Abstract
1. Membrane potential responses to ionophoretically applied noradrenaline and to field stimulation were studied in the mouse anococcygeus muscle using intracellular recording techniques.
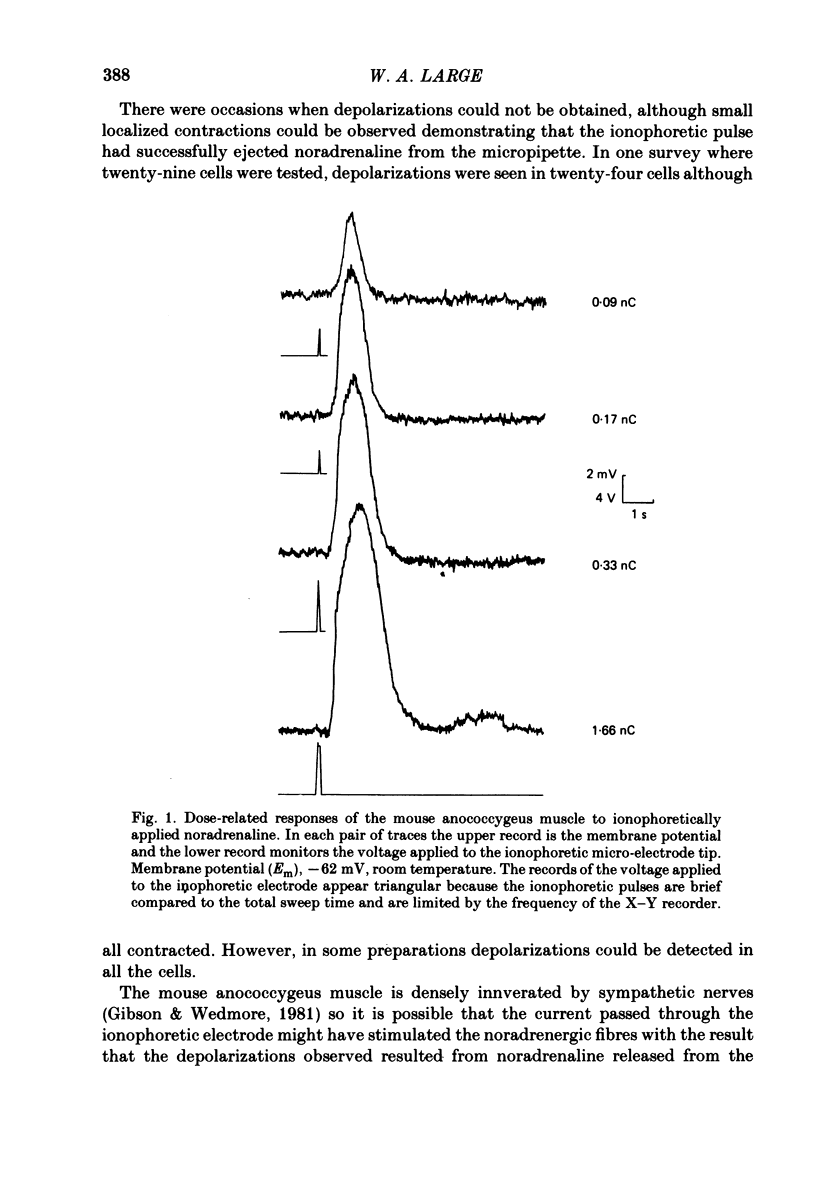
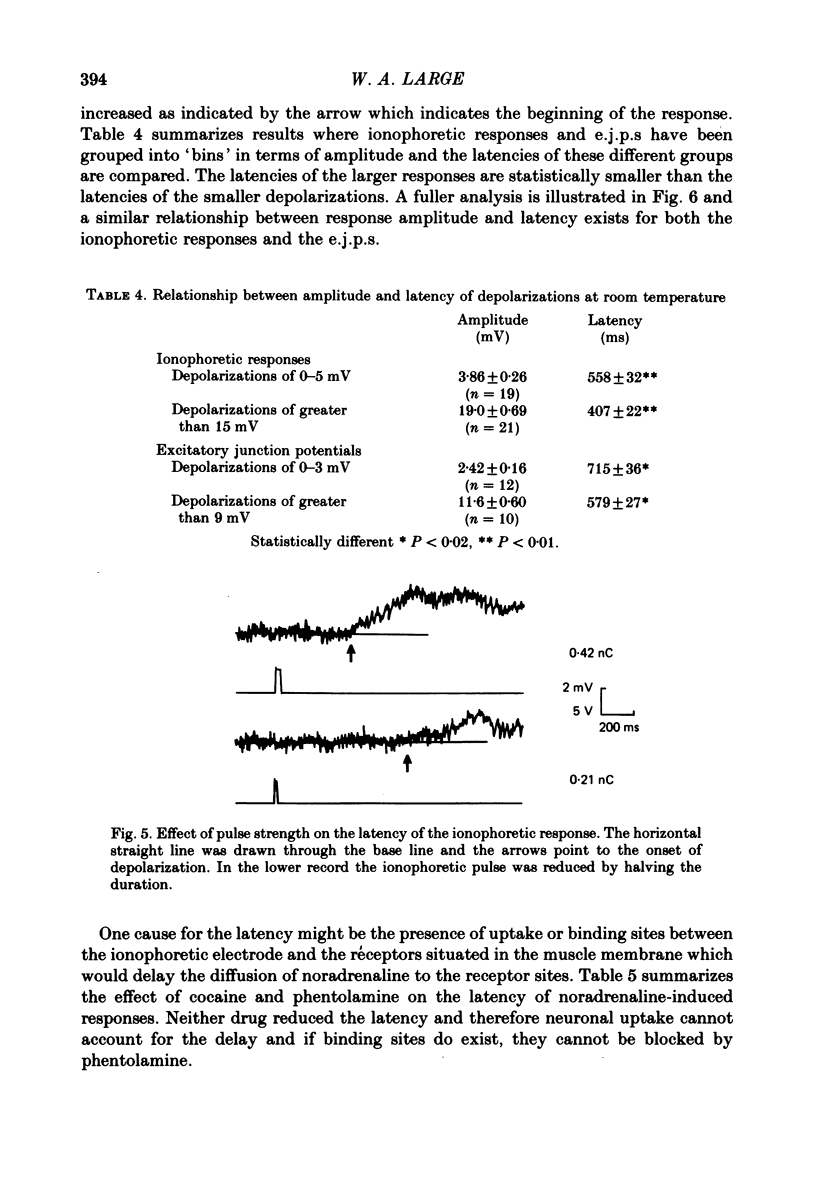
2. The ionophoretic application of noradrenaline produced charge-dependent depolarizations whose total duration was 1-2 s at room temperature and which were characterized by a delay between the start of the ionophoretic pulse and the onset of depolarization (termed the latency of the responses). On occasion ionophoresis of noradrenaline did not depolarize the muscle even though it seemed that successful ejection of noradrenaline had occurred as small localized contractions could be seen.
3. The characteristics of these depolarizations were unaffected by tetrodotoxin (10-7 M) and could not be reproduced when the ionophoretic pipette contained 2 M-NaCl rather than noradrenaline. Moreover noradrenaline still produced depolarizations in denervated muscle and thus it is concluded that the responses were caused by noradrenaline released from the ionophoretic micropipette and not from the intrinsic noradrenergic nerves.
