Abstract
A series of modified oligonucleotides (ONs), characterized by a phosphorothioate (P=S) backbone and a six-membered azasugar (6-AZS) as a sugar substitute in a nucleotide, were newly synthesized and assessed for their ability to inhibit human immunodeficiency virus type 1 (HIV-1) via simple treatment of HIV-1-infected cultures, without any transfection process. While unmodified P=S ONs exhibited only minor anti-HIV-1 activity, the six-membered azasugar nucleotide (6-AZN)-containing P=S oligonucleotides (AZPSONs) exhibited remarkable antiviral activity against HIV-1/simian-human immunodeficiency virus (SHIV) replication and syncytium formation (50% effective concentration = 0.02 to 0.2 μM). The AZPSONs exhibited little cytotoxicity at concentrations of up to 100 μM. DBM 2198, one of the most effective AZPSONs, exhibited antiviral activity against a broad spectrum of HIV-1, including T-cell-tropic, monotropic, and even drug-resistant HIV-1 variants. The anti-HIV-1 activities of DBM 2198 were similarly maintained in HIV-1-infected cultures of peripheral blood mononuclear cells. When we treated severely infected cultures with DBM 2198, syncytia disappeared completely within 2 days. Taken together, our results indicate that DBM 2198 and other AZPSONs may prove useful in the further development of safe and effective AIDS-therapeutic drugs against a broad spectrum of HIV-1 variants.
During the past decade, antisense oligonucleotides (ONs) have gained attention as a possible human immunodeficiency virus type 1 (HIV-1) inhibitor (41). Most antisense studies against HIV-1 have been performed using chemically modified ONs, including phosphorothioate (P=S) ONs (9-11, 16, 21-23, 26, 29-31, 36, 42, 47, 48, 50, 51, 53), methylphosphonate ONs (15, 32, 46), or phosphoramidates ONs (6, 12, 14), in order to improve the stability of the antisense ONs against nucleases. Among these modified ONs, P=S ONs have been studied most extensively due to a host of beneficial properties: strong nuclease resistance (10, 11), higher solubility (26), and relatively intense anti-HIV-1 activity (9, 16, 21-23, 29-31, 36, 42, 47, 48, 50, 51, 53). Nevertheless, the P=S ONs are also associated with some disadvantages, including their propensity for interaction with membrane proteins via a specific mode of action (8, 25, 45), as well as the fact that higher concentrations of these ONs are required than of the existing antiviral drugs.
Several studies have been performed to explain the possible mechanisms underlying the anti-HIV-1 activity of each P=S ON. The mechanisms suggested have included adsorption blocking (9, 16, 47, 50, 51, 53) and inhibition of HIV-1-specific enzymes, such as reverse transcriptase (29-31) or integrase (21-23, 36, 42). However, most studies of the antiviral mechanisms of P=S ONs, as well as recent studies with small interfering RNA (siRNA) against HIV-1 (3, 5, 7, 35, 38, 39, 49), have been conducted by transfection or viral vector-mediated delivery (4, 17, 27, 33, 34), rather than simple treatment of the infected culture. Those transfection steps may impede, to some extent, the application of antisense or siRNA under physiological conditions.
We reported previously that the P=O ONs containing modified adenosine (A), with a six-membered azasugar (6-AZS) instead of a five-membered ribose at the sugar moiety of A, facilitated formation of stable duplexes with mRNA, depending on the location and number of the substitutions (20, 24). In the present study, we synthesized six-membered azasugar nucleotide (6-AZN)-containing P=S oligonucleotides (AZPSONs), designed in specific sequences which are complementary to the HIV-1 RNA genome, primarily to the trans-activation response (TAR) region of the HIV-1 long terminal repeats (LTR), or in random sequences. We then assessed the anti-HIV abilities of these AZPSONs. While the P=S ON itself exhibited little if any anti-HIV-1 activity, most of the AZPSONs exhibited potent anti-HIV-1 activity without the aid of any transfecting agents and were also found to be very stable against enzymatic degradation.
Among the AZPSONs, DBM 2198, containing five 6-AZNs in a random sequence, was found to be the most potent for its anti-HIV-1 activity against T-cell-tropic, monotropic, and drug-resistant HIV-1 variants. The treatment of infected cultures with DBM 2198 did not cause any cytotoxicity to the host cells. The mechanisms underlying the anti-HIV-1 properties of DBM 2198 have been examined through a series of experiments, including sequence-specific LTR promoter inhibition assays, calcium mobilization assays, infection inhibition assays, and flow cytometry analysis (manuscript submitted). In the present manuscript, we report first the azasugar-containing P=S ONs and AZPSONs and their remarkable activities against a broad spectrum of HIV-1 variants.
