Abstract
1. Single unit activity has been recorded in the thalamic, hypothalamic and raphe magnus nuclei of rats anaesthetized with Urethane.
2. Neurones were sought which responded to changes in scrotal skin temperature applied with a water-perfused brass thermode. All sixty-nine neurones in the thalamus and hypothalamus responded with abrupt changes in activity as the scrotum was warmed (`switching response'). The majority responded with an increase in activity from minimal to maximal firing rate as the scrotum was warmed over a range of less than 0·5 °C; in about 20% of the neurones the converse was observed.
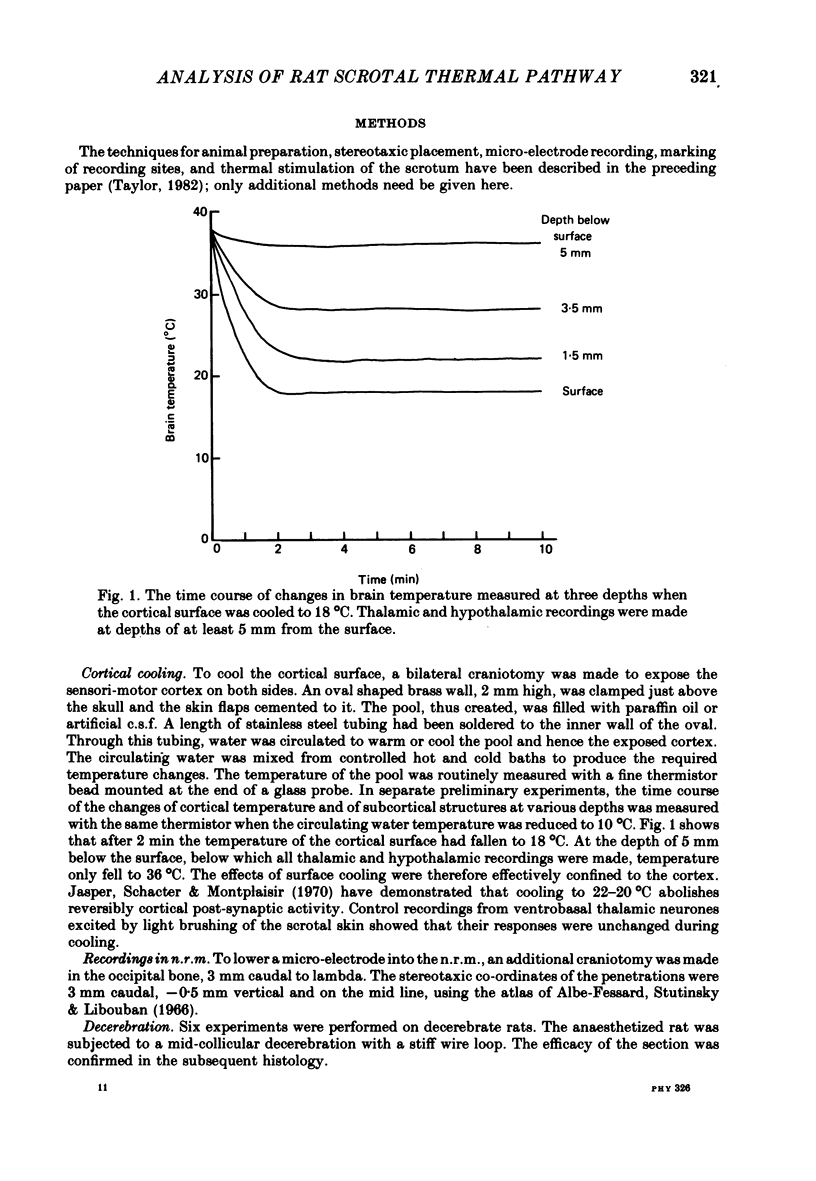
3. To determine whether the switching response of the thalamic and hypothalamic neurones depended upon a cortico-thalamic feed-back loop, the cortical surface was cooled to 18-20 °C to reversibly abolish cortical post-synaptic activity.