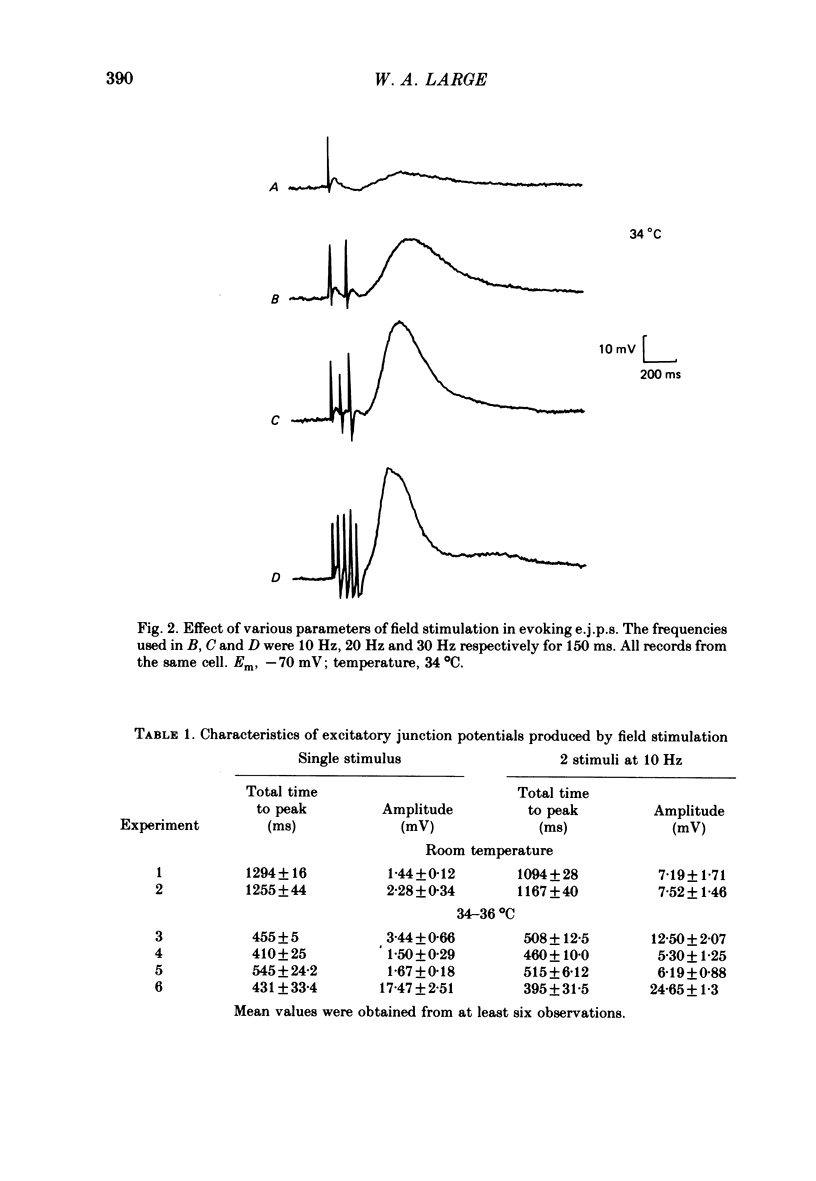
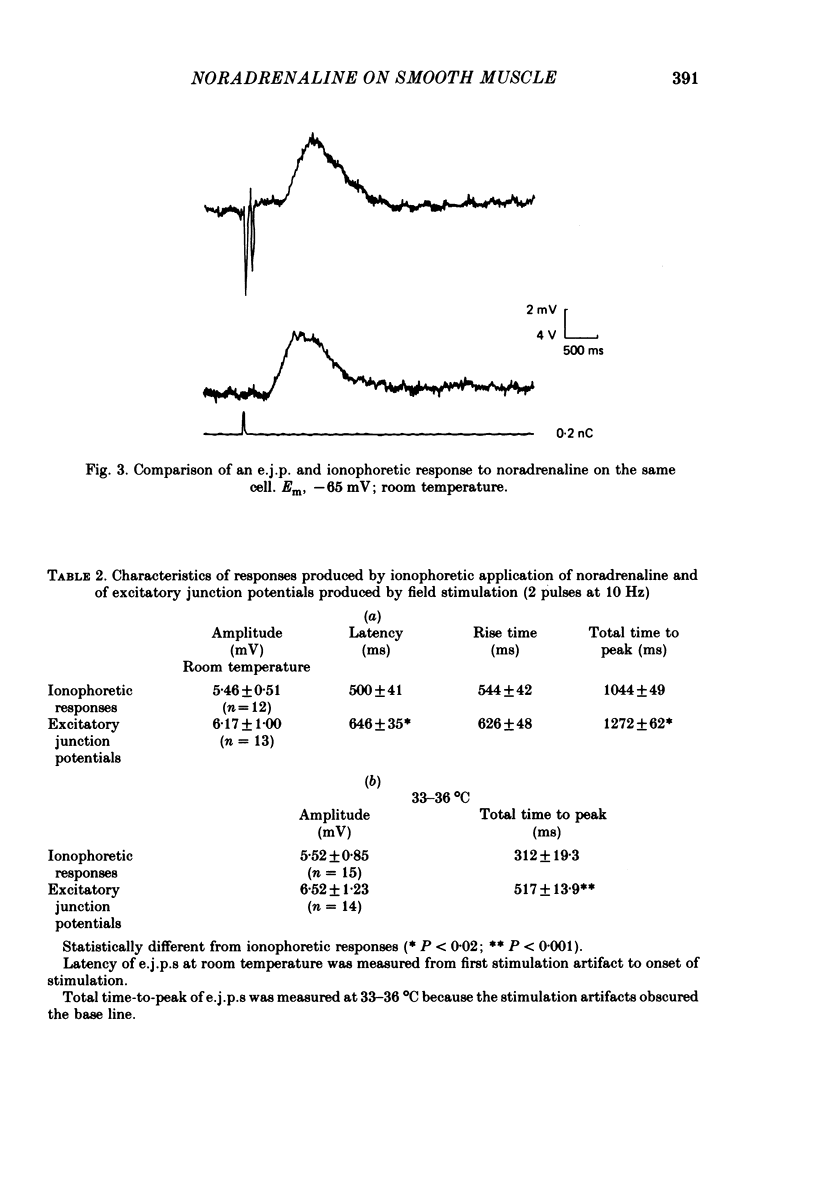
4. Field stimulation of innervated muscle usually evoked excitatory junction potentials (e.j.p.s), but sometimes inhibitory junction potentials (i.j.p.s) or a mixture of e.j.p.s and i.j.p.s were observed. The time course of the e.j.p.s was slightly longer than that of the ionophoretic depolarizations which was accounted for by a smaller latency of the ionophoretically induced responses.
