Abstract
1. The increase in miniature end-plate potential (m.e.p.p.) frequency in response to tetanic stimulation of the motor nerve at frog neuromuscular junctions in Ca2+-free, Mg2+ EGTA-containing (0 Ca2+—Mg2+ EGTA) solutions of varying tonicity has been studied. The response to stimulation is markedly increased in hypertonic solutions and is decreased in hypotonic solutions. Under these conditions changes in tonicity have comparable effects on stimulated and spontaneous quantal release.
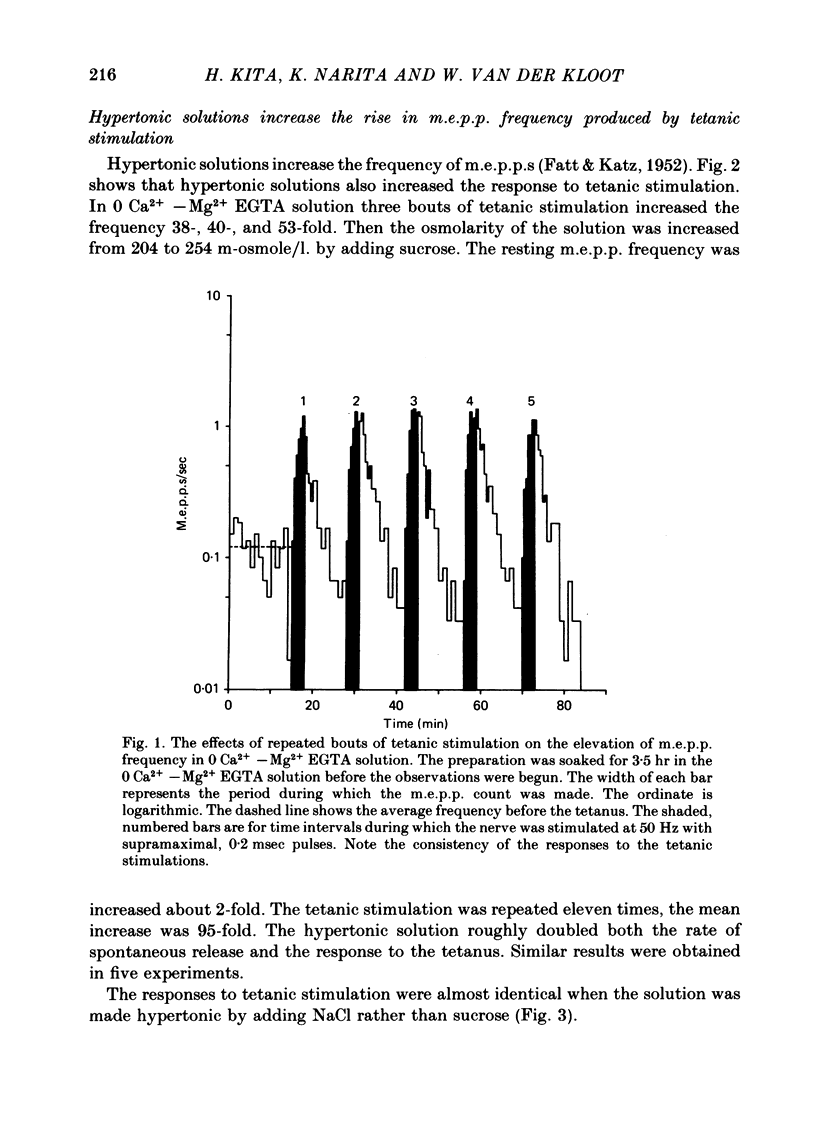
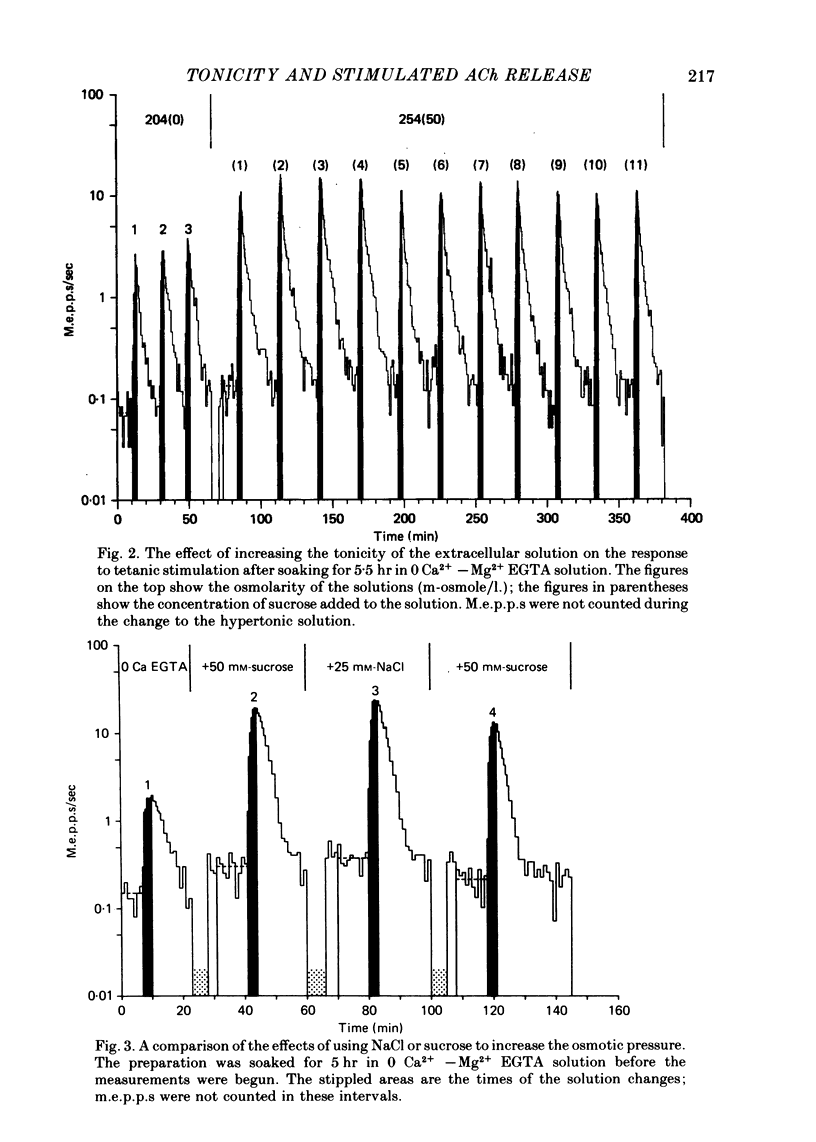
2. The tonicity was raised by adding sucrose, NaCl or glycine to the extracellular solution. The effects of the addition depended primarily on the increase in osmolarity of the solution, not on the chemical species producing it.
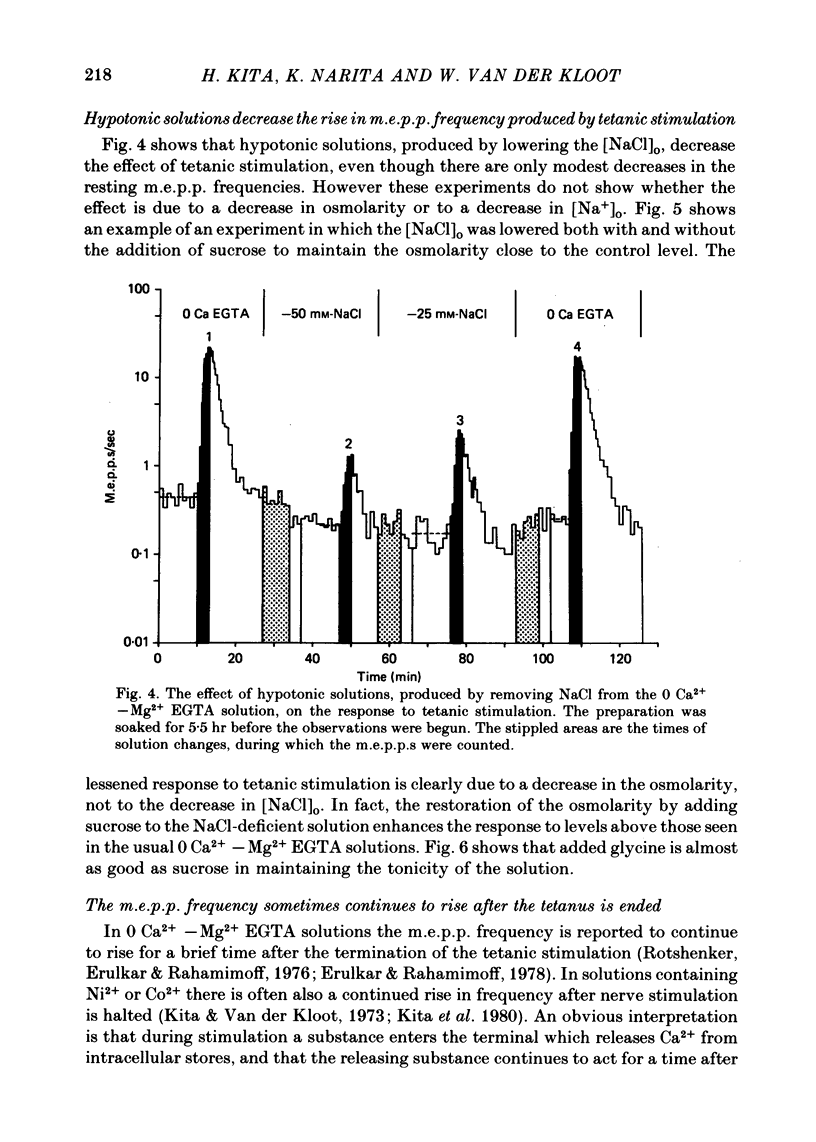