MATERIALS AND METHODS
Synthesis of AZPSONs and other ONs.
The synthesis of modified adenine nucleoside (6-AZN) containing 6-AZS has been previously reported (24). In the present experiments, we synthesized AZPSONs and other ONs with 6-AZN, using an automated DNA synthesizer (Applied Biosystems 392, Foster, CA) based on standard chemistry at a 1-μmole scale. In order to incorporate a modified nucleotide, we employed a coupling time of 600 seconds. The P=S ONs were prepared using tetraethylthiuram disulfide (TETD). The yield of the modified P=S ONs coupling was 90 to 98%, and all ONs were purified by reverse-phase HPLC with an acetonitrile gradient (18 to 48%) in a 100 mM triethylammonium actetate buffer (pH 7.0). Polyacrylamide gel electrophoresis (PAGE) was used to confirm the purity of the ONs.
Cells and viruses.
Jurkat-tat (Tat-expressing Jurkat cells) cells were obtained from J. Sodroski (Dana-Farber Cancer Institute, Harvard Medical School). MT-4, C8166, CEMX-174, HeLa-CD4-LTR-β-gal (Magi) cells, U373-CD4-CXCR4-Magi, and U373-CD4-CCR5-Magi cells were obtained from the AIDS Research and Reference Reagent Program (National Institutes of Health). Jurkat E6 (TIB152), HeLa cells (CCL2), and Vero cells were purchased from the American Type Culture Collection. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors, using Ficoll-Hypaque (Sigma Chem. Co.) density gradient centrifugation, as was described previously (36), and were also used in our experiments. HXBc2 and HXBc2/Δtat (tat-defective HIV-1 laboratory strain) were kindly provided by J. Sodroski (DFCI, Harvard Medical School). HIV-1IIIB, HIV-1MN, HIV-1CC, HIV-1RTMDRI (virus resistant nevirapine, zidovudine, and dideoxyinosine), HIV-1Saquinavir-R, HIV-1Ada-M, HIV-1Ba-L, and SHIV89.6 were all obtained from the AIDS Research and Reference Reagent Program.
Antiviral activity assay.
Jurkat-tat or C8166 cells were infected with different HIV-1 strains at an appropriate multiplicity of infection (MOI; 0.001 to 0.2 depending on the experiment) for 1 h at 37°C, then cultured in media containing different concentrations of AZPSONs and other ONs together with dextran sulfate as a reference compound. The antiviral activity of each AZPSON was assessed according to the inhibition of HIV-1 replication, which was measured by the amount of syncytia and/or reverse transcriptase (RT) activity, or by a visual infection assay (52). The antiviral activity of each DBM ON was also expressed by the concentration required for the inhibition of 50% of virus-mediated cell killing, in comparison with an untreated control (EC50). Cells were infected with a 0.01 multiplicity of infection (MOI) of several different HIV-1 strains, then cultured in the presence of serially diluted DBM ONs. The EC50 values were calculated 4 days postinfection by a tetrazolium-based MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] (Sigma Chemical Co.) assay, as previously described (37).
Reverse transcriptase assay.
Virion-associated RT activity was evaluated as follows: Two-thirds of the culture fraction containing the HIV-1 infected cells was harvested every 3 days, disrupted by vigorous vortexing, then clarified by a quick centrifugation at 15,000 rpm for 15 seconds. The supernatants were then mixed with equal volumes of polyethylene glycol/NaCl solution (30% polyethylene glycol in 0.4 M NaCl), mixed thoroughly, and incubated for 12 h at 4°C. After 45 min of centrifugation at 15,000 rpm, the pellets were resuspended with 10 μl of dissociation buffer (0.25% triton X-100, 20% glycerol, 50 mM Tris-HCl, pH 7.5, 0.1% dithiothreitol, and 250 mM KCl) and then examined for RT activity, as previously described (44).
Visual infection assay for titration of infectious virion.
A visual infection assay was performed as described previously (37), with some minor modifications. In brief, monolayers of U373-CD4-CXCR4-Magi, or U373-CD4-CCR5-Magi cells were infected with serially diluted HIV-1 solutions for 1 h, washed twice with phosphate-buffered saline, then cultured in Dulbecco's modified Eagle's medium (Life Technologies, Inc.), supplemented with 10% fetal bovine serum. Two days after initial infection, the cells were washed and fixed with 1% formaldehyde and 0.2% glutaraldehyde solution, then stained with 0.04% 5-bromo-4-chloro-3-indoryl-β-d-galactopyranoside (X-Gal; Molecular Probes, Eugene, OR) for 2 h at 37°C. Blue cells were counted under an inverted microscope, and expressed as the titer of infectious virus particles in each sample.