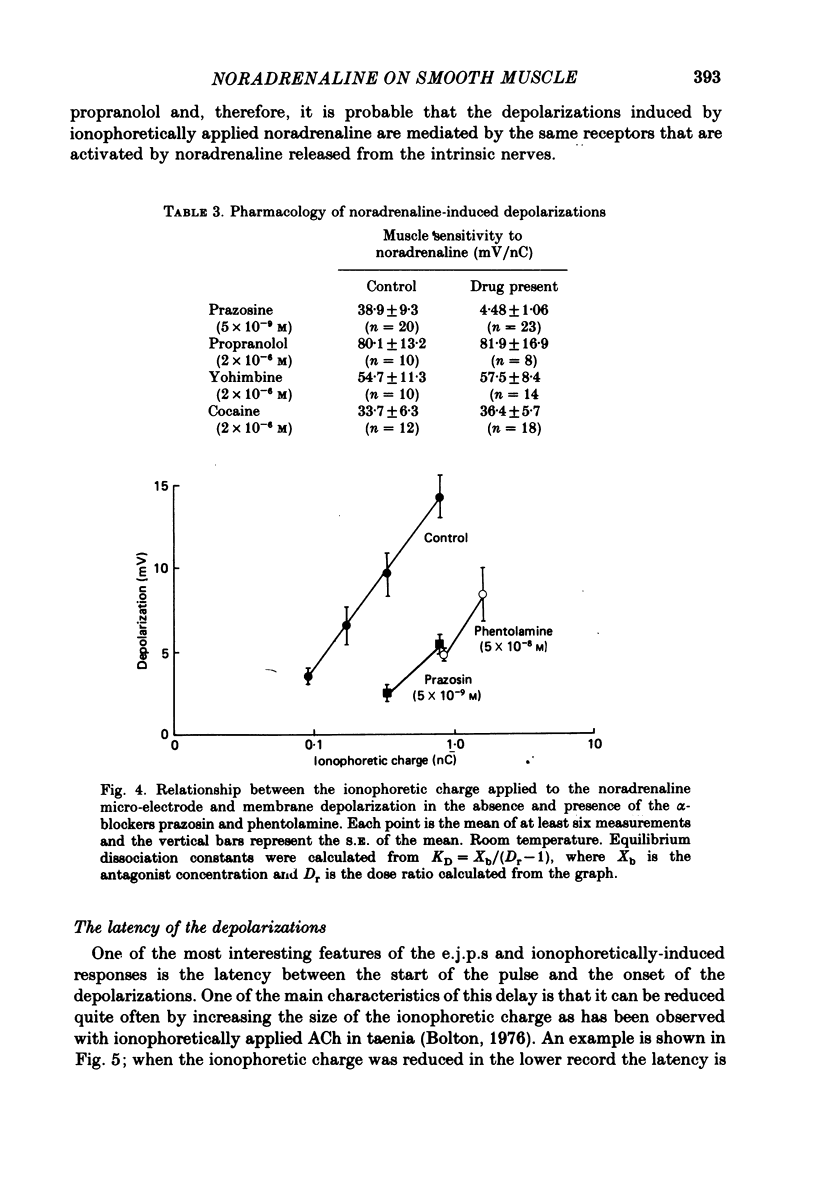
5. The pharmacology of the nerve-evoked e.j.p.s and the ionophoretically induced depolarizations was similar as both types of responses were antagonized by α1-adrenoceptor blocking agents (phentolamine and prazosin) but were unaffected by the β-adrenoceptor antagonist, propranolol. It is probable that noradrenaline released from the intrinsic nerves and that from the ionophoretic micropipette were acting on the same adrenoceptors.
