Abstract
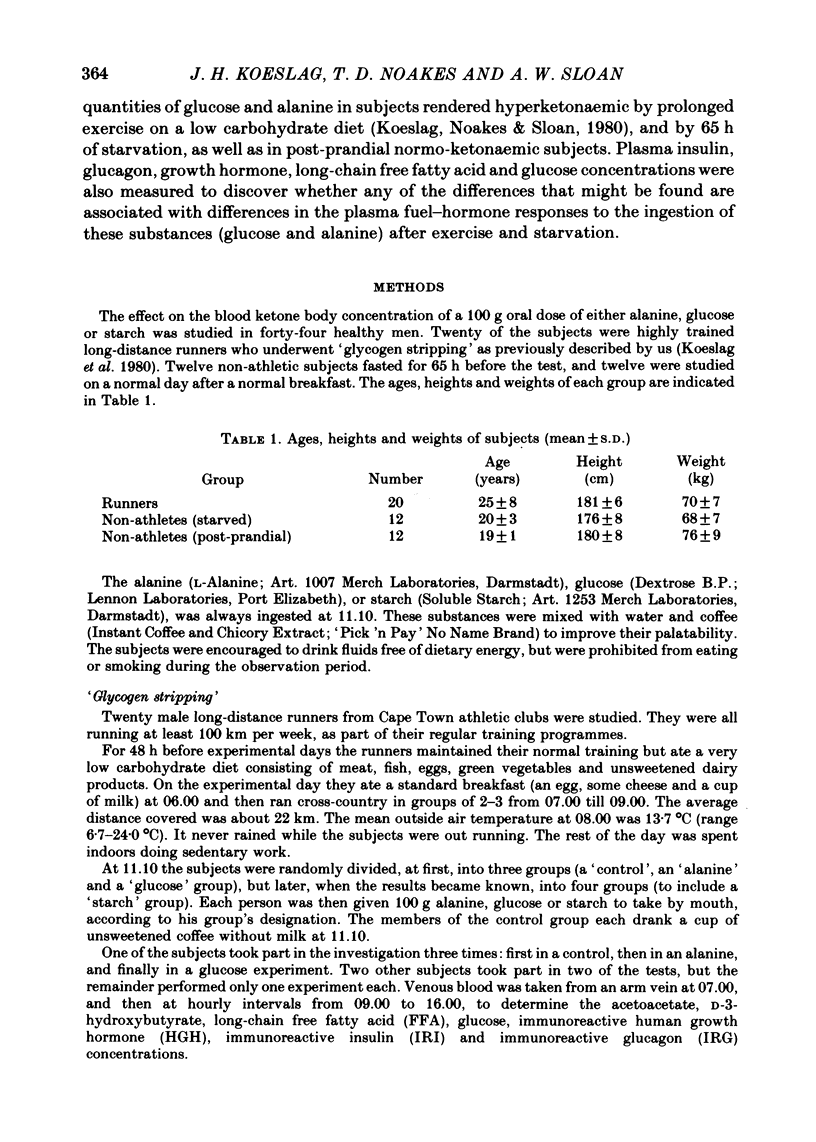
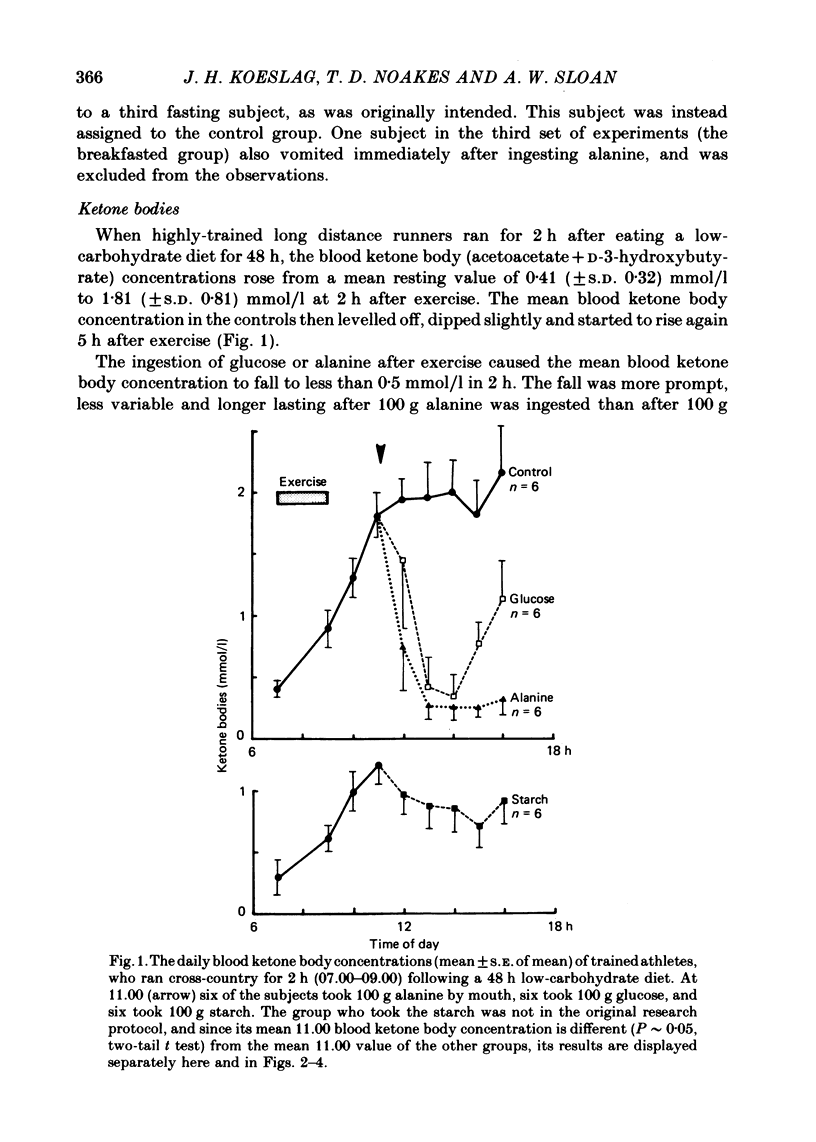
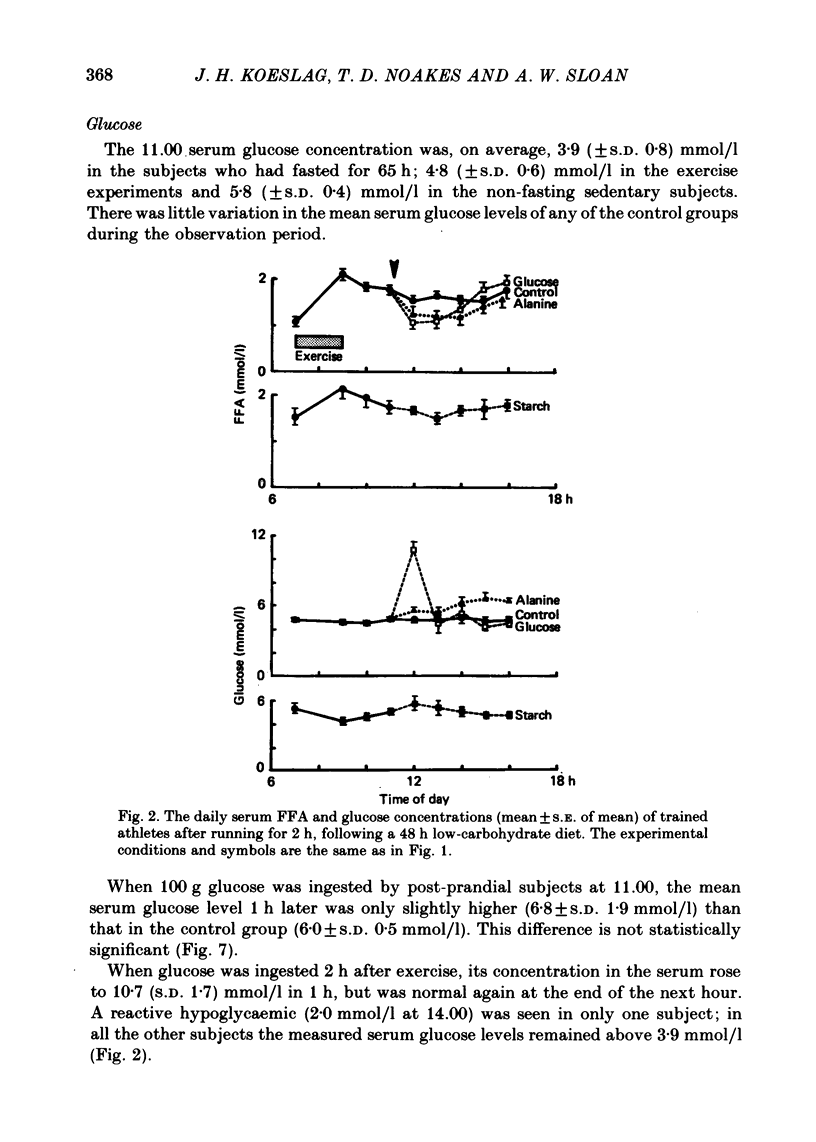
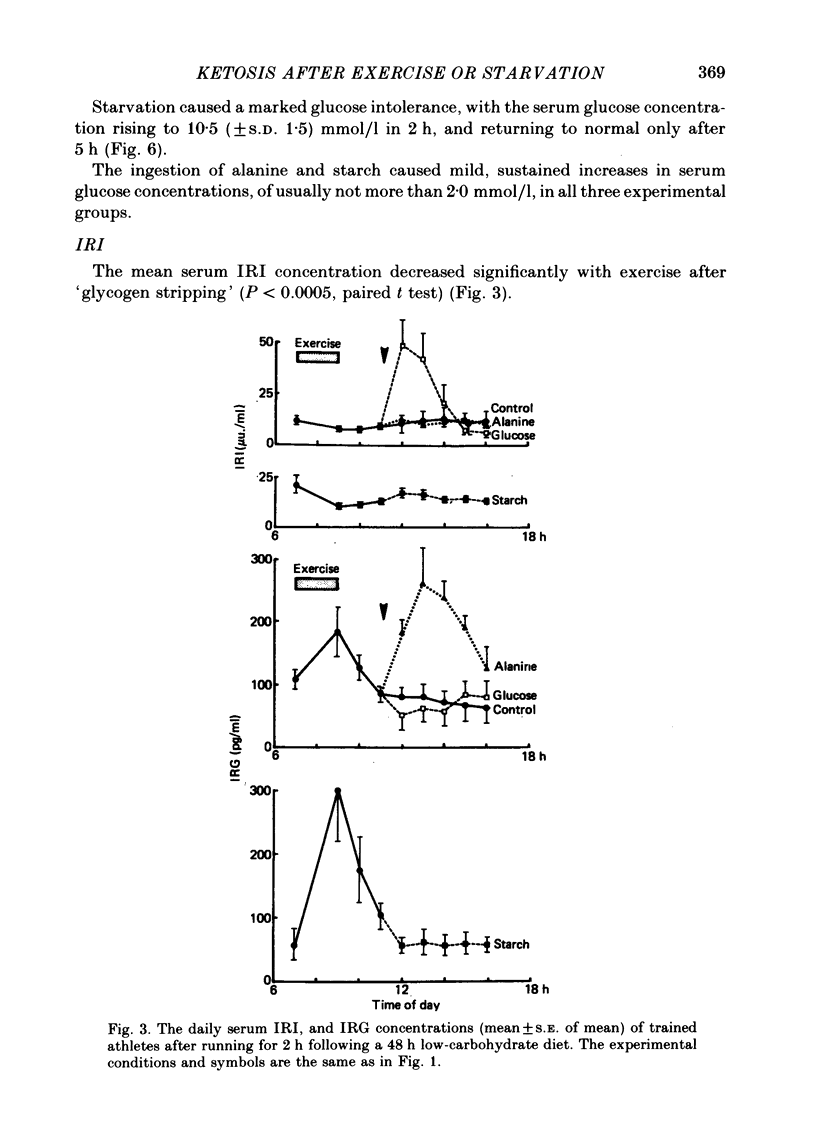
1. Several investigators have found that the development of post-exercise ketosis is not counteracted by glucose ingestion. Post-exercise ketosis might therefore have more in common with diabetic ketoacidosis than with starvation ketosis. 2. The effects of ingesting 100 g of glucose, alanine or starch were therefore studied in subjects rendered hyperketonaemic by prolonged running on a low carbohydrate diet, or by 65 h of starvation. These substances were also ingested by normal post-prandial subjects. 3. The runners developed post-exercise ketosis (1.81 +/- S.D. 0.81 mmol/l), which was counteracted by alanine and glucose, but only minimally by starch. 4. Fasting caused a variable ketosis (2.19 +/- S.D. 1.63 mmol/l), also counteracted by glucose and less by starch, but alanine caused vomiting. 5. Glucose and alanine lowered the blood ketone body levels of the post-prandial subjects. 6. The rising ketone body levels in starvation and after exercise were accompanied by simultaneous increases in the plasma insulin/glucagon ratios; in both, glucose ingestion increased the ratio further, while alanine decreased it. 7. It is concluded that there is no essential difference between established post-exercise and starvation ketosis, and that the blood fuel-hormone changes do not correlate with the changes in blood ketone body concentrations.