Cell cytotoxicity.
The cytotoxicity of the DBM ONs was assessed using MT-4, Jurkat, C8166, Vero, CEMx-174, U937, and HeLa cells. Cells in 96-well plates (2 × 104 cells/well) were incubated with serially diluted DBM ONs for 4 days, and the number of viable cells was quantified via MTT assays. Cytotoxicity was assessed according to the concentration of ONs required to reduce cell viability by 50% (CC50).
Statistical analysis.
Most statistical data were recorded in triplicate, and results were expressed as geometric means ± standard deviation. Statistical significance was evaluated by Student's t tests. Data with a P value of ≤0.01 were considered significant.
RESULTS
AZPSONs exhibit potent anti-HIV-1 activity.
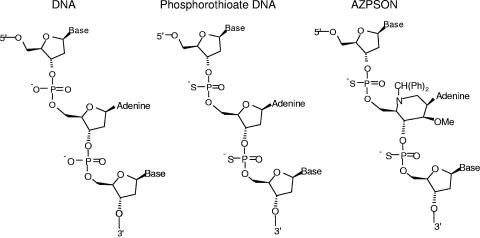
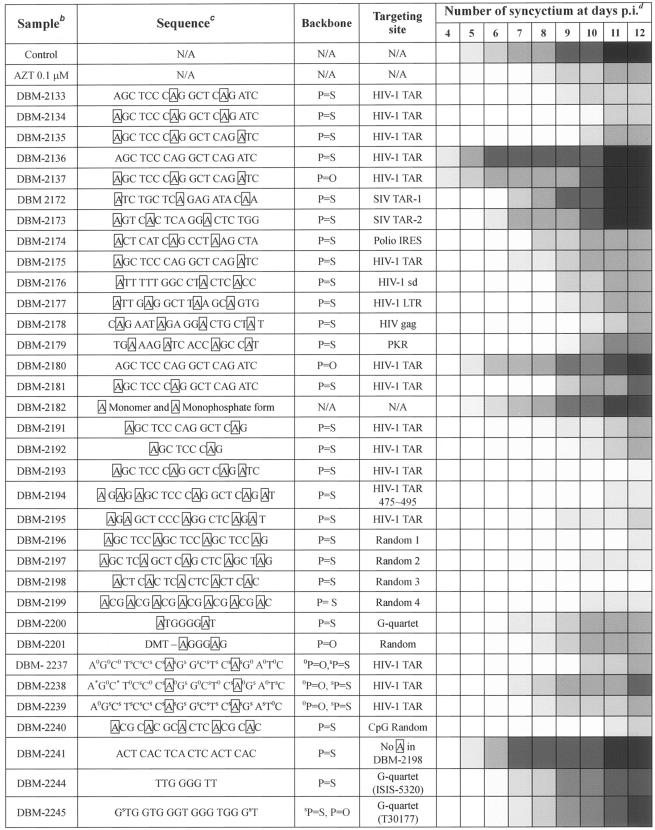
Figure 1 shows the structure of the AZN-containing P=S ONs. Table 1 lists the sequences of the modified and unmodified ONs and exhibits the anti-HIV-1 activity of each ON. The ONs targeting the partial sequences of HIV-1 TAR, upstream LTR, LTR splicing donor site, gag, SIV TAR and poliovirus internal ribosome entry site (IRES) (2), together with random-sequence 18- to 22-mer ONs and a few G-quartet analogue ONs were synthesized with P=S, P=O, or mixed (P=S and P=O) backbones.
FIG. 1.
Chemical structure of AZPSONs and other ONs.
TABLE 1.
DBM ONs and their long-lasting anti-HIV-1 activitya after a single treatment
Antiviral activity represented by the inhibition of syncytium formation.
HIV-1-infected Jurkat-tat cells were cultured on 24-well plates in Opti-MEM medium containing DBM ONs at a final concentration of 0.1 μM.
The boxed A represents the six-membered azasugar-containing deoxyadenosine nucelotide.
The darkness of the box represents the severity of syncytium formations in each well on the indicated days p.i. White, no syncytia; lightest gray, fewer than 10 syncytia; and darkest gray, more than 500 syncytia.
