Abstract
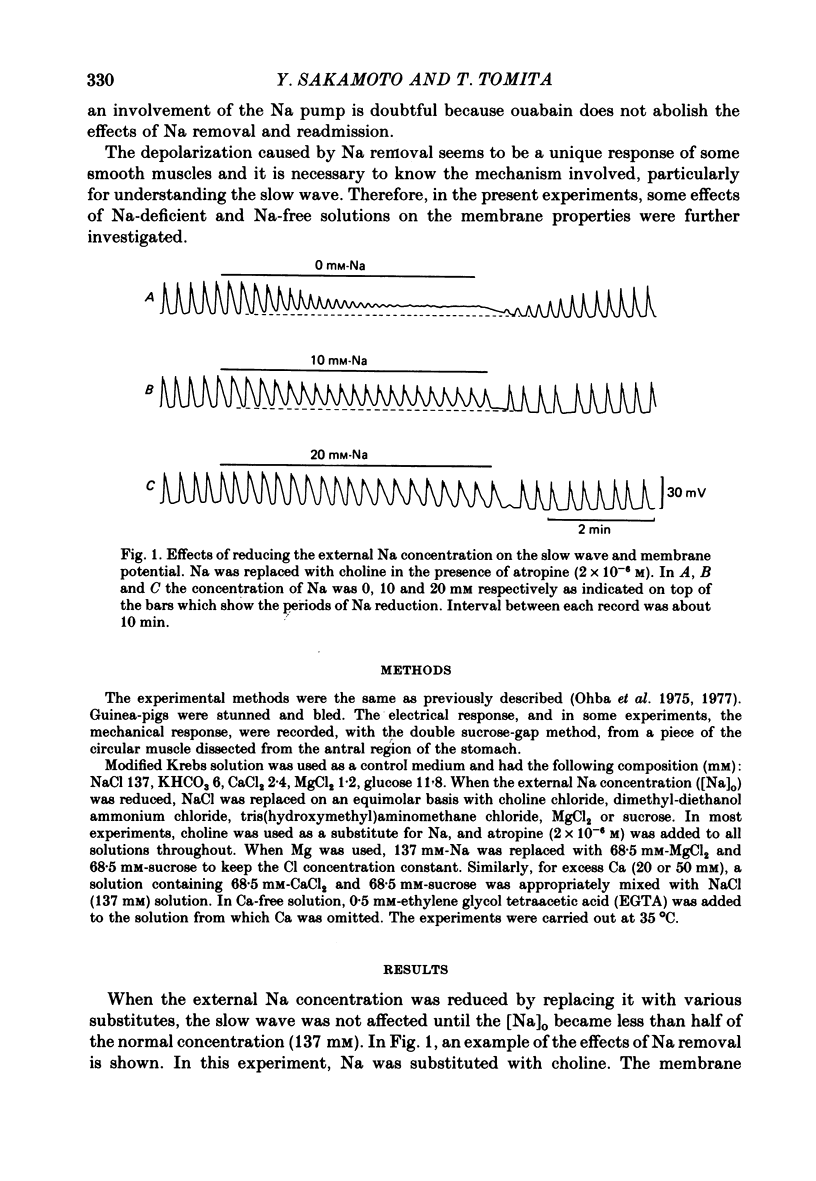
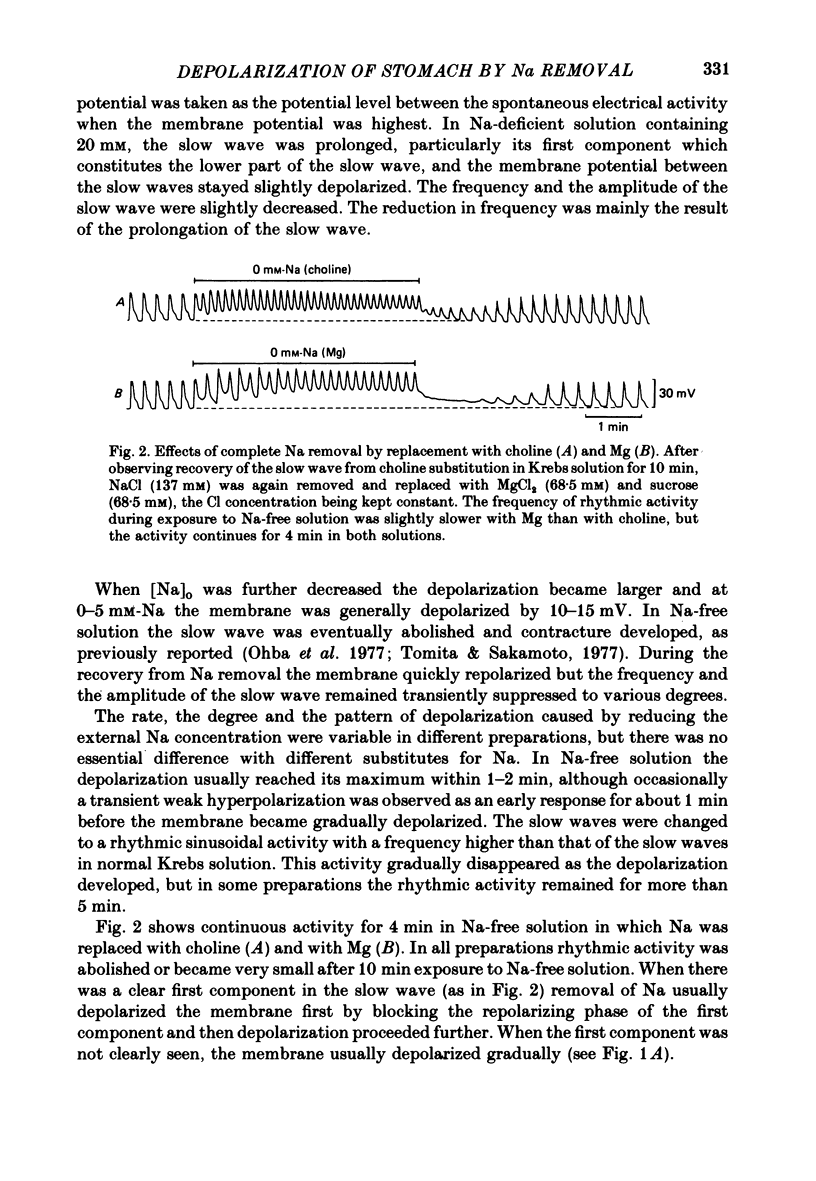
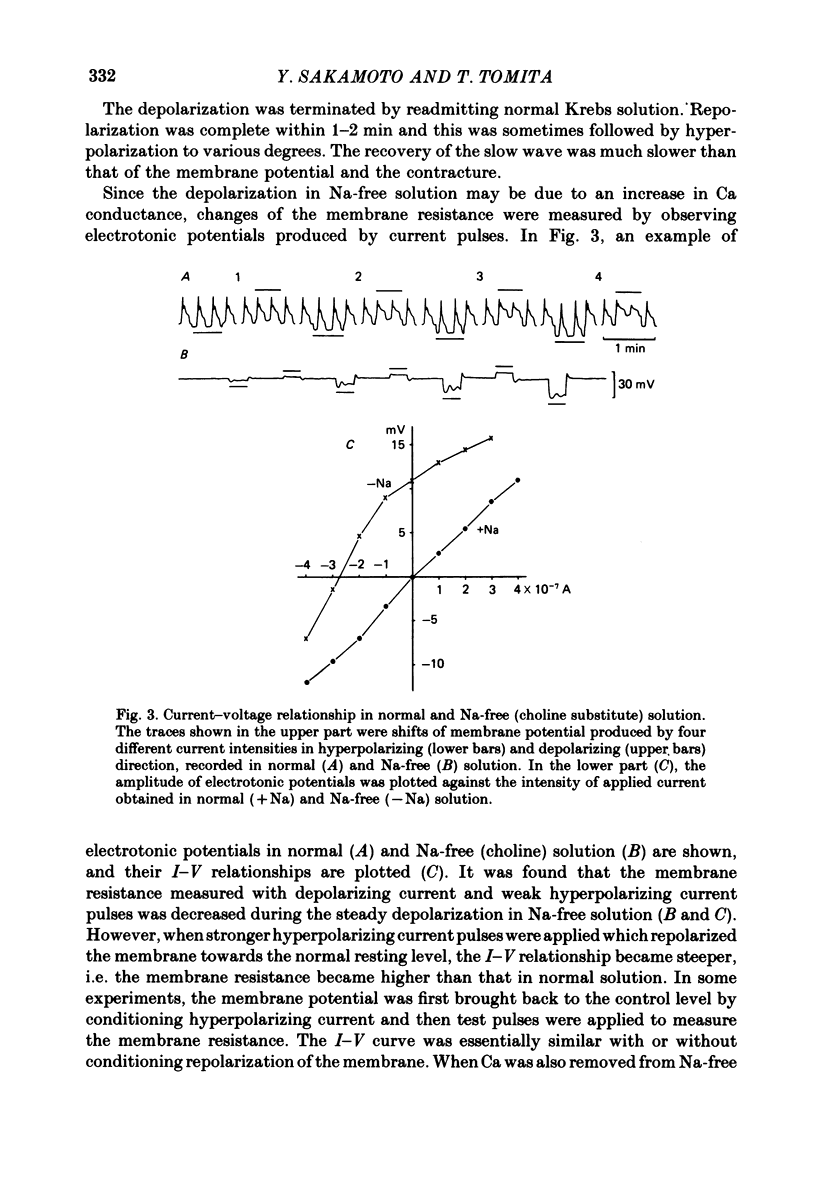
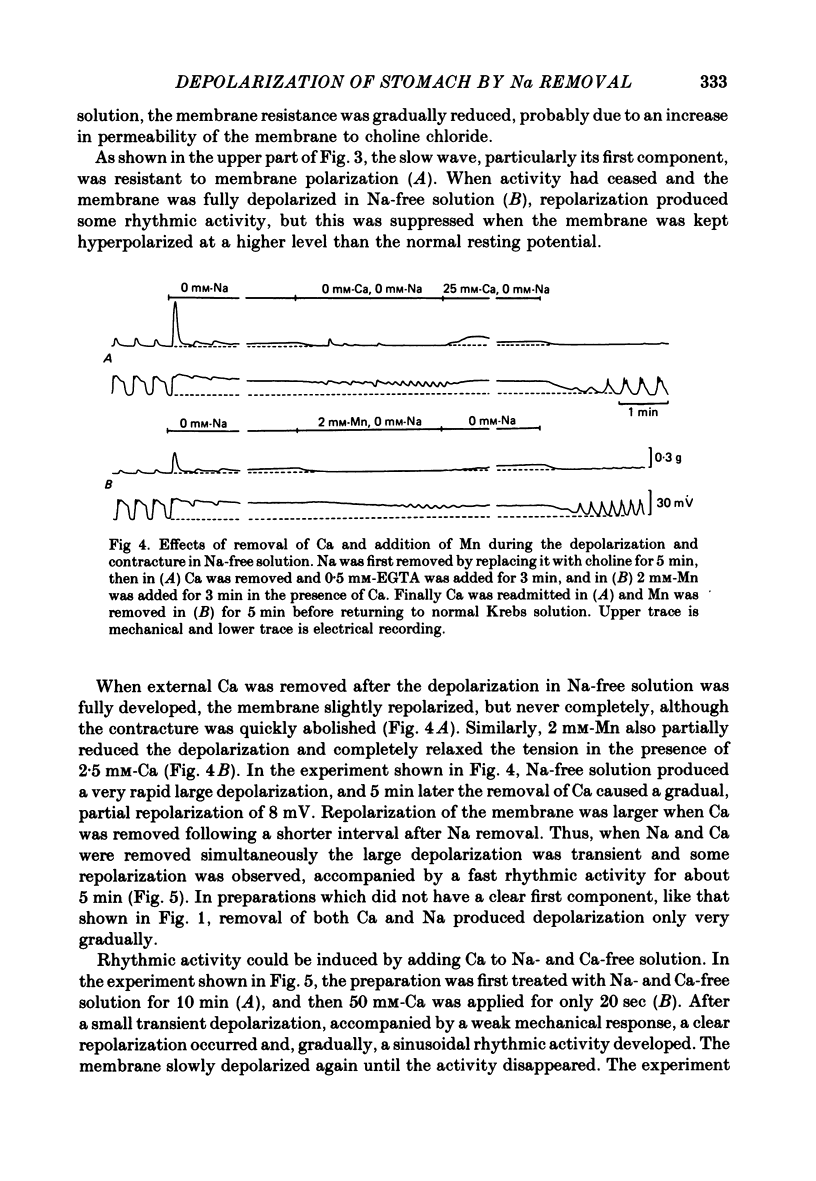
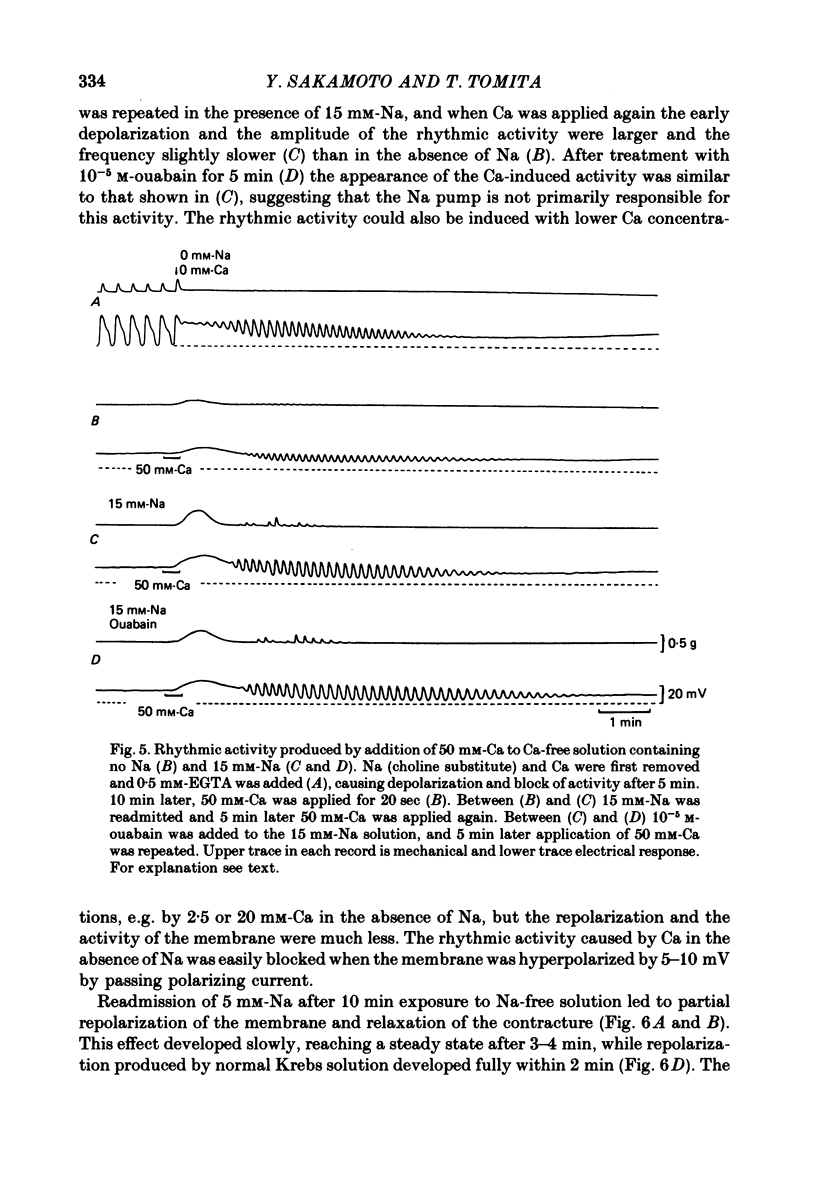
1. Reducing the Na concentration to less than 20 mM depolarized the circular muscle of the guinea-pig stomach and contracture developed. The rate of depolarization varied between preparations. In some, all electrical activity disappeared within 2-3 min in Na-free solution, accompanied by depolarization, but in other preparations a sinusoidal rhythmic activity remained for various lengths of time up to 10 min. 2. When Ca was removed after the