Abstract
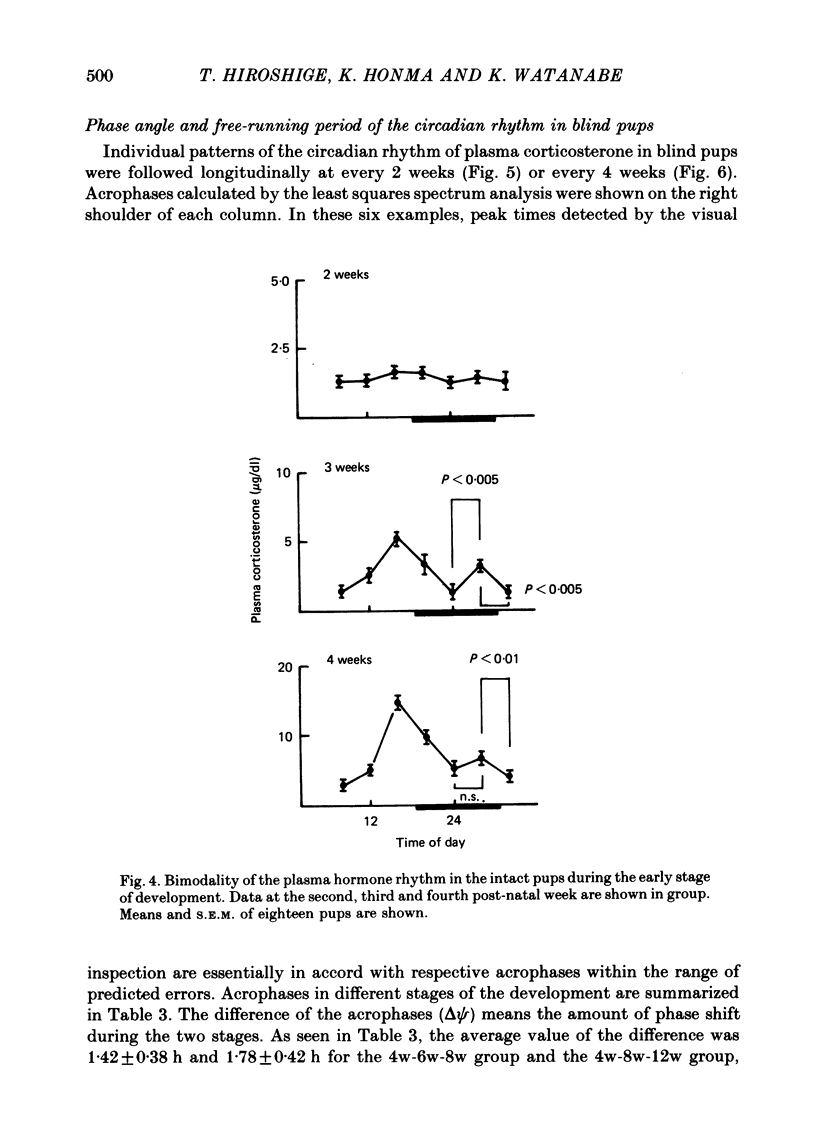
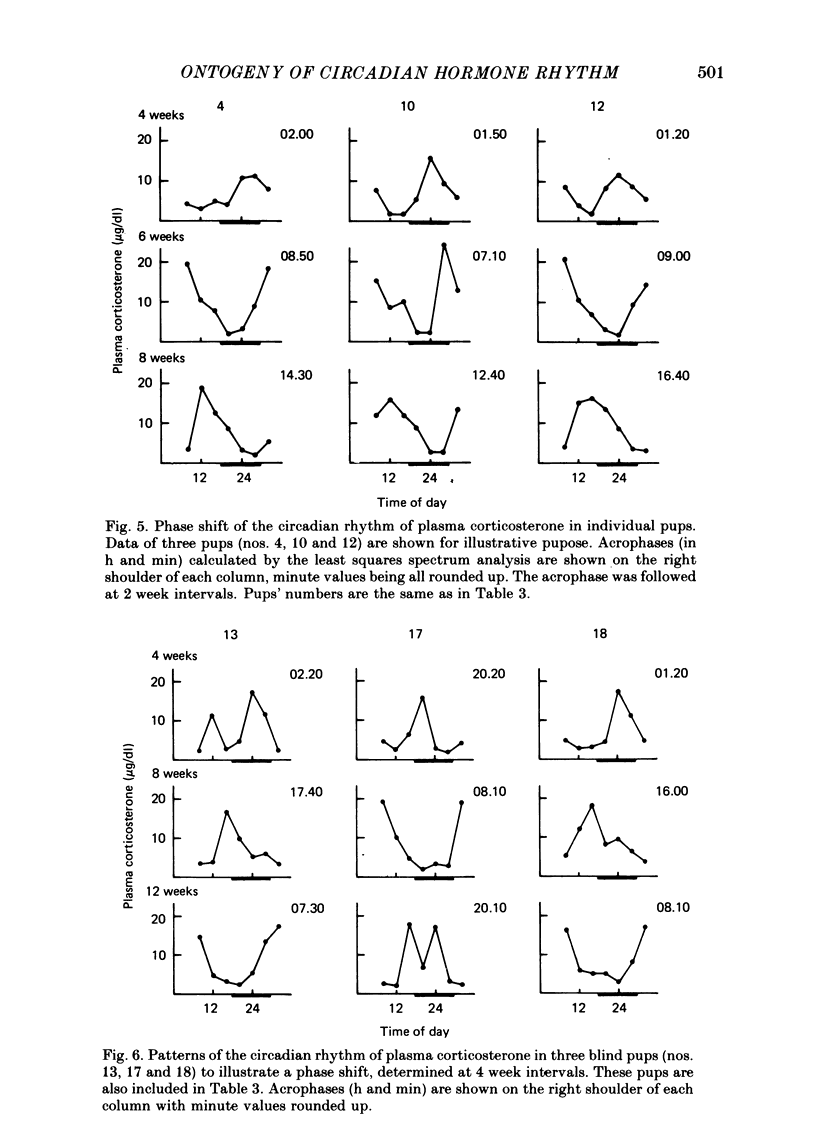
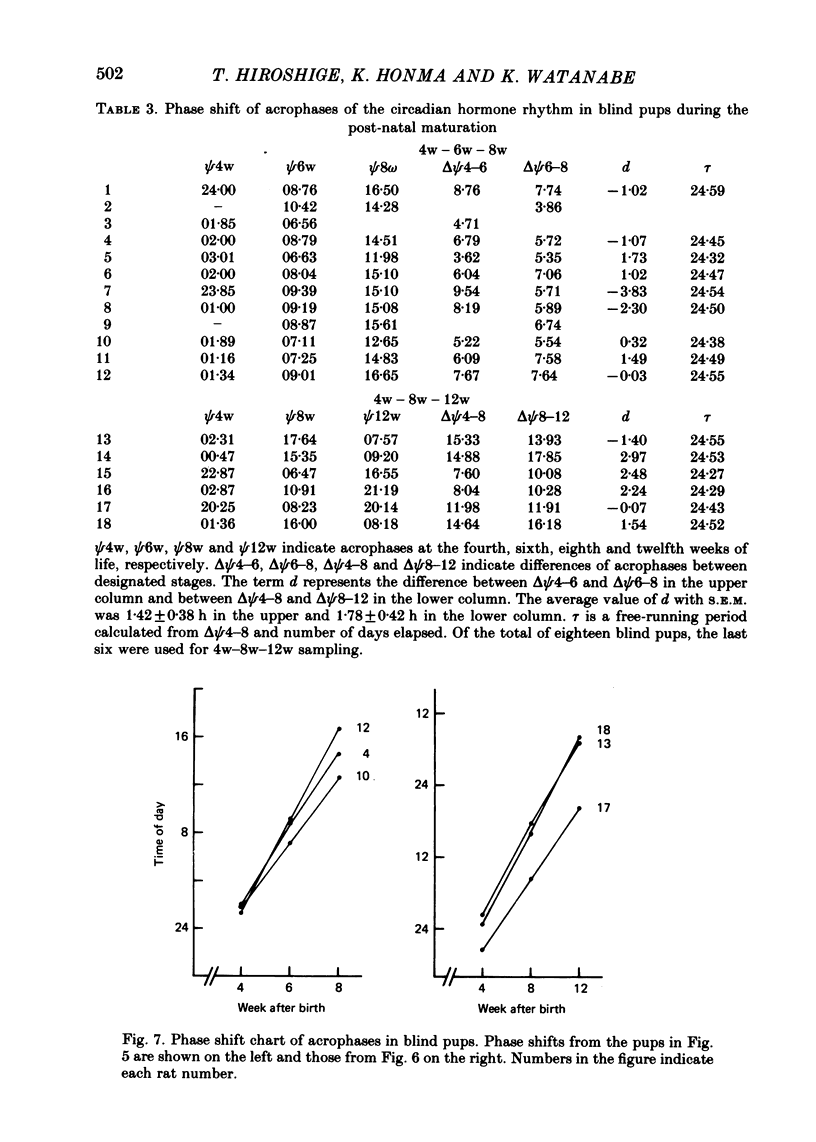
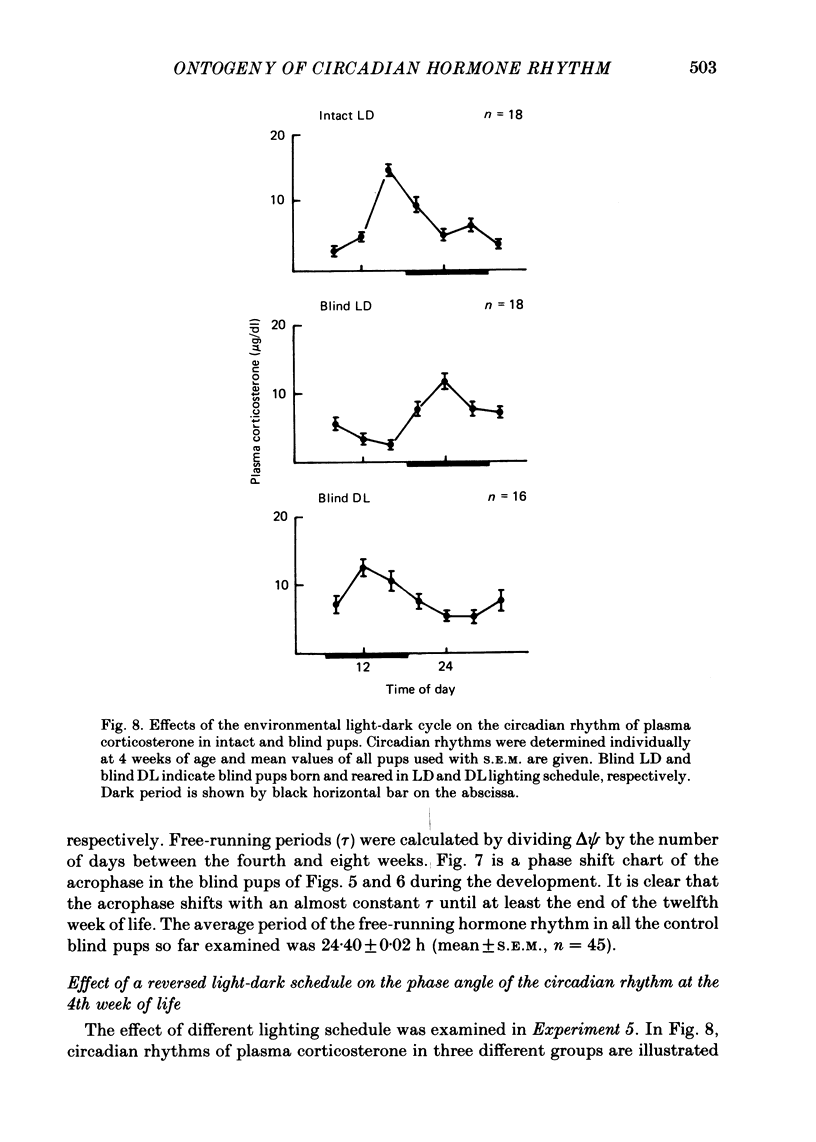
1. Post-natal manifestation of plasma corticosterone rhythm was followed by individual pups until the end of 12 weeks of life. Results obtained were analysed by both visual inspection and a least squares spectrum method. The validity of the approaches used was evaluated. 2. Circadian rhythm of plasma corticosterone in sighted pups under light-dark cycle (LD) first appeared at around the third post-natal week, accompanied often with a bimodal pattern: a second peak being counterphased to the first main peak. The second peak became obscure at the fourth week, disappearing as pups matured. 3. The hormone rhythm in blinded pups whose eyes were removed within 16 h of birth was free-running under LD with a period of 24.40 +/- 0.02 h (mean +/- S.E.M.). The