These ONs were evaluated for their anti-HIV activity according to their ability to inhibit syncytium formation when treated to the culture of HIV-1 (HXBc2/Δtat)-infected Jurkat-tat cells. The anti-HIV-1 activities exhibited by the DBM ONs (AZPSONs and other ONs) were summarized (Table 1). DBM 2133, 2134, 2193, 2194, 2195 (antisense to TAR), 2177 (antisenses to HIV LTR), and 2196, 2197, 2198, 2199, and 2240 (random sequence) induced the significant inhibition of HIV-1-mediated syncytium formation, after a single treatment of the infected culture at a final concentration of 0.1 μM. In particular, DBM 2193, 2196 and 2198 appeared to be much more effective than zidovudine in terms of their long-term term antiviral activities after a single treatment. Neither the 6-AZN monomer (DBM 2182), nor its monophosphate form, exhibited antiviral activity, leading us to dismiss the possibility that the antiviral activity of these AZPSONs might be attributable to the AZN monomer itself, or to a decomposition intermediate of AZN. DBM 2136, however, which harbors no AZN in its P=S linkages, induced rapid and serious syncytium formation, rather than inhibition. The P=O linkage (DBM 2137) or mixed backbone (P=S and P=O) ONs (DBM 2237 to 2239), exhibited unremarkable antiviral activity, although they did harbor 6-AZNs.
The G-quartet ONs were reported to be effective anti-HIV compounds, and some of them had been subjected to preclinical studies (1, 40). We introduced the 6-AZN to the G quartet P=S ON, synthesizing DBM 2200. DBM 2201 contains a dimethoxytrityl group on the 5′-end of the modified G-quadruplex in its P=O linkage, which was synthesized as described in previous reports (13, 16, 53). However, neither of these exhibited any significant anti-HIV-1 activity (Table 1). We also evaluated the abilities of another set of G-quartet ONs (DBM 2244 and 2245), which contained the same sequences as did ISIS-5320 (9, 53) and T30177 (Zintevir) (23, 36), together with AZPSONs. Our results indicated that many AZPSONs (DBM 2134, 2193, 2194, 2196, 2198 and 2240) exhibited much more potent anti-HIV activity than did the G-quartet ONs, at least with regard to long-lasting efficacy after a single treatment at a concentration of 0.1 μM (Table 1).
Dose-dependent inhibitory effects of AZPSONs on HIV-1 replication.
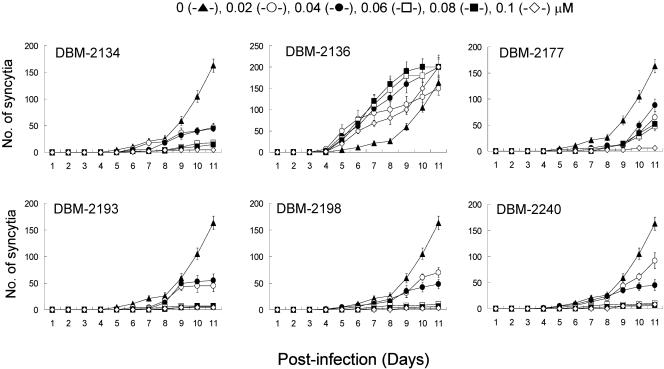
In order to determine whether AZPSONs' antiviral activity shown in Table 1 was specific to the suppression of HIV-1 replication or to the nonspecific side effects, we evaluated the anti-HIV activity of five remarkable AZPSONs and control P=S ONs (DBM 2136), at several different concentrations. As shown in Fig. 2, the selected AZPSONs (DBM 2134, 2177, 2193, 2198, and 2140) resulted in the inhibition of syncytium formation, in a dose-dependent manner. It implies that the antiviral activity of AZPSONs was not attributable to a side effect. Most of them exhibited strong antiviral activity at concentrations greater than 0.06 μM. DBM 2136 exhibited no inhibitory effects on HIV-1 replication; rather, it appeared to enhance viral replication. The effects of DBM 2136 were reexamined, and discussed in the next section.
FIG. 2.
Dose-dependent inhibitory effects of DBM ONs on HIV-1 replication in a serum-free medium. Jurkat-tat cells were infected with HIV-1 (HXBc2/Δtat) at 0.001 MOI and then cultured at 96-well plates in Opti-MEM for 11days in the presence of DBM ONs at the final concentrations shown above. Syncytia were then counted under an inverted microscope.
Serum effects on the antiviral activity of AZPSONs.