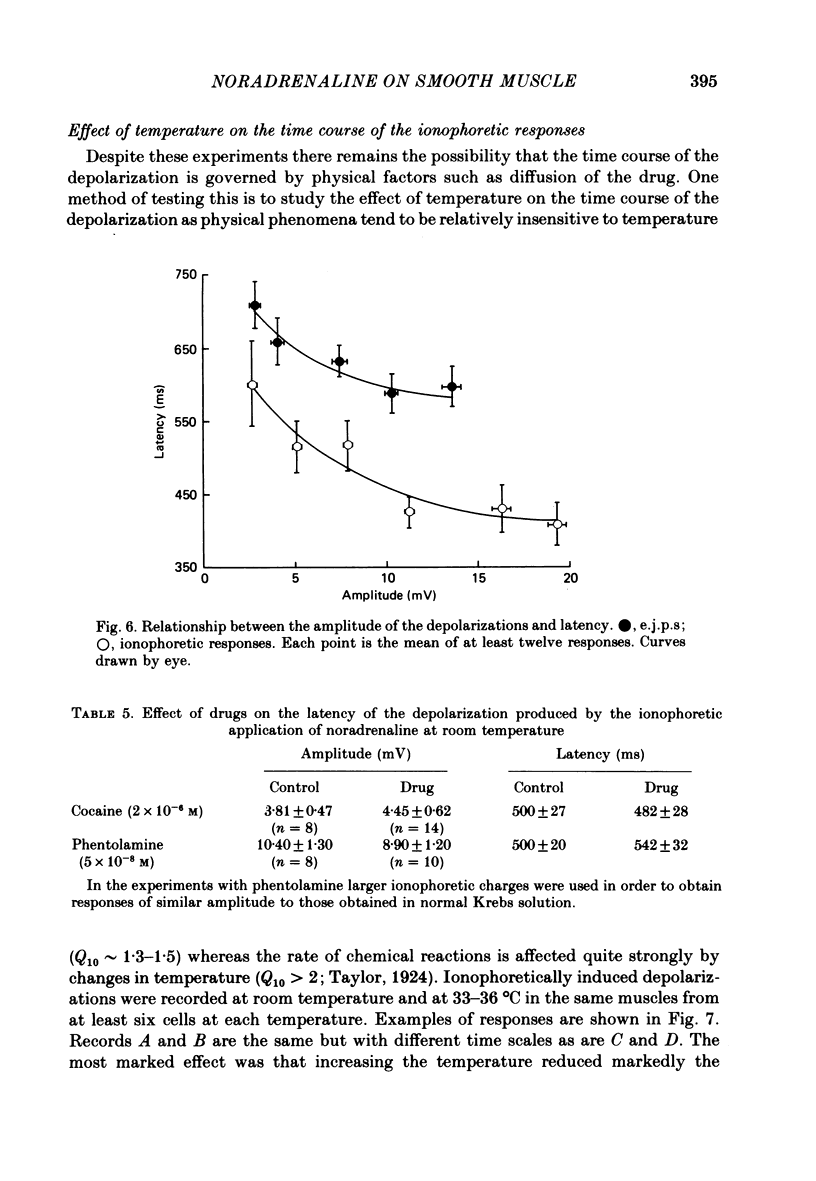
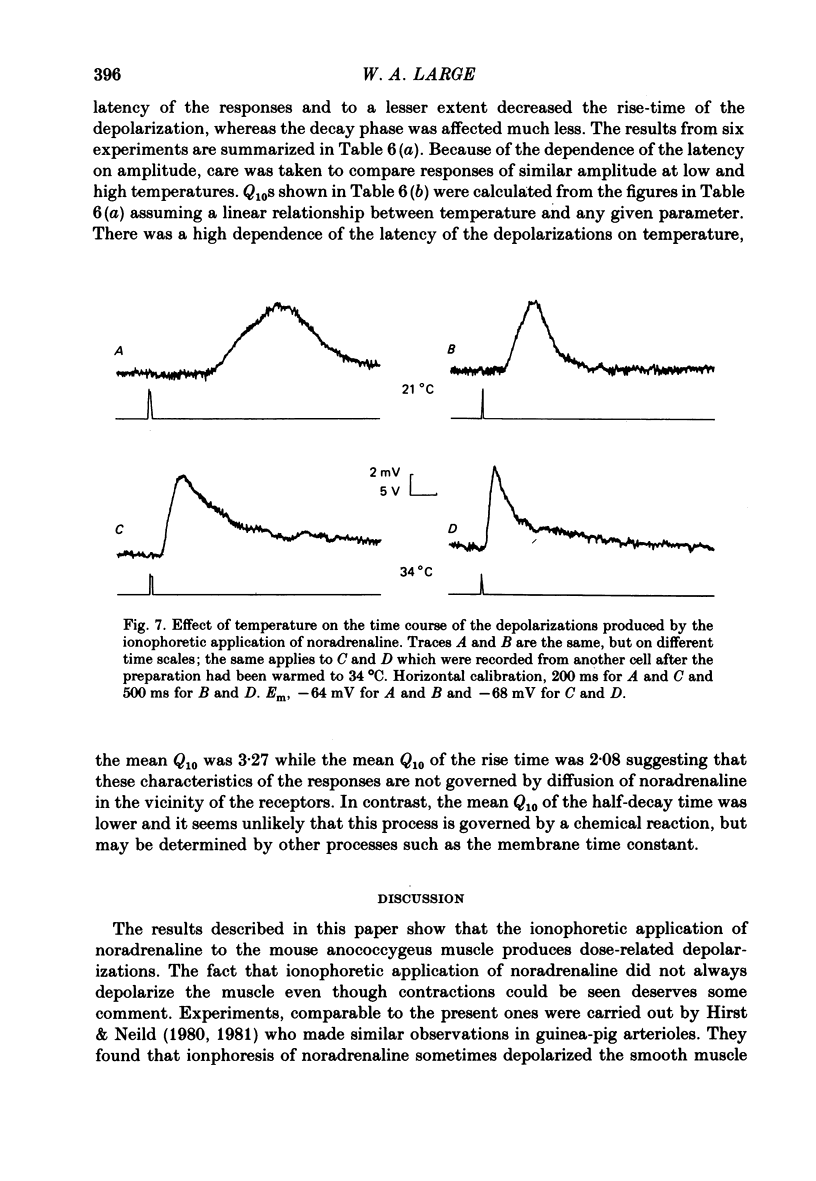
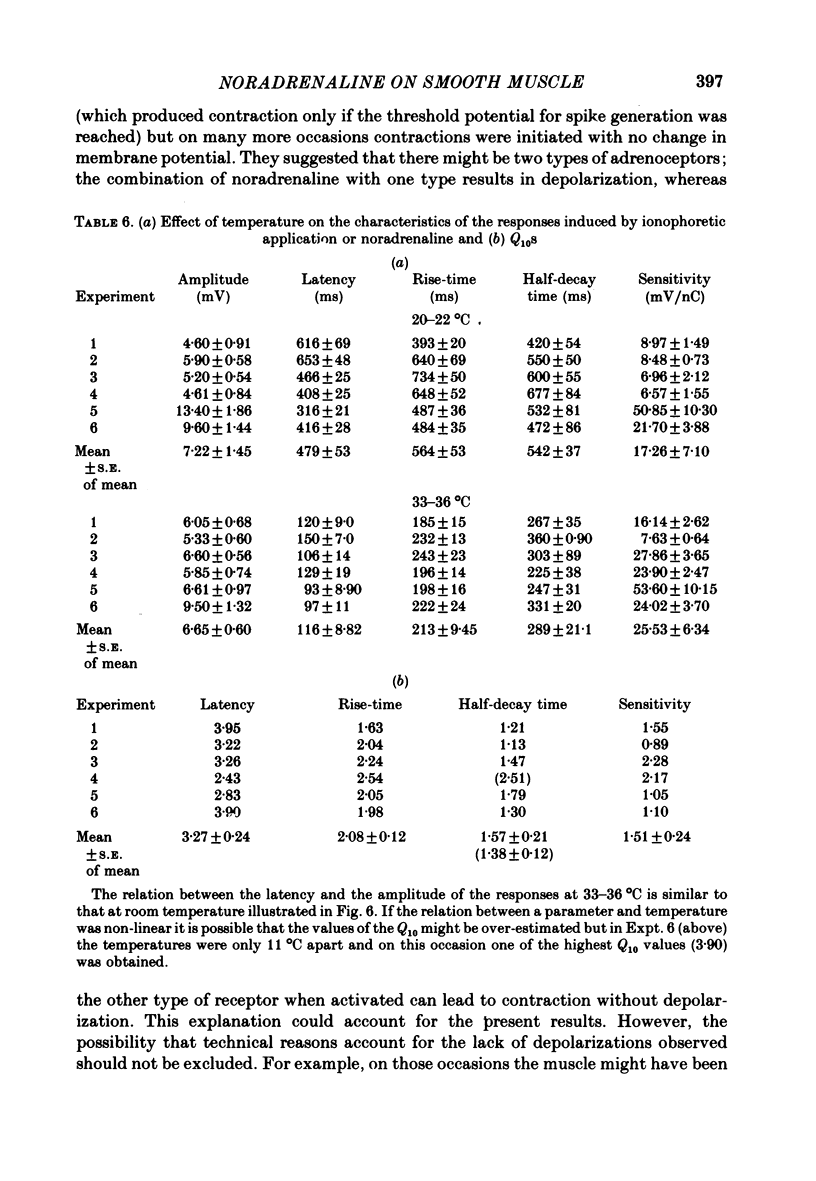
6. The latency and to a lesser extent the rise-time of the depolarizations produced by the ionophoretic application of noradrenaline was highly sensitive to changes in temperature of the bathing fluid (Q10s > 2) whereas the half-decay time was relatively insensitive to temperature changes (Q10 ∼ 1·5). In addition the latency of the depolarizations was not altered by inhibiting the noradrenaline-uptake mechanism with cocaine (2 × 10-6 M) or by α-adrenoceptor blocking agents. Thus it seems likely that the latency of the responses is a property of the noradrenaline—receptor interaction rather than being caused by other phenomena such as diffusion of noradrenaline.