free-running period was stable until at least the end of the twelfth week of life. 4. The acrophase of the circadian rhythm in blind pups born and reared under LD was 180 degrees out of phase to that born and reared under DL, a reversed light-dark condition. In each case, the acrophase shifted about 8 h from that of sighted pups at the fourth week of life. 5. It is suggested that an underlying oscillator for the hormone rhythm starts to oscillate earlier than the fourth week of life and the environmental light-dark cycle prior to the fourth week determines the phase angle of the circadian rhythm in blind pups.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ader R. Early experiences accelerate maturation of the 24-hour adrenocortical rhythm. Science. 1969 Mar 14;163(3872):1225–1226. doi: 10.1126/science.163.3872.1225. [DOI] [PubMed] [Google Scholar]
- Allen C., Kendall J. W. Maturation of the circadian rhythm of plasma corticosterone in the rat. Endocrinology. 1967 May;80(5):926–930. doi: 10.1210/endo-80-5-926. [DOI] [PubMed] [Google Scholar]
- Hiroshige T., Sato T. Postnatal development of circadian rhythm of corticotropin-releasing activity in the rat hypothalamus. Endocrinol Jpn. 1970 Feb;17(1):1–6. doi: 10.1507/endocrj1954.17.1. [DOI] [PubMed] [Google Scholar]
- Honma K. I., Hiroshige T. Internal synchronization among several circadian rhythms in rats under constant light. Am J Physiol. 1978 Nov;235(5):R243–R249. doi: 10.1152/ajpregu.1978.235.5.R243. [DOI] [PubMed] [Google Scholar]
- Krieger D. T. Effect of ocular enucleation and altered lighting regimens at various ages on the circadian periodicity of plasma corticosteroid levels in the rat. Endocrinology. 1973 Nov;93(5):1077–1091. doi: 10.1210/endo-93-5-1077. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- Ramaley J. A. The adrenal rhythm and puberty onset in the female rat. Life Sci. 1978 Nov 20;23(21):2079–2087. doi: 10.1016/0024-3205(78)90181-9. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Hanada K., Kobayashi K., Hayafuji C., Otani S., Takahashi Y. Development of the circadian adrenocortical rhythm in rats: studied by determination of 24- or 48-hour patterns of blood corticosterone levels in individual pups. Endocrinology. 1979 Apr;104(4):954–961. doi: 10.1210/endo-104-4-954. [DOI] [PubMed] [Google Scholar]
- Underwood R. H., Williams G. H. The simultaneous measurement of aldosterone, cortisol, and corticosterone in human peripheral plasma by displacement analysis. J Lab Clin Med. 1972 May;79(5):848–862. [PubMed] [Google Scholar]
- Wilson M. M., Rice R. W., Critchlow V. Evidence for a free-running circadian rhythm in pituitary-adrenal function in blinded adult female rats. Neuroendocrinology. 1976;20(4):289–295. doi: 10.1159/000122495. [DOI] [PubMed] [Google Scholar]