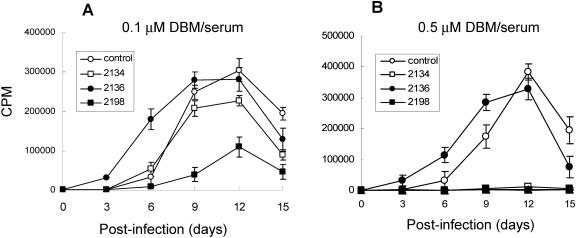
P=S ONs have been reported to bind plasma proteins, such as albumin (53). To see whether serum proteins would also attenuate the antiviral activity of AZPSONs, DBM 2134 and 2198 were examined in a serum-containing RPMI medium (RPMI10). As shown in Fig. 3, while DBM 2134 was markedly attenuated in its antiviral activity in the presence of serum, compared with that in serum-free medium that of DBM 2198 was minimally attenuated by serum at a final concentration of 0.1 μM (Fig. 3A). Nevertheless, these AZPSONs (DBM 2134 and 2198) still showed complete inhibition of HIV-1 replication at a final concentration of 0.5 μM, even in the presence of serum (Fig. 3B). Serum effects on the other AZPSONs (DBM 2193, 2196, and 2240) were similar to those of DBM 2134 or DBM 2198, respectively (data not shown). The kinds of sera (fetal bovine, human, and horse serum) and concentrations of sera (5%, 10%, and 50% of fetal bovine serum) did not cause any additional attenuation of the antiviral activity of the AZPSONs (data not shown), suggesting that effective AZPSONs, such as DBM 2198, can be used as anti-HIV-1 drugs in physiological conditions.
FIG. 3.
Serum effects on the antiviral activity of AZPSONs. Jurkat-tat cells were infected with 0.001 MOI of HXBc2/Δtat and cultured in RPMI10 containing 0.1 μM (A) and 0.5 μM (B) of each DBM ON. Two-thirds of the culture was harvested every 3 days, and the same amount of fresh medium containing DBM ON was added to the culture. The titer of HIV-1 in the harvest was measured by RT assay.
EC50 of AZPSONs.
The EC50 values of the selected AZPSONs, together with zidovudine, dideoxycytosine, and dideoxyinosine, and dextrans sulfate, a well-documented fusion inhibitor, were determined using three different HIV-1 variants and susceptible host cells, as described in the Materials and Methods section, and summarized in Table 2. The treatment of infected cells with AZPSONs (DBM 2134, 2193, 2196, 2198, and 2240) inhibited the replication of all three HIV-1 variants, with EC50s ranging from 0.05 to 0.23 μM (Table 2). The AZPSONs were much more effective against HIV-1IIIB than were dideoxycytosine or dideoxyinosine, but were not as effective as zidovudine on a molar scale. Notably, the AZPSONs also proved to be very effective against the SIV-HIV chimeric recombinant virus SHIV89.6, which exhibits resistance to zidovudine even at an extreme concentration (400 μM). Among the AZPSONs, DBM 2198 proved to be the most effective against these T-cell-tropic and monotropic HIV-1 variants (0.05 < EC50 < 0.10 μM), while 2 to 10 μM of DBM 2136 (no AZN in the P=S ON backbone) was required for a 50% inhibition of HIV-1 replication (EC50). dextran sulfate was very efficient in inhibiting the T-cell-tropic HIV-1 variants, but was not effective at all in the inhibition of the monotropic Ba-L strain, as described previously (18, 19).
TABLE 2.
EC50 of each DBM ON on each HIV-1 strain
| HIV-1/host cells | Mean EC50 (μM)a ± SD with:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2134 | 2136 | 2193 | 2196 | 2198 | 2240 | AZT | ddC | ddI | DxS | |
| HXBc2/Δtat/Jurkat-tat | 0.12 ± 0.02 | >10 | 0.06 ± 0.01 | 0.08 ± 0.02 | 0.05 ± 0.01 | 0.08 ± 0.03 | 0.01 ± 0.003 | ND | ND | 0.01 ± 0.01 |
| HIV-1IIIB/MT-4 | 0.13 ± 0.02 | >2.0 | 0.14 ± 0.02 | 0.10 ± 0.01 | 0.08 ± 0.02 | 0.12 ± 0.02 | 0.007 ± 0.003 | 0.66 ± 0.25 | 35.9 ± 12.3 | 0.04 ± 0.02 |
| SHIV89.6p/C8166 | 0.23 ± 0.03 | >2.0 | 0.16 ± 0.03 | 0.12 ± 0.02 | 0.10 ± 0.02 | 0.16 ± 0.03 | >400 | 2.67 ± 1.27 | 70.7 ± 21.2 | 0.05 ± 0.03 |
| HIVcc/C8166 | 0.15 ± 0.05 | >2.0 | 0.16 ± 0.03 | 0.12 ± 0.02 | 0.10 ± 0.02 | 0.14 ± 0.03 | 0.009 ± 0.004 | 1.54 ± 0.55 | ND | 0.05 ± 0.01 |
| Ba-L/U373-CD4-CCR5b | 0.20 ± 0.05 | >2.0 | 0.13 ± 0.03 | 0.10 ± 0.02 | 0.07 ± 0.02 | 0.12 ± 0.02 | ND | ND | ND | NI |
EC50 was determined by MTT assay. ND, not determined. AZT, zidovudine; ddC, dideoxycytosine; ddI, dideoxyinosine; DxS, dextran sulfate. Values represents the mean of triplicates, P < 0.01.
