Abstract
1. Uptake of calcium was studied in spontaneously contracting monolayers of cultured chick embryo ventricular cells. Ca exchange could be separated into two components: a rapid phase with a rate constant of 3·91/min, accounting for 1·6 nmol/mg protein; and a slower phase with a rate constant of 0·069/min, accounting for 2·7 nmol/mg protein.
2. Negatively inotropic concentrations of the slow Ca channel blocker verapamil inhibited the rapid phase of Ca uptake partially, with a maximum inhibition of 30-40% observed at concentrations of verapamil which completely inhibited contraction.
3. The component of Ca uptake not inhibited by verapamil could be stimulated up to 25-fold by elevation of intracellular Na concentration and reduction of extracellular Na concentration, and thus appeared to represent at least in part Ca uptake via Na—Ca exchange.
4. Ca uptake by cultured cells could be almost completely inhibited by exposure to LaCl3, 1 mmol/l, within 5 s. This same concentration of La completely inhibited contraction within 5 s.
5. During efflux of 45Ca from cells, exposure to La (1 mmol/l) slightly inhibited efflux initially with more marked inhibition of Ca influx after 3 min of La exposure. There was no evidence for a component of superficial La-displaceable Ca, and thus the rapid phase of Ca uptake probably is due to an intracellular rapidly exchanging Ca pool. Efflux of Ca from this rapidly exchanging intracellular Ca pool was not significantly altered by exposure to Na-free choline chloride solutions.
6. We conclude that rapid Ca uptake in these cultured myocardial cells is due primarily to Ca influx via the slow Ca channel and via Na—Ca exchange. In the presence of physiological [Ca2+]i, efflux of Ca from this intracellular Ca pool does not appear to be due to Na—Ca exchange.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Eisner D., Cohen I. Voltage clamp and tracer flux data: effects of a restricted extra-cellular space. Q Rev Biophys. 1979 Aug;12(3):213–261. doi: 10.1017/s0033583500005448. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
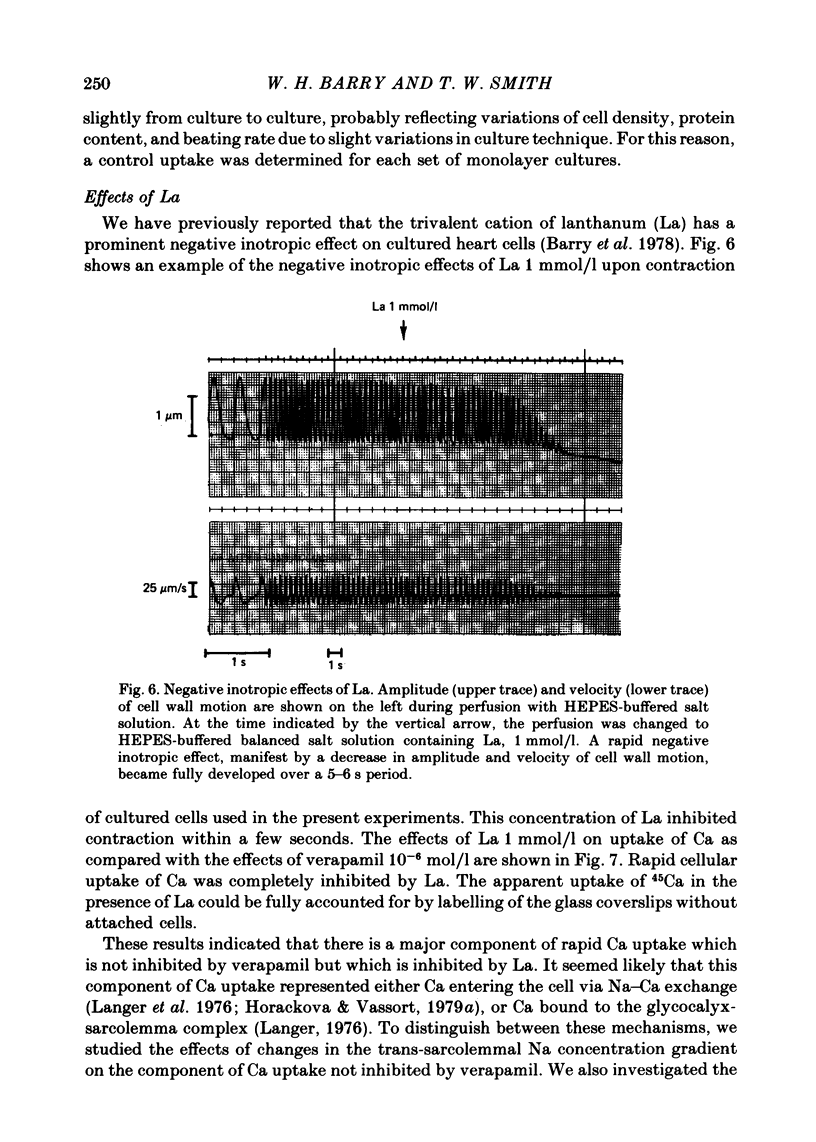
- Barry W. H., Goldminz D., Kimball T., Fitzgerald J. W. Influence of cell dissociation and culture of chick embryo ventricle on inotropic responses to calcium and lanthanum. J Mol Cell Cardiol. 1978 Oct;10(10):967–979. doi: 10.1016/0022-2828(78)90342-5. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Pitzen R., Protas K., Harrison D. C. Inotropic effects of different calcium ion concentration on the embryonic chick ventricle. Comparison of single cultured cells and intact muscle strips. Circ Res. 1975 Jun;36(6):727–734. doi: 10.1161/01.res.36.6.727. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Pober J., Marsh J. D., Frankel S. R., Smith T. W. Effects of graded hypoxia on contraction of cultured chick embryo ventricular cells. Am J Physiol. 1980 Nov;239(5):H651–H657. doi: 10.1152/ajpheart.1980.239.5.H651. [DOI] [PubMed] [Google Scholar]