Full text
PDF




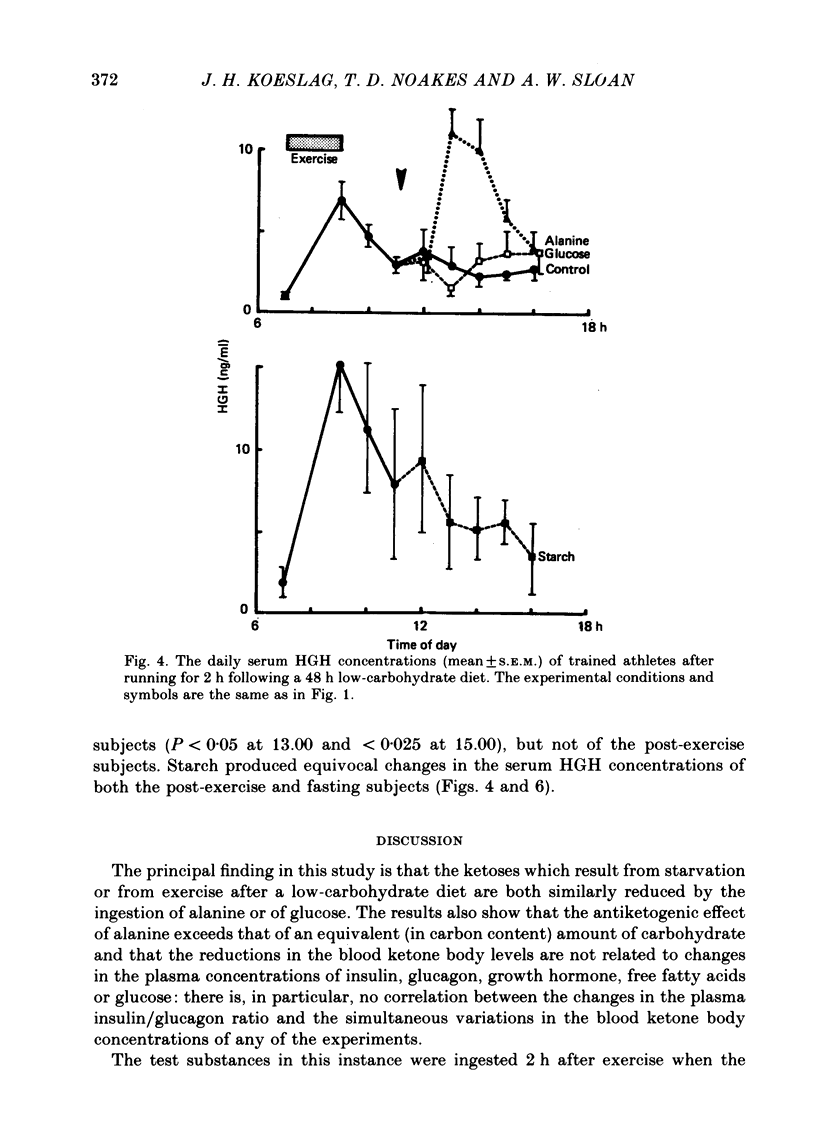
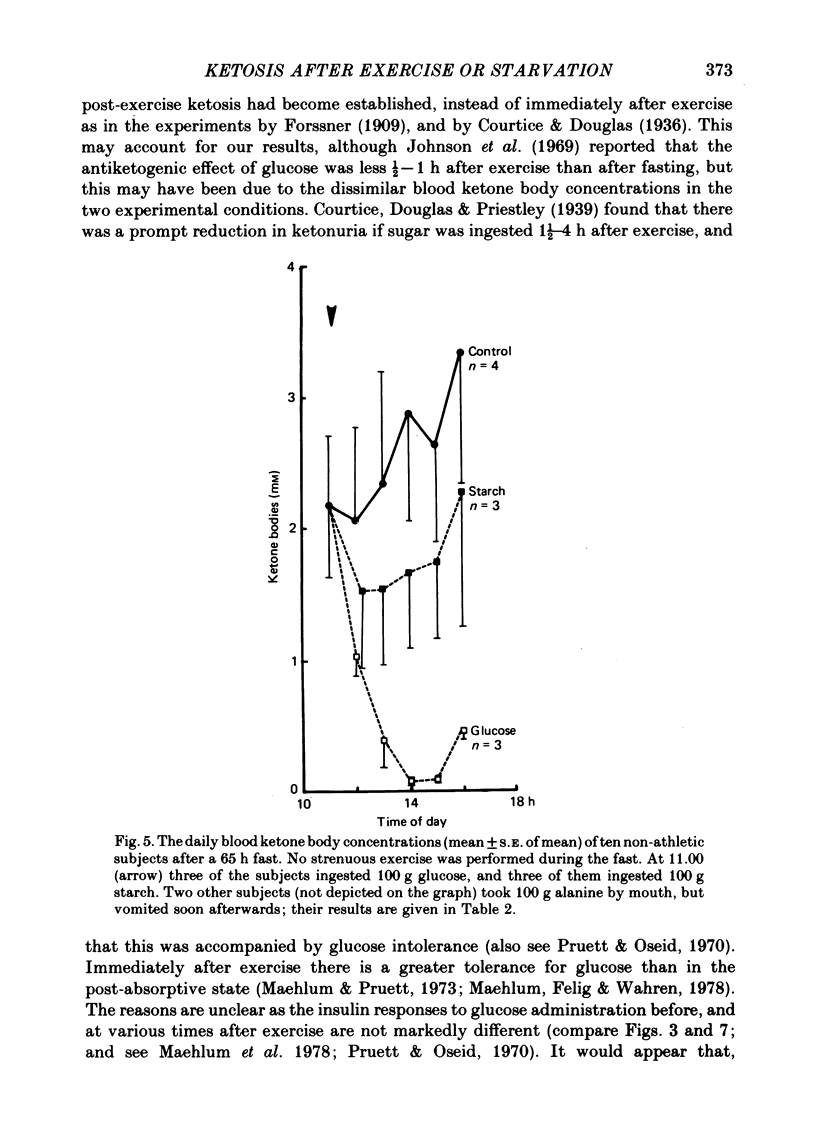
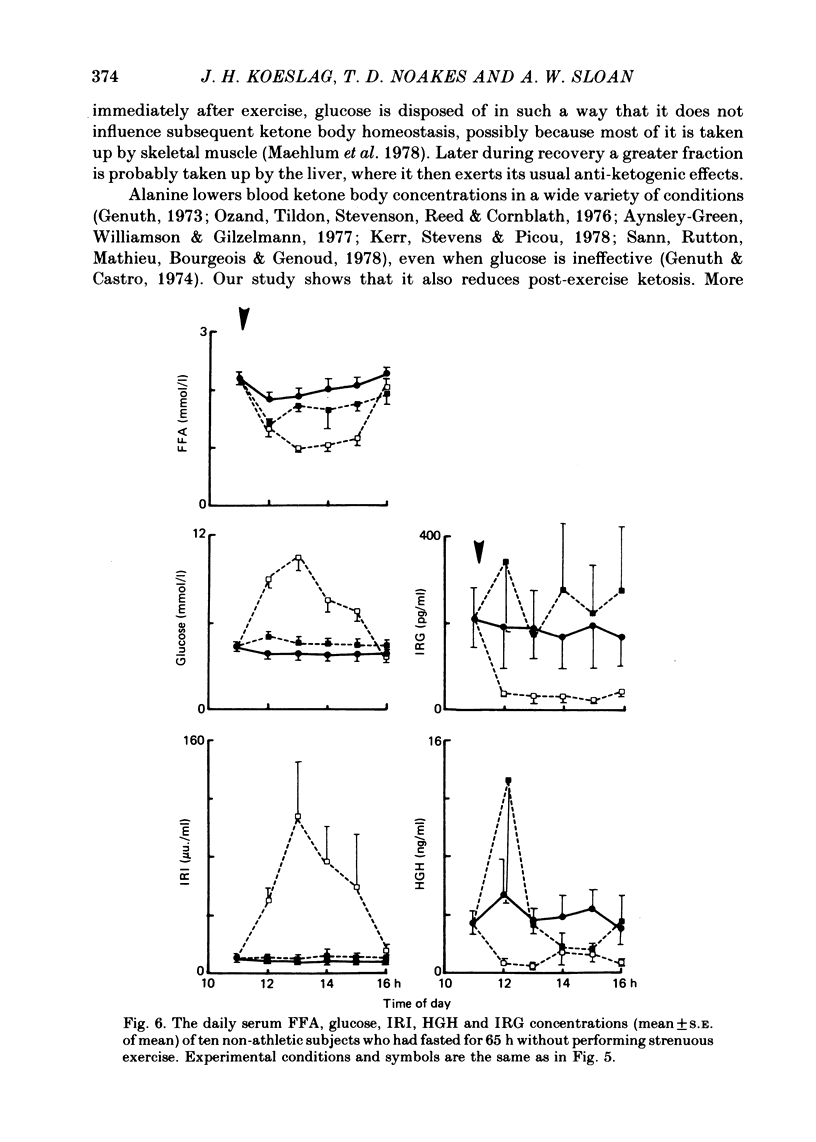
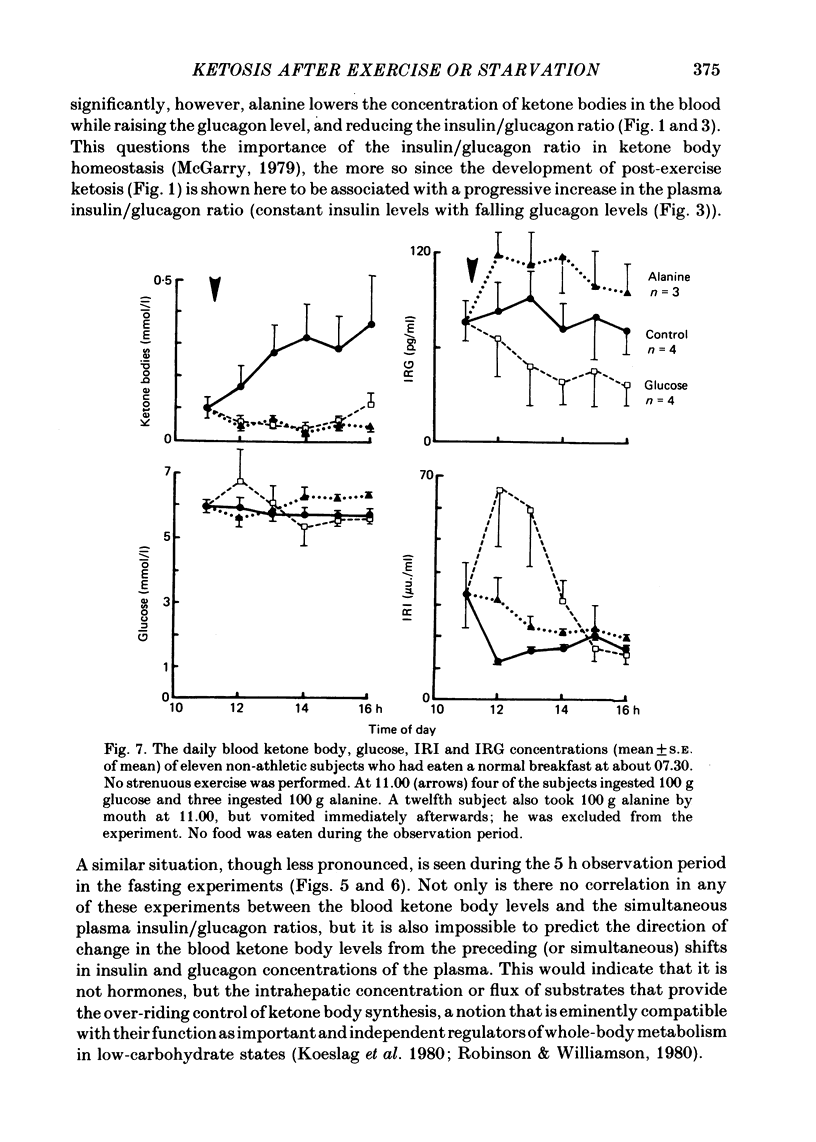

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aynsley-Green A., Williamson D. H., Gitzelmann R. Hepatic glycogen synthetase deficiency. Definition of syndrome from metabolic and enzyme studies on a 9-year-old girl. Arch Dis Child. 1977 Jul;52(7):573–579. doi: 10.1136/adc.52.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Genuth S. M., Castro J. Effect of oral alanine on blood beta-hydroxybutyrate and plasma glucose, insulin, free fatty acids, and growth hormone in normal and diabetic subjects. Metabolism. 1974 Apr;23(4):375–386. doi: 10.1016/0026-0495(74)90056-0. [DOI] [PubMed] [Google Scholar]