EC50 with strain Ba-L was determined by visual infection assay. NI, no inhibition.
Cell cytotoxicity and therapeutic indices of AZPSONs.
The cytotoxicity of each AZPSON was determined by means of MTT assays, and the CC50s of the AZPSONs are summarized in Table 3. While P=S ON without AZN (DBM 2136) showed relatively lower CC50s (<100 μM) in four out of six cell types, most of the AZPSONs exhibited CC50s over 100 μM in more than half of the tested cell lines, suggesting that the AZN would attenuate the toxicity of the P=S ONs.
TABLE 3.
Cytotoxicity of DBM ONs in vitro
| DBM ON | Mean cytotoxicity by CC50 (μM)a ± SD
|
|||||
|---|---|---|---|---|---|---|
| MT-4 | Jurkat | C8166 | Vero | CEMx174 | HeLa | |
| 2134 (3)b | >100 | 80 ± 9.4 | 80 ± 4.7 | >100 | >100 | >100 |
| 2136 (0) | >100 | 80 ± 9.4 | 90 ± 4.7 | 60 ± 9.4 | 80 ± 9.4 | >100 |
| 2177 (4) | >100 | >100 | 60 ± 9.4 | >100 | >100 | >100 |
| 2180 (P=O) | 80 ± 9.4 | >100 | >100 | >100 | >100 | >100 |
| 2193 (4) | >100 | >100 | 67 ± 14.1 | >100 | >100 | >100 |
| 2196 (4) | >100 | >100 | >100 | >100 | >100 | >100 |
| 2198 (5) | >100 | >100 | >100 | 80 ± 9.4 | >100 | >100 |
| 2240 (5) | >100 | 80 ± 4.7 | >100 | 80 ± 4.7 | >100 | >100 |
CC50, concentration required to inhibit cell survival 50%. Cells were incubated for 4 days in the presence of DBM ONs at different concentrations and then tested for their viability by MTT assay. Each value represents the mean cytotoxicity of triplicates, P < 0.01.
The number of azasugar units in each ON. All have the P=S backbone except 2180.
The therapeutic indices (TI = CC50/EC50) of the tested AZPSONs were at least higher than 400 (TI of DBM 2134: CC50 = 67 μM and EC50 = 0.16 μM for SHIV89.6 in C8166). Whereas, the most effective AZPSON, DBM 2198, proved to have TI scores higher than 2,000 for HXBc2/Δtat in Jurkat-tat cells, 1250 for HIV-1IIIB in MT-4 cells, and 1,000 for HIV-1cc and SHIV89.6 in C8166 cells. These data strongly suggest that the AZPSONs are very safe and effective for the inhibition of HIV-1 replication.
DBM 2198 inhibits a broad-spectrum of HIV-1 variants.