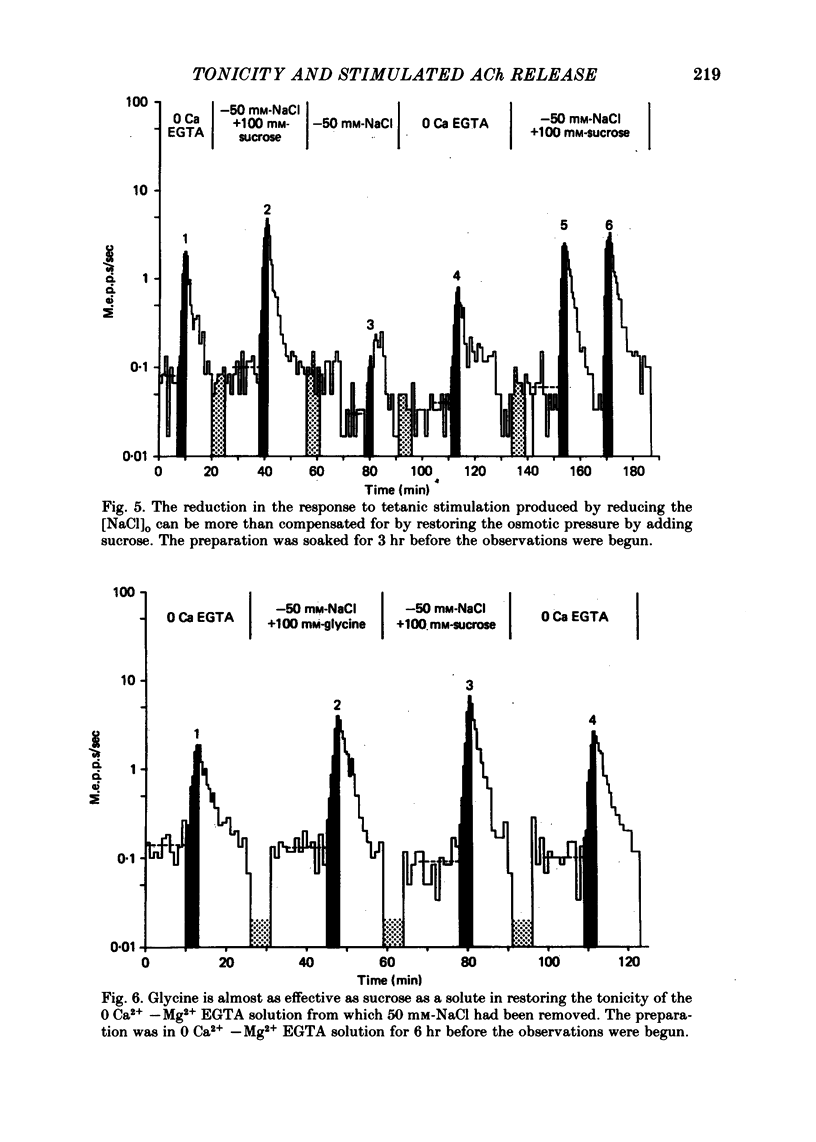
3. The tonicity was decreased by lowering [NaCl]o. The hypotonic solution decreased the response to tetanic stimulation. When the tonicity of the solution with the low [NaCl]o was restored to normal by adding sucrose, the response was restored to its usual level. These results suggest that in 0 Ca2+—Mg2+ EGTA solutions stimulation does not enhance the probability of quantal release by raising [Na+]i.