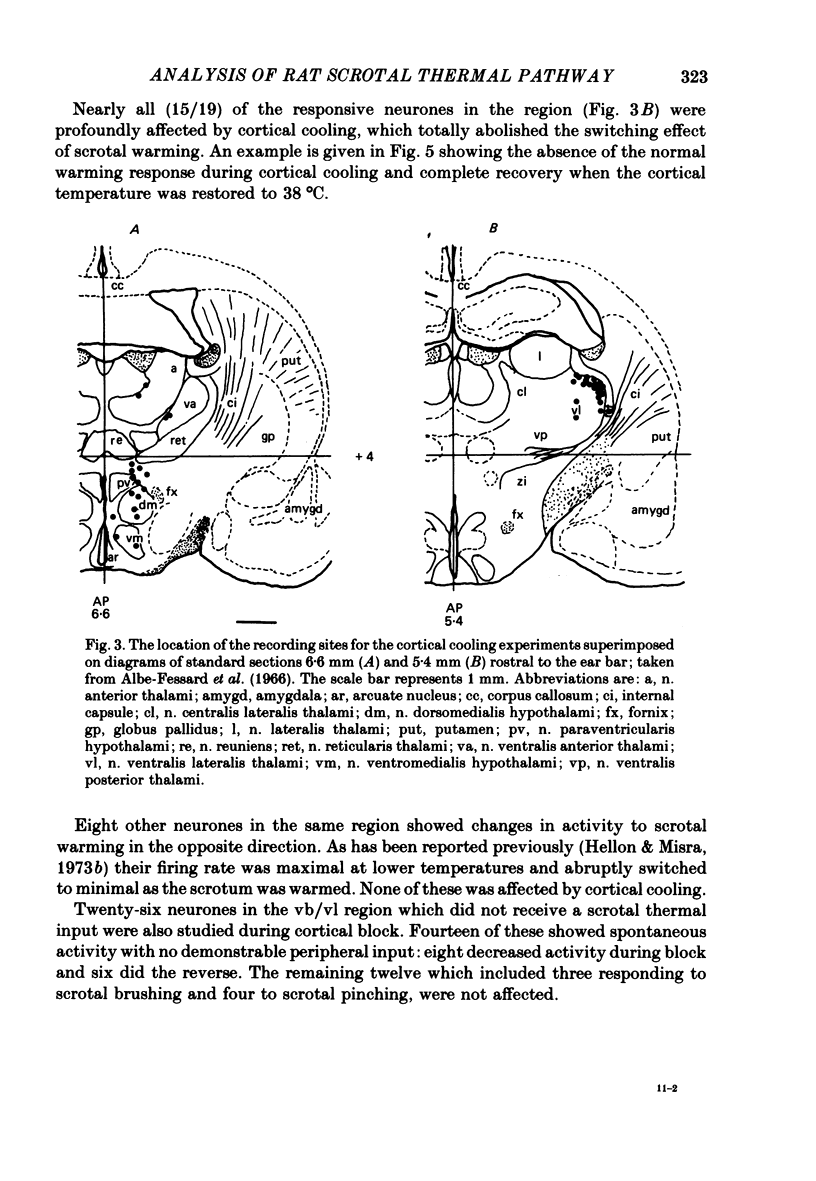
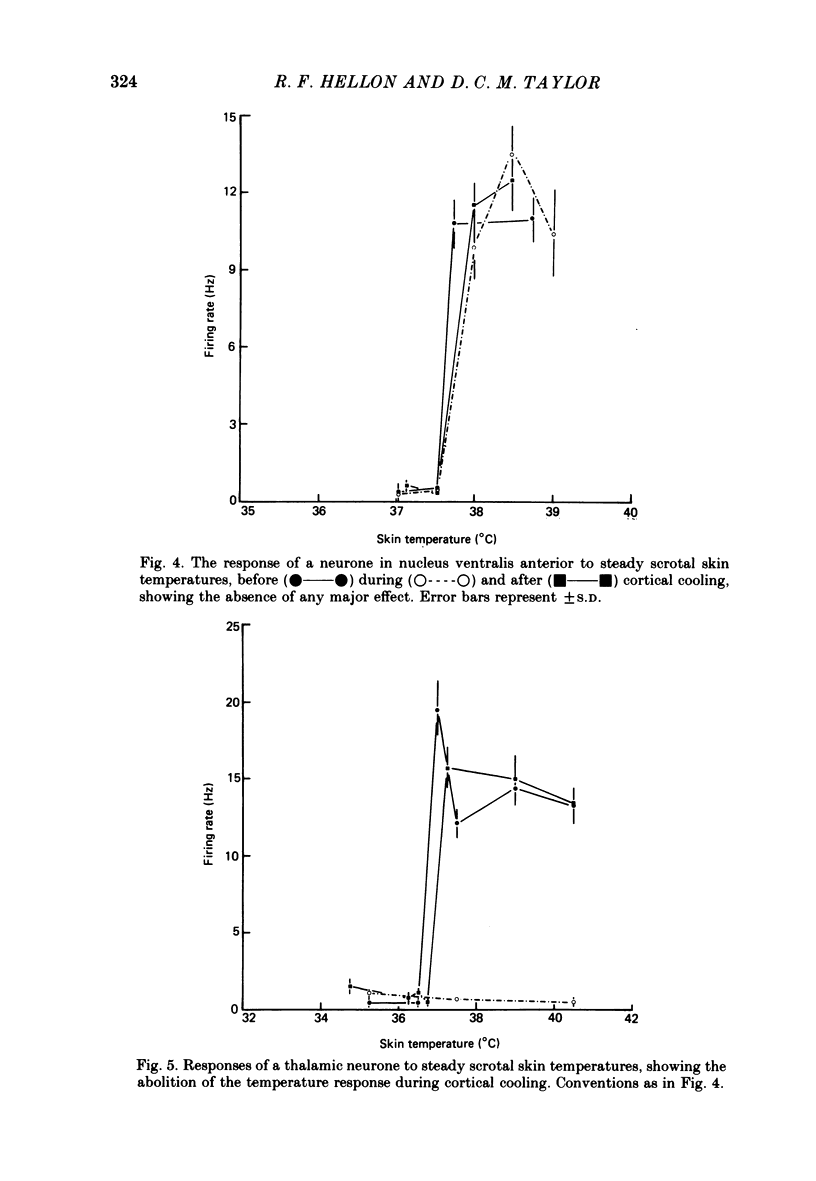
4. Cortical cooling abolished the positive switching response of nearly all (15/19) ventrobasal thalamic neurones to scrotal warming. All eight