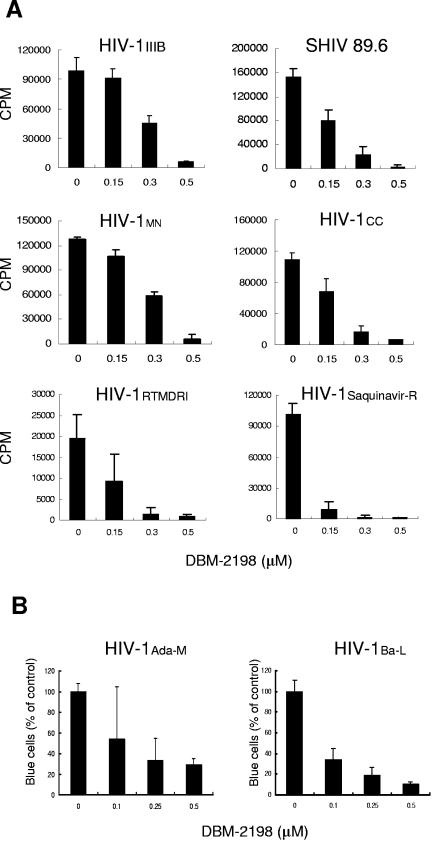
DBM 2198 was the most potent among the tested APZONs with regard to anti-HIV-1 activity against a laboratory strain (HIV-1IIIB), a recombinant virus (HXBc2/Δtat), and the chimeric recombinant SHIV89.6, as illustrated in Table 2. In the next phase of our study, we attempted to determine whether DBM 2198 exhibits similar anti-HIV-1 activity when encountering other HIV-1 variants. As shown in Fig. 4A, all six T-cell-tropic variants were inhibited by over 95% as the result of a single treatment of DBM 2198, at a final concentration of 0.5 μM. Interestingly, the drug-resistant HIV-1 variants (Saquinavir-resistant and zidovudine, dideoxyinosine, nevirapine-resistant) were found to be much more susceptible to DBM 2198-induced inhibition than were the primary isolates or recombinants (Fig. 4A). DBM 2198's potent anti-HIV effects were also observed in trials with monotropic virus-infected cells. As shown in Fig. 4B, the number of infected cells (in the visual infection assay) was markedly reduced, in a dose-dependent manner, when U373-CD4-CCR5-Magi cells were infected with 0.01 MOI of monotropic HIV-1 variants (Ada-M and Ba-L), in the presence of DBM 2198. These results clearly suggest that DBM 2198 works against a broad spectrum of HIV-1 variants, including drug-resistant HIV-1 strains.
FIG.4.
Antiviral activity of DBM 2198 against several HIV-1 variants. (A) C8166 cells were infected with 0.01 MOI of the HIV-1 strains shown in the legend for 1 h. Infected cells were washed twice with phosphate-buffered saline and then cultured for 3 days (for MN and CC strains) or 4 days (for other strains) in RPMI10 containing DBM 2198 at final concentrations of 0.15, 0.3, and 0.5 μM. Cell-free solutions (0.5 ml) were harvested from each culture and tested for their RT activity. HIV-1RTMDRI (zidovudine-, dideoxyinosine-, and nevirapine-resistant) and HIV-1Saquinavir-R (saquinavir-resistant) are drug-resistant mutant strains isolated from AIDS patients. (B) U373-CD4-CCR5-Magi cells were infected with 0.01 MOI of monotropic HIV-1 virus (Ada-M or Ba-L) in the presence of three different concentrations of DBM 2198. Two days after infection, cells were fixed and stained with X-Gal, as described in Materials and Methods. Cells stained blue were counted, and the inhibition capacity was expressed as a percentage of the untreated positive control.
DBM 2198 works against acute HIV-1 infection.
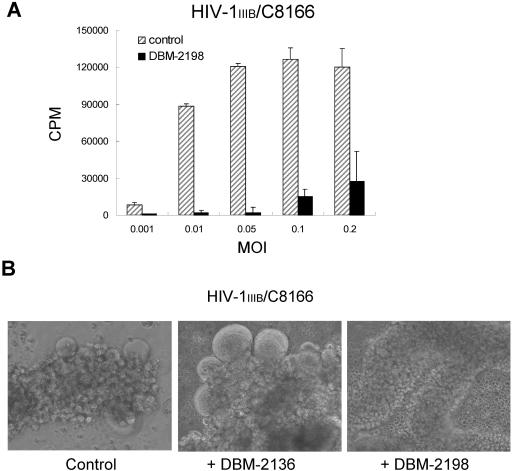
In order to determine whether DBM 2198 remains effective acute HIV-1 infection, we assessed the antiviral activity of DBM 2198 in cultures infected with a high MOI of HIV-1 IIIB, as described previously (42). HIV-1 replication was observed to be minimal or undetectable when the cells were infected with 0.05 MOI or lower in the presence of DBM 2198. However, the HIV-1 titers were scored at about 15% and 30% of that of the 2198-untreated controls when infected with 0.1 and 0.2 MOI, respectively, at a concentration of 0.5 μM DBM 2198 (Fig. 5A). This indicates that once the cells were infected with the virus, DBM 2198 could not impede the first round of HIV-1 replication, which was followed by the production of progeny viruses in proportion to the initial MOI. However, further propagation did appear to be inhibited by DBM 2198-mediated blocking of the second infection, resulting in a very much lower titer of virus in the culture, compared with that of the untreated controls.
