Abstract
1. Intracellular recordings from an in vitro preparation of turtle olfactory bulb were used to determine the pathway responsible for producing synaptic inhibition of mitral cells.
2. Inhibitory post-synaptic potentials (i.p.s.p.s) could be elicited in mitral cells by both olfactory nerve (orthodromic) and mitral cell axon (antidromic) stimulation and by suprathreshold depolarizing current pulses injected intracellularly through the recording micro-electrode. Reversing the chloride gradient by either intracellular injection of chloride or lowering the external chloride concentration reversed the i.p.s.p.s into depolarizing potentials. The GABA antagonists, bicuculline and picrotoxin, blocked the i.p.s.p.s.
3. A large increment in the size of the orthodromic and antidromic i.p.s.p. was associated with an action potential. Grading the stimulus intensity on either side of threshold resulted in graded changes in the size of the i.p.s.p. The increment associated with an action potential and the ability to evoke an i.p.s.p. by direct stimulation of a mitral cell suggested that these phenomena were due to activation of the dendrodendritic reciprocal synapses between mitral and granule cells.
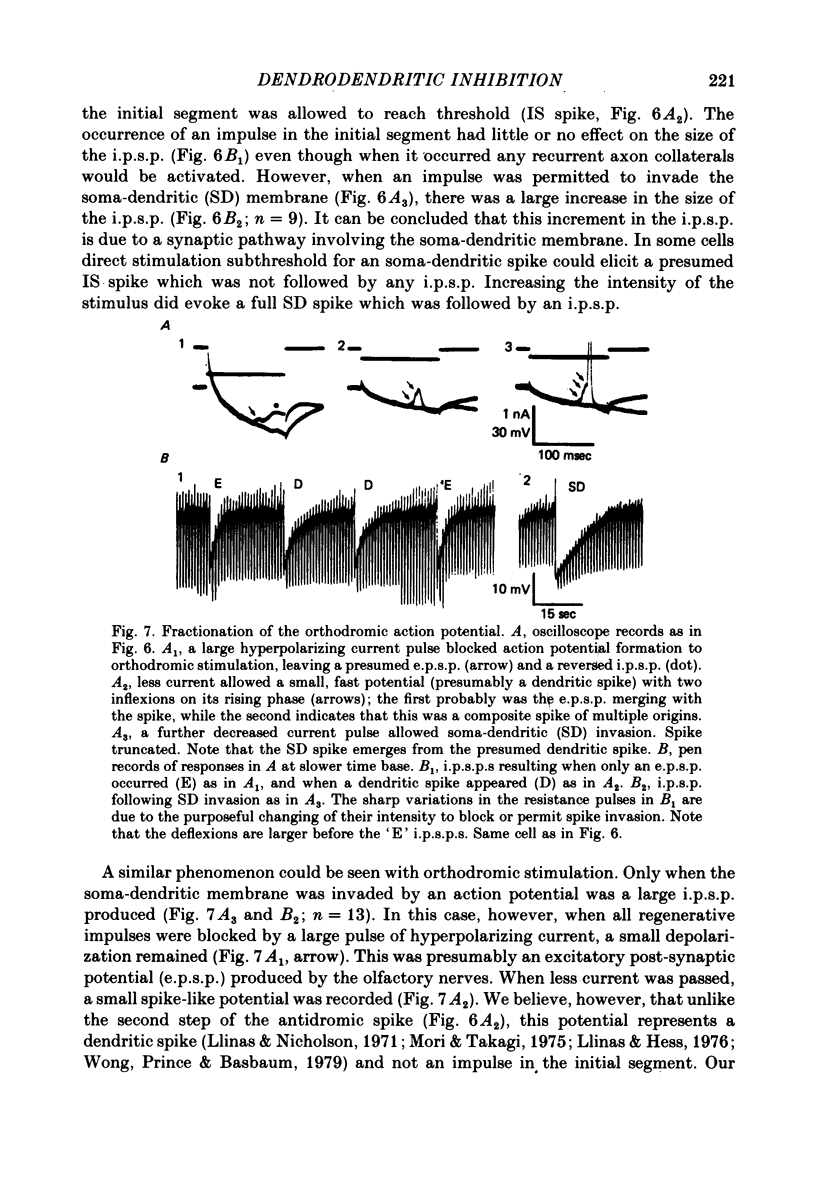
4. Orthodromic, antidromic and directly produced action potentials could be fractionated such that regenerative activation of the soma-dendritic membrane could be blocked. Only when this membrane was allowed to reach threshold was a large i.p.s.p. recorded. This indicated that the increment in the i.p.s.p. was due to activation of a synaptic pathway involving the soma-dendritic membrane.