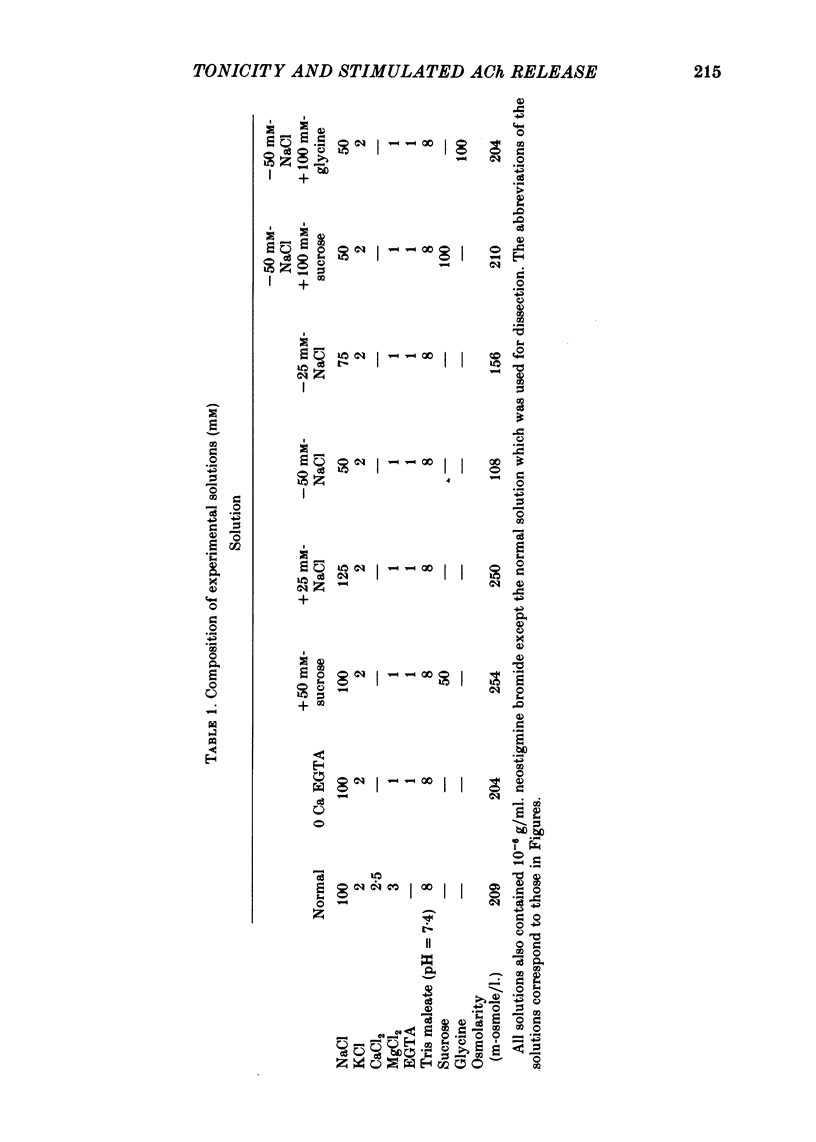
4. Repeated bouts of tetanic stimulation produced almost identical responses. In some instances the frequency continued to rise after the end of the tetanic stimulation, as reported by Erulkar & Rahamimoff (1978). This suggests that the stimulation of the nerve leads to the elevation within the terminal of a substance that in turn liberates an activator for quantal release.