- Biedert S., Barry W. H., Smith T. W. Inotropic effects and changes in sodium and calcium contents associated with inhibition of monovalent cation active transport by ouabain in cultured myocardial cells. J Gen Physiol. 1979 Oct;74(4):479–494. doi: 10.1085/jgp.74.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Santiago E. M. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977 Oct;20(1):79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Spangler S. G., Mullins L. J. Calcium and EDTA fluxes in dialyzed squid axons. J Gen Physiol. 1975 Aug;66(2):223–250. doi: 10.1085/jgp.66.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busselen P., van Kerkhove E. The effect of sodium, calcium and metabolic inhibitors on calcium efflux from goldfish heart ventricles. J Physiol. 1978 Sep;282:263–283. doi: 10.1113/jphysiol.1978.sp012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. An ATP-dependent Ca2+-pumping system in dog heart sarcolemma. Nature. 1980 Feb 21;283(5749):765–767. doi: 10.1038/283765a0. [DOI] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Use of chlorotetracycline fluorescence to demonstrate Ca2+-induced release of Ca2+ from the sarcoplasmic reticulum of skinned cardiac cells. Nature. 1979 Sep 13;281(5727):146–148. doi: 10.1038/281146a0. [DOI] [PubMed] [Google Scholar]
- Fosset M., De Barry J., Lenoir M. C., Lazdunski M. Analysis of molecular aspects of Na+ and Ca2+ uptakes by embryonic cardiac cells in culture. J Biol Chem. 1977 Sep 10;252(17):6112–6117. [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graca A. S., van Zwieten P. A. The influence of iproveratril on calcium movements in isolated heart muscle. Eur J Pharmacol. 1971 Jun;15(1):137–140. doi: 10.1016/0014-2999(71)90089-6. [DOI] [PubMed] [Google Scholar]
- Harding S. E., Halliday J. Removal of sialic acid from cardiac sarcolemma does not affect contractile function in electrically stimulated guinea pig left atria. Nature. 1980 Aug 21;286(5775):819–821. doi: 10.1038/286819a0. [DOI] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Slow inward current and contraction in frog atrial muscle at various extracellular concentrations of Na and Ca ions. J Mol Cell Cardiol. 1979 Aug;11(8):733–753. doi: 10.1016/0022-2828(79)90400-0. [DOI] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Sodium-calcium exchange in regulation of cardiac contractility. Evidence for an electrogenic, voltage-dependent mechanism. J Gen Physiol. 1979 Apr;73(4):403–424. doi: 10.1085/jgp.73.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Glycocalyx is not required for show inward calcium current in isolated rat heart myocytes. Nature. 1980 Mar 27;284(5754):358–360. doi: 10.1038/284358a0. [DOI] [PubMed] [Google Scholar]