ventrobasal thalamic neurones with negative switching responses, and all twenty-two scrotal temperature-responsive neurones in other thalamic and hypothalamic nuclei were unaffected.
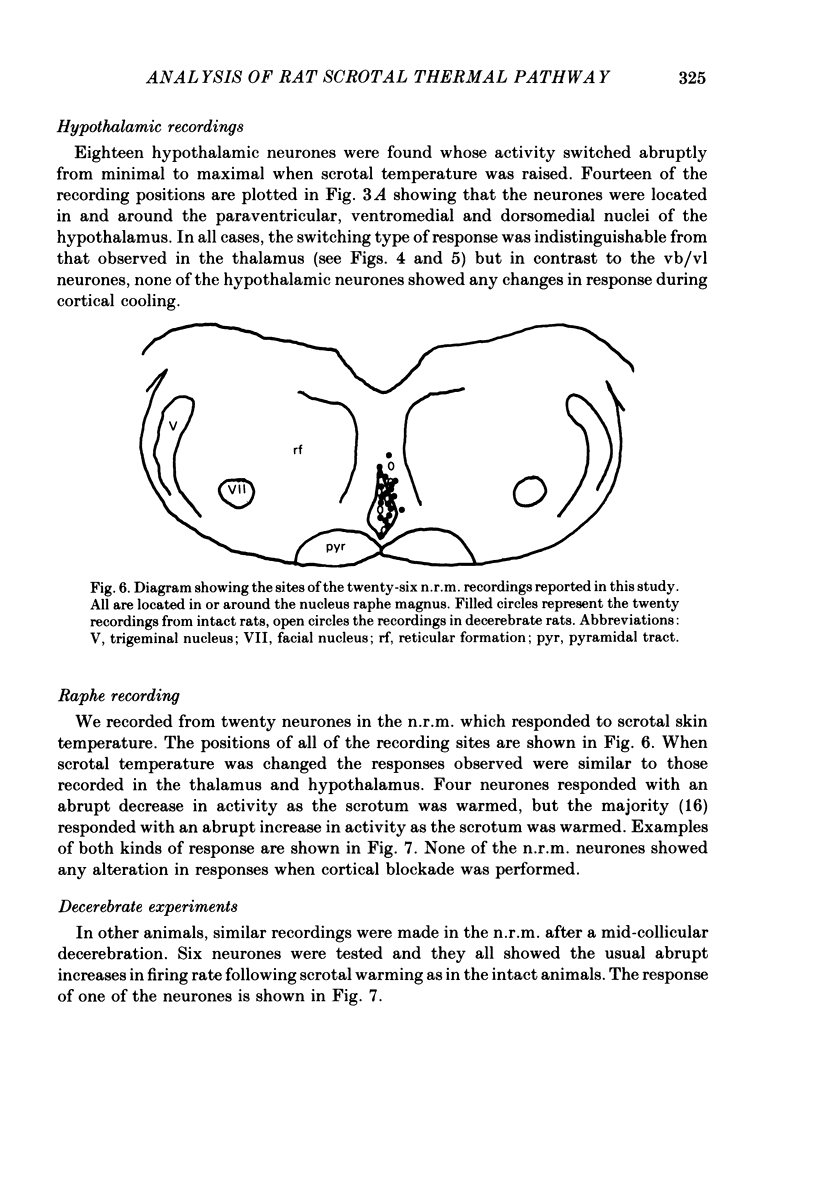
