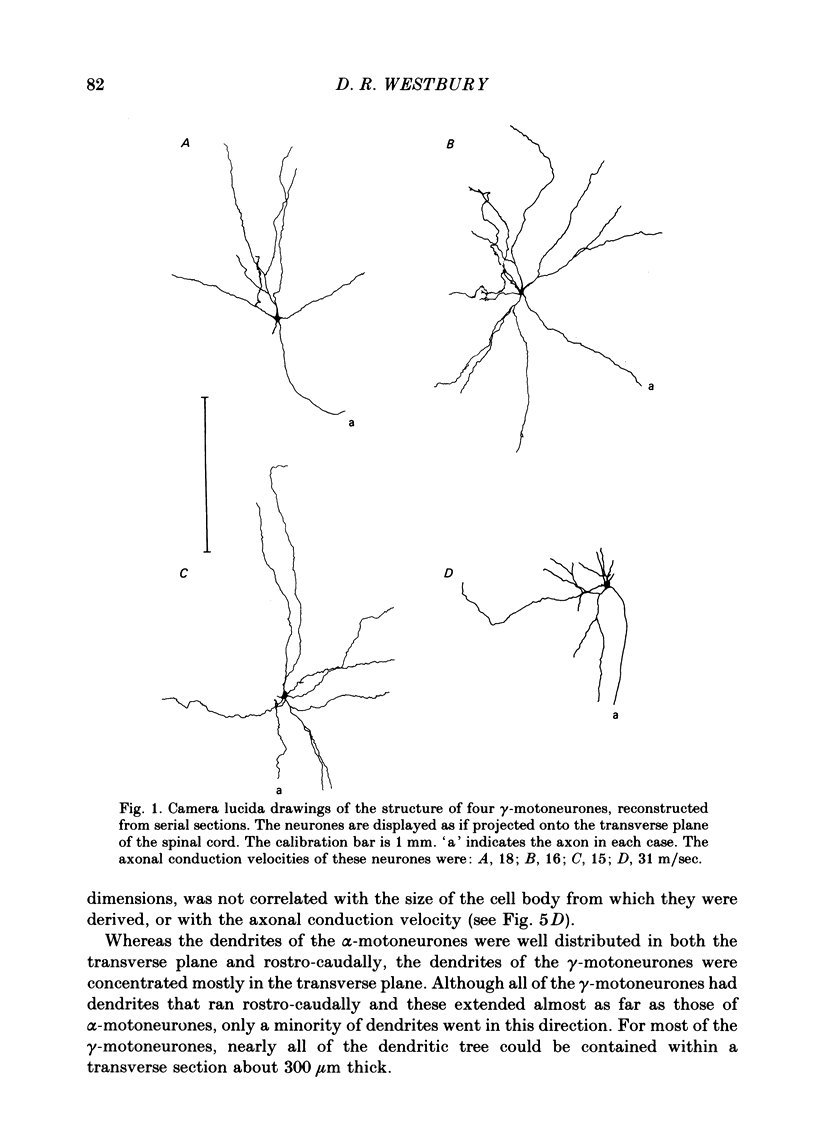
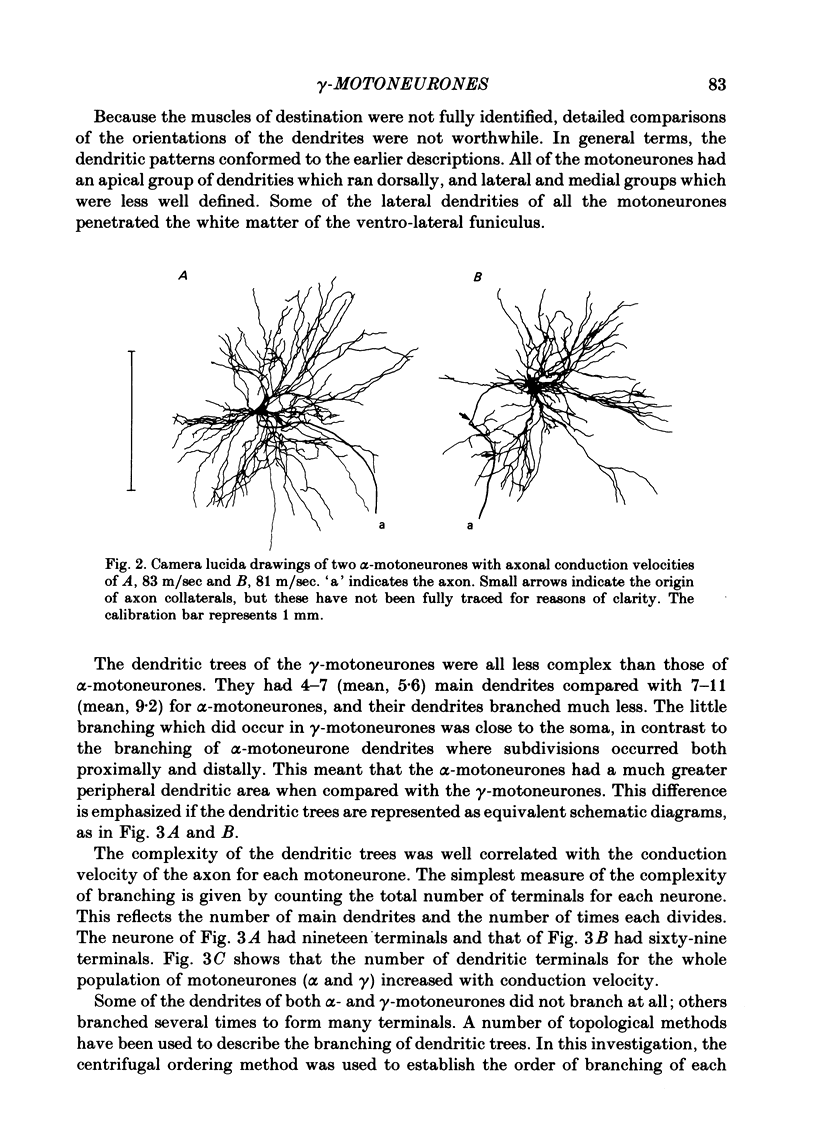
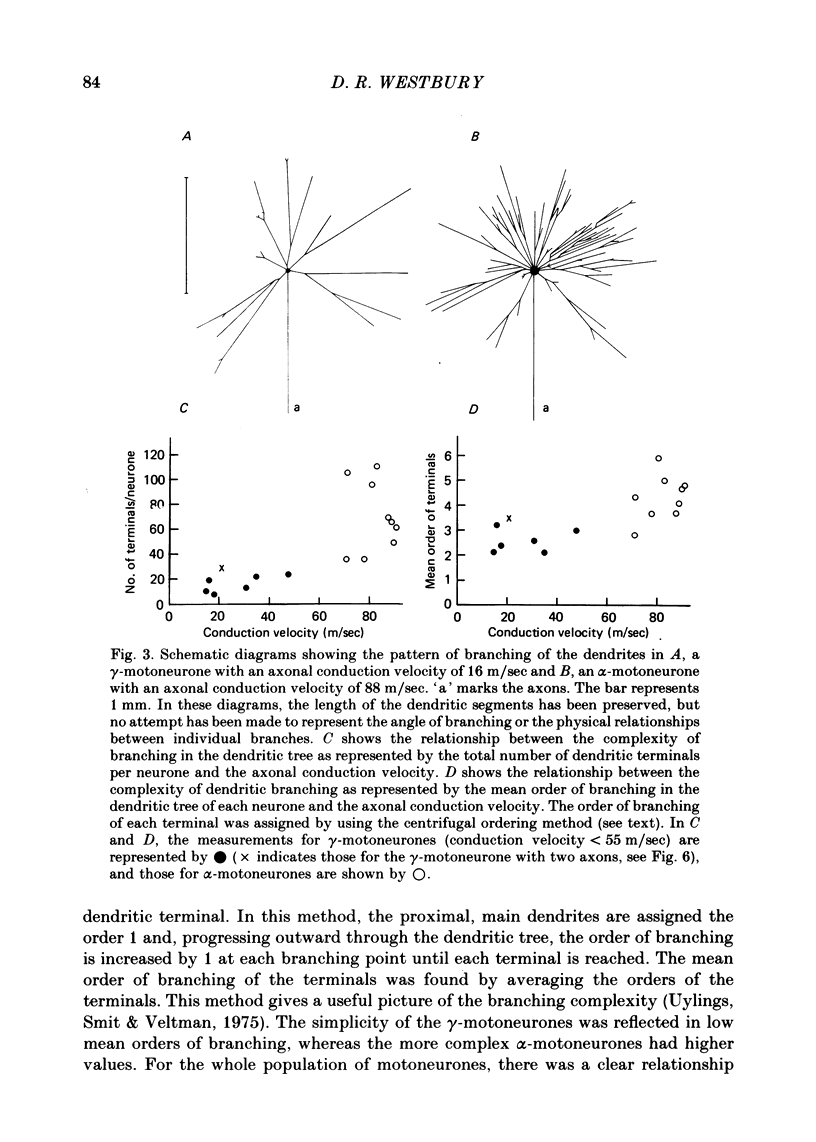
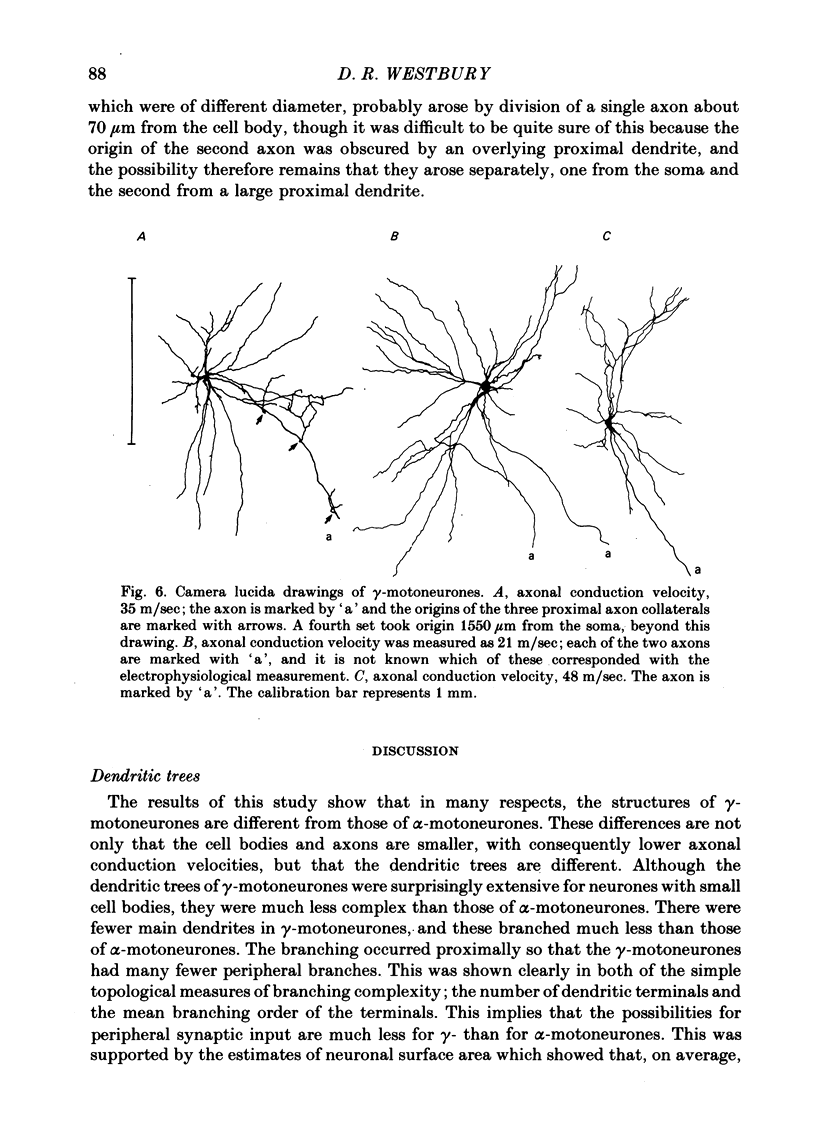
3. Despite the smaller size of the cell bodies of the γ-motoneurones, their dendritic trees extended as far as those of the α-motoneurones. However, γ-motoneurones had fewer main dendrites than the α-motoneurones and these branched much less, so that the dendritic trees of the γ-motoneurones were much simpler than those of α-motoneurones. Although the extents of the dendritic trees were not related to axonal conduction velocity, the complexity of the dendritic trees was clearly related to axonal conduction velocity and to cell body size.