- Ishida A., Mashima H., Tanaka S. Intracellular distribution of calcium in cardiac muscles studied by electron microscope autoradiography. Jpn J Physiol. 1979;29(1):37–48. doi: 10.2170/jjphysiol.29.37. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung B. G., Reuter H., Porzig H. Lanthanum inhibits Ca inward current but not Na-Ca exchange in cardiac muscle. Experientia. 1973 Sep 15;29(9):1073–1075. doi: 10.1007/BF01946727. [DOI] [PubMed] [Google Scholar]
- Kitazawa T. Physiological significance of Ca uptake by mitochondria in the heart in comparison with that by cardiac sarcoplasmic reticulum. J Biochem. 1976 Nov;80(5):1129–1147. doi: 10.1093/oxfordjournals.jbchem.a131369. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer G. A. Events at the cardiac sarcolemma: localization and movement of contractile-dependent calcium. Fed Proc. 1976 May 1;35(6):1274–1278. [PubMed] [Google Scholar]
- Langer G. A., Frank J. S. Lanthanum in heart cell culture. Effect on calcium exchange correlated with its localization. J Cell Biol. 1972 Sep;54(3):441–455. doi: 10.1083/jcb.54.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A., Nudd L. M., Ricchiuti N. V. The effect of sodium deficient perfusion on calcium exchange in cardiac tissue culture. J Mol Cell Cardiol. 1976 Apr;8(4):321–328. doi: 10.1016/0022-2828(76)90006-7. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The structure and function of the myocardial cell surface. Am J Physiol. 1978 Nov;235(5):H461–H468. doi: 10.1152/ajpheart.1978.235.5.H461. [DOI] [PubMed] [Google Scholar]
- Marban E., Rink T. J., Tsien R. W., Tsien R. Y. Free calcium in heart muscle at rest and during contraction measured with Ca2+ -sensitive microelectrodes. Nature. 1980 Aug 28;286(5776):845–850. doi: 10.1038/286845a0. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Shigenobu K., Sperelakis N. Two pharmacological types of cardiac slow Na+ channels as distinguished by verapamil. Eur J Pharmacol. 1974 May;26(2):379–382. doi: 10.1016/0014-2999(74)90250-7. [DOI] [PubMed] [Google Scholar]
- Nayler W. G. An effect of ouabain on the superficially-located stores of calcium in cardiac muscle cells. J Mol Cell Cardiol. 1973 Feb;5(1):101–110. doi: 10.1016/0022-2828(73)90039-4. [DOI] [PubMed] [Google Scholar]
- Pitts B. J. Stoichiometry of sodium-calcium exchange in cardiac sarcolemmal vesicles. Coupling to the sodium pump. J Biol Chem. 1979 Jul 25;254(14):6232–6235. [PubMed] [Google Scholar]
- Reeves J. P., Sutko J. L. Sodium-calcium ion exchange in cardiac membrane vesicles. Proc Natl Acad Sci U S A. 1979 Feb;76(2):590–594. doi: 10.1073/pnas.76.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]