- Genuth S. M. Effects of oral alanine administration in fasting obese subjects. Metabolism. 1973 Jul;22(7):927–937. doi: 10.1016/0026-0495(73)90065-6. [DOI] [PubMed] [Google Scholar]
- Johnson R. H., Walton J. L., Krebs H. A., Williamson D. H. Post-exercise ketosis. Lancet. 1969 Dec 27;2(7635):1383–1385. doi: 10.1016/s0140-6736(69)90931-3. [DOI] [PubMed] [Google Scholar]
- Kerr D. S., Stevens M. C., Picou D. I. Fasting metabolism in infants: II. The effect of severe undernutrition and infusion of alanine on glucose production estimated with U-13C-glucose. Metabolism. 1978 Jul;27(7):831–848. doi: 10.1016/0026-0495(78)90218-4. [DOI] [PubMed] [Google Scholar]
- Koeslag J. H., Noakes T. D., Sloan A. W. Post-exercise ketosis. J Physiol. 1980 Apr;301:79–90. doi: 10.1113/jphysiol.1980.sp013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehlum S., Felig P., Wahren J. Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am J Physiol. 1978 Sep;235(3):E255–E260. doi: 10.1152/ajpendo.1978.235.3.E255. [DOI] [PubMed] [Google Scholar]
- Maehlum S., Pruett E. D. Muscular exercise and metabolism in male juvenile diabetics. II. Glucose tolerance after exercise. Scand J Clin Lab Invest. 1973 Oct;32(2):149–153. doi: 10.3109/00365517309084342. [DOI] [PubMed] [Google Scholar]
- McGarry J. D. Lilly Lecture 1978. New perspectives in the regulation of ketogenesis. Diabetes. 1979 May;28(5):517–523. doi: 10.2337/diab.28.5.517. [DOI] [PubMed] [Google Scholar]
- Ozand P. T., Tildon J. T., Stevenson J. H., Reed W. D., Cornblath M. Ability of exogenous alanine to lower blood beta-hydroxybutyrate during development in rats. Am J Physiol. 1976 May;230(5):1385–1388. doi: 10.1152/ajplegacy.1976.230.5.1385. [DOI] [PubMed] [Google Scholar]
- Pruett E. D., Oseid S. Effect of exercise on glucose and insulin response to glucose infusion. Scand J Clin Lab Invest. 1970 Nov;26(3):277–285. doi: 10.3109/00365517009046234. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980 Jan;60(1):143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Sann L., Ruitton A., Mathieu M., Bourgeois J., Genoud J. Effect of intravenous L-alanine administration on plasma glucose, insulin and glucagon, blood pyruvate, lactate and beta-hydroxybutyrate concentrations in newborn infants. Study in term and preterm newborn infants. Acta Paediatr Scand. 1978 May;67(3):297–302. doi: 10.1111/j.1651-2227.1978.tb16324.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]