5. The Q10 for the increase in probability of quantal release is as high as 7. High Q10 values have also been reported for spontaneous m.e.p.p. frequencies. Tonicity and temperature appear to affect spontaneous and stimulated quantal release similarly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Erulkar S. D., Rahamimoff R. The role of calcium ions in tetanic and post-tetanic increase of miniature end-plate potential frequency. J Physiol. 1978 May;278:501–511. doi: 10.1113/jphysiol.1978.sp012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. An examination of the effects of osmotic pressure changes upon transmitter release from mammalian motor nerve terminals. J Physiol. 1968 Aug;197(3):639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Narita K., Van der Kloot W. Tetanic stimulation increases the frequency of miniature end-plate potentials at the frog neuromuscular junction in Mn2+-, CO2+-, and Ni2+-saline solutions. Brain Res. 1981 Jan 26;205(1):111–121. doi: 10.1016/0006-8993(81)90723-x. [DOI] [PubMed] [Google Scholar]
- Kita H., Narita K., van der Kloot W. Effects of temperature on the decline in miniature end-plate potential frequency following a tetanus. Brain Res. 1980 May 26;190(2):435–445. doi: 10.1016/0006-8993(80)90286-3. [DOI] [PubMed] [Google Scholar]
- Kita H., Van Der Kloot W. Effects of the ionophore X-537A on acetylcholine release at the frog neuromuscular junction. J Physiol. 1976 Jul;259(1):177–198. doi: 10.1113/jphysiol.1976.sp011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Action of Co and Ni at the frog neuromuscular junction. Nat New Biol. 1973 Sep 12;245(141):52–53. doi: 10.1038/newbio245052a0. [DOI] [PubMed] [Google Scholar]
- Kita H., van der Kloot W. Time course and magnitude of effects of changes in tonicity on acetylcholine release at frog neuromuscular junction. J Neurophysiol. 1977 Mar;40(2):212–224. doi: 10.1152/jn.1977.40.2.212. [DOI] [PubMed] [Google Scholar]
- Landau E. M., Nachshen D. A. The interaction of pH and divalent cations at the neuromuscular junction. J Physiol. 1975 Oct;251(3):775–790. doi: 10.1113/jphysiol.1975.sp011121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Calcium transients recorded with arsenazo III in the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):197–211. doi: 10.1098/rspb.1981.0034. [DOI] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Kuba K. Inhibitory action of Ca2+ on spontaneous transmitter release at motor nerve terminals in a high K+ solution. Pflugers Arch. 1980 Jul;386(1):29–34. doi: 10.1007/BF00584183. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Meiri H., Erulkar S. D., Barenholz Y. Changes in transmitter release induced by ion-containing liposomes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5214–5216. doi: 10.1073/pnas.75.10.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S., Erulkar S. D., Rahamimoff R. Reduction in the frequency of miniature end-plate potentials by nerve stimulation in low calcium solutions. Brain Res. 1976 Jan 16;101(2):362–365. doi: 10.1016/0006-8993(76)90277-8. [DOI] [PubMed] [Google Scholar]
- Shimoni Y., Alnaes E., Rahamimoff R. Is hyperosmotic neurosecretion from motor nerve endings a calcium-dependent process? Nature. 1977 May 12;267(5607):170–172. doi: 10.1038/267170a0. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Motor end-plate 'desensitization' by repetitive nerve stimuli. J Physiol. 1959 Oct;148:659–664. doi: 10.1113/jphysiol.1959.sp006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]