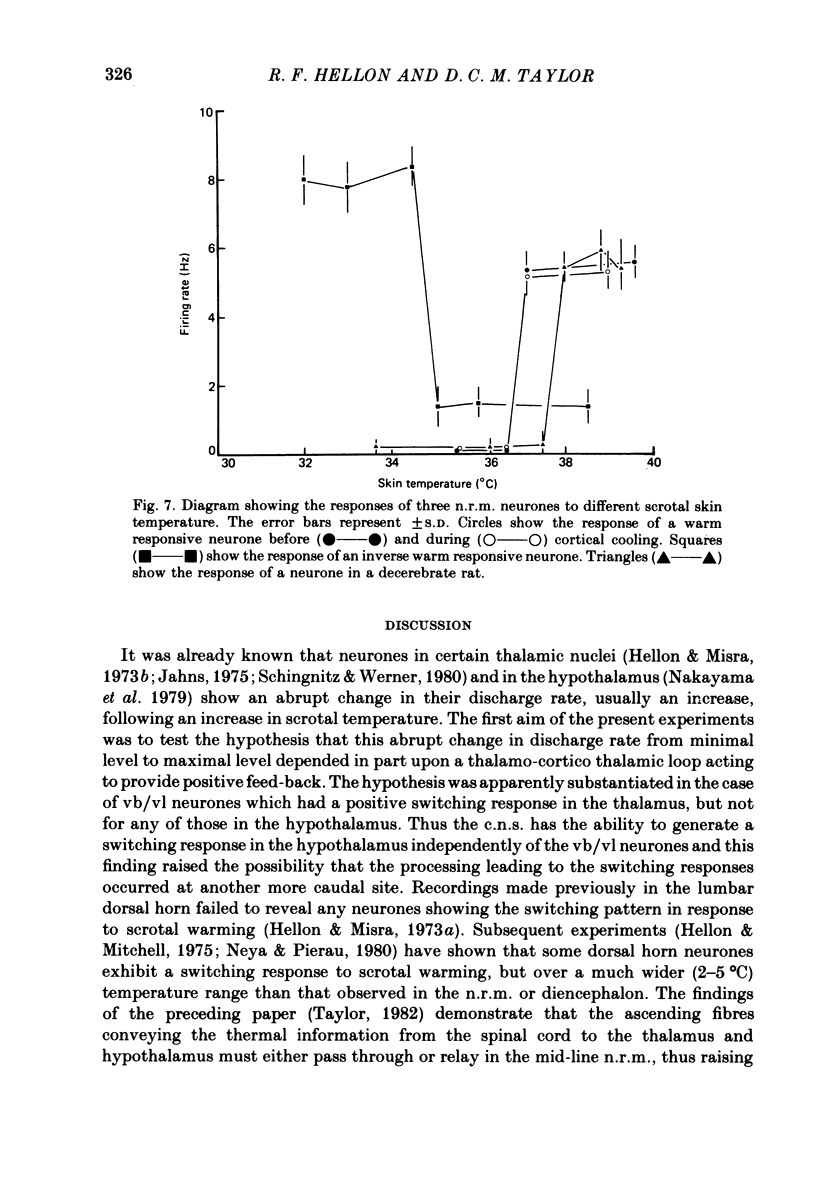
5. Twenty recordings were also made from scrotal temperature-responsive neurones in the nucleus raphe magnus. All possessed switching responses similar to those observed in the thalamus and hypothalamus.