5. When spike propagation in the mitral cell axons was blocked by tetrodotoxin (TTX), an i.p.s.p. could still be produced by direct stimulation, indicating that the mitral cell soma-dendritic membrane is functionally both pre- and post-synaptic. TTX blocked the fast, high amplitude somatic spikes and revealed higher threshold, broader spikes of lower amplitude that were blocked by cobalt and calcium-free Ringer.
6. Tetraethylammonium (TEA) increased the duration and the amplitude of the calcium spike. The amplitude was also increased by barium which prolonged the spike only if TEA was present. In the presence of TEA, bicuculline also prolonged the calcium spike. This suggests that three ionic conductances limit the duration of the calcium action potential: a voltage-dependent potassium conductance, a calcium-dependent potassium conductance, and the chloride conductance associated with the i.p.s.p.
7. Spontaneous, bicuculline-sensitive, depolarizing potentials were recorded in mitral cells impaled with KCl-filled electrodes. Orthodromic or antidromic stimulation increased the frequency of these small potentials for the duration of the i.p.s.p., indicating prolonged GABA release.
8. Stimulation of the olfactory nerves, the mitral cell axons, and direct stimulation could elicit action potentials in granule layer cells. Orthodromic and antidromic activation was followed by a hyperpolarization of about the same duration as the mitral cell i.p.s.p. and was probably the result of dysfacilitation.
9. Paired stimulation of the mitral cell axons resulted in the diminution of the granule cell e.p.s.p. evoked by the second shock, indicating that the predominant excitatory input to the granule cells is through the mitral cell dendrites.
10. It is concluded that both synaptic inhibition of mitral cells and excitation of granule cells is mediated primarily by the dendrodendritic reciprocal pathway.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES K. H. DER FEINBAU DES BULBUS OFACTORIUS DER RATTE UNTER BESONDERER BERUECKSICHTIGUNG DER SYNAPTISCHEN VERBINDUNGEN. Z Zellforsch Mikrosk Anat. 1965 Feb 9;65:530–561. [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes N. Werman R:The cooperativity of -aminobutyric action on the membrane of locust muscle fibers. Mol Pharmacol. 1973 Jul;9(4):571–579. [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. GABA, bicuculline and central inhibition. Nature. 1970 Jun 27;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- EDWARDS C., OTTOSON D. The site of impulse initiation in a nerve cell of a crustacean stretch receptor. J Physiol. 1958 Aug 29;143(1):138–148. doi: 10.1113/jphysiol.1958.sp006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN J. D., MANCIA M., von BAUMGARTEN Recurrent inhibition in the olfactory bulb. I. Effects of antidromic stimulation of the lateral olfactory tract. J Neurophysiol. 1962 Jul;25:467–488. doi: 10.1152/jn.1962.25.4.467. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRATA Y. SOME OBSERVATIONS ON THE FINE STRUCTURE OF THE SYNAPSES IN THE OLFACTORY BULB OF THE MOUSE, WITH PARTICULAR REFERENCE TO THE ATYPICAL SYNAPTIC CONFIGURATIONS. Arch Histol Jpn. 1964 Feb;24:293–302. doi: 10.1679/aohc1950.24.293. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Halász N., Ljungdahl A., Hökfelt T., Johansson O., Goldstein M., Park D., Biberfeld P. Transmitter histochemistry of the rat olfactory bulb. I. Immunohistochemical localization of monoamine synthesizing enzymes. Support for intrabulbar, periglomerular dopamine neurons. Brain Res. 1977 May 13;126(3):455–474. doi: 10.1016/0006-8993(77)90597-2. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Nicoll R. A. Dendrodendritic inhibition: demonstration with intracellular recording. Science. 1980 Mar 28;207(4438):1473–1475. doi: 10.1126/science.7361098. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K., Nishi S. Calcium and action potentials of bullfrog sympathetic ganglion cells. J Gen Physiol. 1969 May;53(5):608–623. doi: 10.1085/jgp.53.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidbrink P., Jonsson G., Fuxe K. Selective reserpine-resistant accumulation of catecholamines in central dopamine neurones after DOPA administration. Brain Res. 1974 Mar 8;67(3):439–456. doi: 10.1016/0006-8993(74)90493-4. [DOI] [PubMed] [Google Scholar]