depolarization in Na-free solution was fully developed, the membrane partly repolarized and the contracture was abolished. Addition of 2 mM-Mn in the presence of Ca had the same effect. 3. After complete block of electrical activity in Ca-free and Na-free solution, readmission of only Ca (2.5-50 mM) produced sinusoidal rhythmic activity. This activity, observed in the absence of Na, was abolished when the membrane was hyperpolarized by applying inward current. 4. The membrane depolarized in Na-free solution was repolarized by readmission of only 5 mM-Na. This repolarization was greater and faster when the exposure to Na-free solution was prolonged. 5. The possibility was considered that an electrogenic Na-Ca exchange may be involved in the generation of the first component of the slow wave, and that removal of Na blocked the repolarizing phase of this component. In the absence of Na, Ca can evoke rhythmic activity and the underlying mechanism may be the same as that of the second component of slow wave, i.e. an increase in Ca conductance.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Ohba M., Sakamoto Y., Tomita T. Effects of sodium, potassium and calcium ions on the slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1977 May;267(1):167–180. doi: 10.1113/jphysiol.1977.sp011806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M., Sakamoto Y., Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1975 Dec;253(2):505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa T. Effect of removing the external sodium on the electrical and mechanical activities of the pregnant mouse myometrium. Jpn J Physiol. 1971 Dec;21(6):607–625. doi: 10.2170/jjphysiol.21.607. [DOI] [PubMed] [Google Scholar]
- Osa T. The effects of sodium, calcium and manganese on the electrical and mechanical activities of the myometrial smooth muscle of pregnant mice. Jpn J Physiol. 1973 Apr;23(2):113–133. doi: 10.2170/jjphysiol.23.113. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]