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton T. B. On the latency and form of the membrane responses of smooth muscle to the iontophoretic application of acetylcholine or carbachol. Proc R Soc Lond B Biol Sci. 1976 Aug 27;194(1114):99–119. doi: 10.1098/rspb.1976.0068. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- Gibson A., Gillespie J. S. The effect of immunosympathectomy and of 6-hydroxydopamine on the responses of the rat anococcygeus to nerve stimulation and to some drugs. Br J Pharmacol. 1973 Feb;47(2):261–267. doi: 10.1111/j.1476-5381.1973.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Wedmore C. V. Responses of the isolated anococcygeus muscle of the mouse to drugs and to field stimulation. J Auton Pharmacol. 1981 Jun;1(3):225–233. doi: 10.1111/j.1474-8673.1981.tb00451.x. [DOI] [PubMed] [Google Scholar]
- Green R. D., 3rd, Miller J. W. Evidence for the active transport of epinephrine and norepinephrine by the uterus of the rat. J Pharmacol Exp Ther. 1966 Apr;152(1):42–50. [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Smith I., Purves R. D. Synaptic delay in the heart: an ionophoretic study. J Physiol. 1978 Jun;279:31–54. doi: 10.1113/jphysiol.1978.sp012329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. J. Temperature and inhibitory junctional transmission in guinea-pig ileum. Br J Pharmacol. 1979 Jul;66(3):355–357. doi: 10.1111/j.1476-5381.1979.tb10836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves R. D. Muscarinic excitation: a microelectrophoretic study on cultured smooth muscle cells. Br J Pharmacol. 1974 Sep;52(1):77–86. doi: 10.1111/j.1476-5381.1974.tb09689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]