- Llinas R., Baker R., Precht W. Blockage of inhibition by ammonium acetate action on chloride pump in cat trochlear motoneurons. J Neurophysiol. 1974 May;37(3):522–532. doi: 10.1152/jn.1974.37.3.522. [DOI] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The effects of strontium and barium ions at synapses in sympathetic ganglia. J Physiol. 1977 May;267(2):497–518. doi: 10.1113/jphysiol.1977.sp011823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Mori K., Kogure S., Takagi S. Alternating responses of olfactory bulb neurons to repetitive lateral olfactory tract stimulation. Brain Res. 1977 Sep 9;133(1):150–155. doi: 10.1016/0006-8993(77)90056-7. [DOI] [PubMed] [Google Scholar]
- Mori K., Shepherd G. M. Synaptic excitation and long-lasting inhibition of mitral cells in the in vitro turtle olfactory bulb. Brain Res. 1979 Aug 17;172(1):155–159. doi: 10.1016/0006-8993(79)90904-1. [DOI] [PubMed] [Google Scholar]
- Mori K., Takagi S. F. Activation and inhibition of olfactory bulb neurones by anterior commissure volleys in the rabbit. J Physiol. 1978 Jun;279:589–604. doi: 10.1113/jphysiol.1978.sp012363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Takagi S. F. An intracellular study of dendrodendritic inhibitory synapses on mitral cells in the rabbit olfactory bulb. J Physiol. 1978 Jun;279:569–588. doi: 10.1113/jphysiol.1978.sp012362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Takagi S. F. Spike generation in the mitral cell dendrite of the rabbit olfactory bulb. Brain Res. 1975 Dec 26;100(3):685–689. doi: 10.1016/0006-8993(75)90170-5. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science. 1981 May 22;212(4497):957–959. doi: 10.1126/science.6262912. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Inhibitory mechanisms in the rabbit olfactory bulb: dendrodendritic mechanisms. Brain Res. 1969 Jun;14(1):157–172. doi: 10.1016/0006-8993(69)90037-7. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Olfactory nerves and their excitatory action in the olfactory bulb. Exp Brain Res. 1972;14(2):185–197. doi: 10.1007/BF00234798. [DOI] [PubMed] [Google Scholar]
- OCHI J. Olfactory bulb response to antidromic olfactory tract stimulation in the rabbit. Jpn J Physiol. 1963 Apr 15;13:113–128. doi: 10.2170/jjphysiol.13.113. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., POWELL T. P., SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO STIMULATION OF THE LATERAL OLFACTORY TRACT IN THE RABBIT. J Physiol. 1963 Aug;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching A. J., Powell T. P. Experimental studies on the axons intrinsic to the glomerular layer of the olfactory bulb. J Cell Sci. 1972 May;10(3):637–655. doi: 10.1242/jcs.10.3.637. [DOI] [PubMed] [Google Scholar]
- Price J. L., Powell T. P. The mitral and short axon cells of the olfactory bulb. J Cell Sci. 1970 Nov;7(3):631–651. doi: 10.1242/jcs.7.3.631. [DOI] [PubMed] [Google Scholar]
- Priestley J. V., Kelly J. S., Cuello A. C. Uptake of [3H]dopamine in periglomerular cells of the rat olfactory bulb: an autoradiographic study. Brain Res. 1979 Apr 6;165(1):149–155. doi: 10.1016/0006-8993(79)90054-4. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M., Reese T. S., Brightman M. W. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966 Jan;14(1):44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- SHEPHERD G. M. NEURONAL SYSTEMS CONTROLLING MITRAL CELL EXCITABILITY. J Physiol. 1963 Aug;168:101–117. doi: 10.1113/jphysiol.1963.sp007180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO OLFACTORY NERVE VOLLEYS IN THE RABBIT. J Physiol. 1963 Aug;168:89–100. doi: 10.1113/jphysiol.1963.sp007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci U S A. 1980 Jan;77(1):629–633. doi: 10.1073/pnas.77.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westecker M. E. Excitatory and inhibitory interactions in the olfactory bulb involving dendrodendritic synapses between mitral cells and granular cells. Pflugers Arch. 1970;317(2):173–186. doi: 10.1007/BF00592500. [DOI] [PubMed] [Google Scholar]
- White E. L. Synaptic organization of the mammalian olfactory glomerulus: new findings including an intraspecific variation. Brain Res. 1973 Oct 12;60(2):299–313. doi: 10.1016/0006-8993(73)90792-0. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A., Basbaum A. I. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci U S A. 1979 Feb;76(2):986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO C., YAMAMOTO T., IWAMA K. The inhibitory systems in the olfactory bulb studied by intracellular recording. J Neurophysiol. 1963 May;26:403–415. doi: 10.1152/jn.1963.26.3.403. [DOI] [PubMed] [Google Scholar]