6. None of the scrotal temperature-responsive neurones in the nucleus raphe magnus was affected by cortical cooling. Six neurones were observed in decerebrate rats with properties apparently identical to those in intact rats.
7. We conclude that the switching response of thalamic and hypothalamic scrotal temperature-responsive neurones is probably generated in the nucleus raphe magnus and passed in parallel to the thalamus and hypothalamus. In addition, thalamic neurones depend on an intact link with the cerebral cortex for the generation of their switching responses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Junge K., Sveen O. Cortico-thalamic facilitation of somatosensory impulses. Nature. 1967 Jun 3;214(5092):1011–1012. doi: 10.1038/2141011a0. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H. Specific responses of rat raphé neurones to skin temperature. J Physiol. 1977 Dec;273(1):277–293. doi: 10.1113/jphysiol.1977.sp012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Hensel H., Schäfer K. Thermal receptors in the scrotum of the rat. J Physiol. 1975 Jun;248(2):349–357. doi: 10.1113/jphysiol.1975.sp010978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Neurones in the dorsal horn of the rat responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):375–388. doi: 10.1113/jphysiol.1973.sp010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Neurones in the ventrobasal complex of the rat thalamus responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):389–399. doi: 10.1113/jphysiol.1973.sp010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K., Provins K. A. Neurones in the somatosensory cortex of the rat responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):401–411. doi: 10.1113/jphysiol.1973.sp010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Mitchell D. Convergence in a thermal afferent pathway in the rat. J Physiol. 1975 Jun;248(2):359–376. doi: 10.1113/jphysiol.1975.sp010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol. 1969 Feb;200(2):403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns R. Different projections of cutaneous thermal inputs to single units of the midbrain raphe nuclei. Brain Res. 1976 Jan 16;101(2):355–361. doi: 10.1016/0006-8993(76)90276-6. [DOI] [PubMed] [Google Scholar]
- Jahns R. Types of neuronal responses in the rat thalamus to peripheral temperature changes. Exp Brain Res. 1975 Aug 14;23(2):157–166. doi: 10.1007/BF00235458. [DOI] [PubMed] [Google Scholar]
- Jasper H. H., Shacter D. G., Montplaisir J. The effect of local cooling upon spontaneous and evoked electrical activity of cerebral cortex. Can J Physiol Pharmacol. 1970 Sep;48(9):640–652. doi: 10.1139/y70-094. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Ishikawa Y., Tsurutani T. Projection of scrotal thermal afferents to the preoptic and hypothalamic neurons in rats. Pflugers Arch. 1979 May 15;380(1):59–64. doi: 10.1007/BF00582613. [DOI] [PubMed] [Google Scholar]
- Neya T., Pierau F. K. Activity patterns of temperature-reactive dorsal horn neurons and their reactions to peripheral receptor stimulation by Ca. Jpn J Physiol. 1980;30(6):921–934. doi: 10.2170/jjphysiol.30.921. [DOI] [PubMed] [Google Scholar]
- Pierau F-K, Torrey P., Carpenter D. O. Afferent new fiber activity responding to temperature changes of scrotal skin of the rat. J Neurophysiol. 1975 May;38(3):601–612. doi: 10.1152/jn.1975.38.3.601. [DOI] [PubMed] [Google Scholar]
- Taylor D. C. The effects of nucleus raphe magnus lesions on an ascending thermal pathway in the rat. J Physiol. 1982 May;326:309–318. doi: 10.1113/jphysiol.1982.sp014194. [DOI] [PMC free article] [PubMed] [Google Scholar]