4. The total surface area of each cell, taken as an indication of the area available for synaptic contact, was much smaller for γ- than for α-motoneurones, and was related to axonal conduction velocity.
5. Only one of the seven γ-motoneurones studied had axon collaterals whereas five of the nine α-motoneurones had well developed collaterals. This finding is consistent with the relative contribution that each group of motoneurone axons makes to recurrent inhibition.
6. One of the γ-motoneurones had two axons, of different diameter, which emerged from the spinal cord at the same level but in different ventral rootlets.
7. These features of motoneurone structure are related to aspects of their physiological properties.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AITKEN J. T., BRIDGER J. E. Neuron size and neuron population density in the lumbosacral region of the cat's spinal cord. J Anat. 1961 Jan;95:38–53. [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Specific membrane properties of cat motoneurones. J Physiol. 1974 Jun;239(2):301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat's lumbosacral spinal cord. J Physiol. 1981;313:121–140. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. N., Trevino D. L., Willis W. D. Evidence for a common location of alpha and gamma motoneurons. Brain Res. 1972 Mar 10;38(1):193–196. doi: 10.1016/0006-8993(72)90602-6. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Strick P. L., Kanda K., Kim C. C., Walmsley B. Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. J Neurophysiol. 1977 May;40(3):667–680. doi: 10.1152/jn.1977.40.3.667. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different functional types of muscle unit. J Physiol. 1978 Aug;281:301–313. doi: 10.1113/jphysiol.1978.sp012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different hind-limb muscles. J Physiol. 1978 Aug;281:285–299. doi: 10.1113/jphysiol.1978.sp012422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat sciatic alpha-motoneurons after intracellular staining with horseradish peroxidase. J Comp Neurol. 1978 Apr 1;178(3):537–557. doi: 10.1002/cne.901780309. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Ulfhake B. Observations on the morphology of intracellularly stained gamma-motoneurons in relation to their axon conduction velocity. Neurosci Lett. 1979 Jun;13(1):47–50. doi: 10.1016/0304-3940(79)90073-9. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger C., Baker R., McCrea R. A. Axon collaterals of cat medial rectus motoneurons. Brain Res. 1979 Sep 28;174(1):153–160. doi: 10.1016/0006-8993(79)90810-2. [DOI] [PubMed] [Google Scholar]
- Grantyn R., Grantyn A. Morphological and electrophysiological properties of cat abducens motoneurons. Exp Brain Res. 1978 Feb 15;31(2):249–274. doi: 10.1007/BF00237603. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., PAINTAL A. S. Spinal reflex regulation of fusimotor neurones. J Physiol. 1958 Sep 23;143(2):195–212. doi: 10.1113/jphysiol.1958.sp006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. The reflex activity of mammalian small-nerve fibres. J Physiol. 1951 Dec 28;115(4):456–469. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C., QUILLIAM J. P. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951 Jan;14(1):29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- Kemm R. E., Westbury D. R. Some properties of spinal gamma-motoneurones in the cat, determined by micro-electrode recording. J Physiol. 1978 Sep;282:59–71. doi: 10.1113/jphysiol.1978.sp012448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Zwaagstra B. Input conductance axonal conduction velocity and cell size among hindlimb motoneurones of the cat. Brain Res. 1981 Jan 12;204(2):311–326. doi: 10.1016/0006-8993(81)90591-6. [DOI] [PubMed] [Google Scholar]
- Laporte Y., Emonet-Dénand F. The skeleto-fusimotor innervation of cat muscle spindle. Prog Brain Res. 1976;44:99–109. doi: 10.1016/s0079-6123(08)60726-8. [DOI] [PubMed] [Google Scholar]
- Murthy K. S. Vertebrate fusimotor neurones and their influences on motor behavior. Prog Neurobiol. 1978;11(3-4):249–307. doi: 10.1016/0301-0082(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Snow P. J., Rose P. K., Brown A. G. Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science. 1976 Jan 23;191(4224):312–313. doi: 10.1126/science.54936. [DOI] [PubMed] [Google Scholar]
- Westbury D. R. Lack of a contribution from gamma motoneurone axons to Renshaw inhibition in the cap spinal cord. Brain Res. 1980 Mar 17;186(1):217–221. doi: 10.1016/0006-8993(80)90269-3. [DOI] [PubMed] [Google Scholar]