FIG. 5.
Therapeutic effects of DBM 2198 on acute infection. (A) C8166 cells were infected with HIV-1IIIB at the indicated MOIs for 1 h. Infected cells were washed twice with phosphate-buffered saline, followed by culture for 4 days in RPMI10 containing 0.5 μM of DBM 2198. Cell-free virus solutions of 0.5 ml from each sample were tested for their RT activity. (B) C8166 cells were infected with 0.01 MOI of HIV-1IIIB and then cultured for 5 days in RPMI10. Cultures showing severe syncytium formation (Control) were treated with DBM 2136 and 2198 at a final concentration of 0.5 μM for 2 days. Cells were photographed at a magnification of ×100.
To see whether DBM 2198 was also effective for blocking HIV-1 spread in severely infected cultures, Jurkat-tat cells exhibiting strong syncytium formation were treated with DBM 2198 at a final concentration of 0.5 μM, and then monitored for 2 days. In repeated experiments, most of the syncytia in the DBM 2198-treated disappeared within 2 days (Fig. 5B). Possible mechanisms are suggested in the Discussion section. On the contrary, DBM 2136 (P=S ON) exacerbated, rather than inhibited, the rate and severity of syncytium formation (Fig. 5B), which is consistent with our results shown above (Table 1, Fig. 2 and Fig. 3).
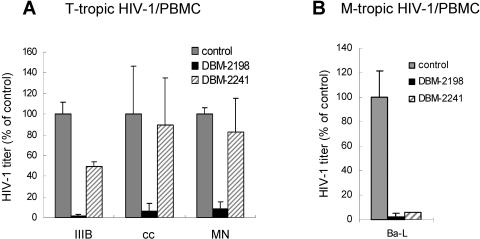
DBM 2198 maintains its potency in the inhibition of HIV-1 replication in PBMC.
DBM 2198 proved effective, blocking HIV-1 replication in PBMCs regardless of the host tropisms (Fig. 6). T-cell-tropic viruses in the infected PBMC cultures were dramatically inhibited by DBM 2198 treatment, while DBM 2241 (the same sequence as DBM 2198 but harboring no AZN) proved ineffective in the inhibition of these viruses (Fig. 6A). This indicates that the AZNs in DBM 2198 are essential for the inhibition of T-cell-tropic viruses. However, DBM 2241 exhibited remarkable, although incomplete, inhibitory effects on the monotropic Ba-L strain, as shown in the cultures treated with DBM 2198 (Fig. 6B).
FIG. 6.
Anti-HIV-1 activity of DBM 2198 in PBMCs. PBMCs (106 cells) pretreated with 0.25 μg/ml of phytohemagglutinin P (Sigma Chemical Inc.) for 3 days were infected with three different T-cell-tropic (A) and monotropic (B) HIV-1 strains at an MOI of 0.01 and then cultured for 4 days in the presence of DBM 2198 and 2241at a final concentration of 0.5 μM. The amounts of T-cell-tropic and monotropic viruses in the culture supernatants were measured by visual infection assays with U373-CD4-CXCR4-Magi and U373-CD4-CCR5-Magi cells, respectively.
DISCUSSION
In this study, we demonstrated that newly designed 6-membered Azasugar-containing P=S ONs (AZPSONs) exhibit potent antiviral activity against HIV-1 infection. These anti-HIV-1 properties of AZPSONs require only a simple administration into an infected culture without any extra transfection processes. The substitution of sugar moieties with six-membered azasugars (6-AZS) in P=S ONs appear to be an essential factor in the potent anti-HIV-1 properties of the AZPSONs, as little, if any antiviral activity was exhibited by P=S ONs such as DBM 2136 or DBM 2241 alone, although they carry nucleotide sequences identical to those of the AZPSONs DBM 2134 and DBM 2198, respectively (Table 1, Fig. 2). The P=S ONs themselves have been studied extensively, due to their remarkable anti-HIV-1 properties. In the present study, however, the P=S ON itself was not as effective as was previously reported when administered in cultures without transfection. Whereas AZPSONs containing specific substitutions of the sugar moiety of adenosine in their P=S ONs proved to exert much more potent anti-HIV-1 effects than did the P=S ONs alone (Table 1). Nevertheless, the P=O backbone only exhibited no intrinsic anti-HIV-1 activity, nor did the mixed backbone (P=O and P=S) oligonucleotides, even though those ONs contained 6-AZNs. These results indicate that the P=S backbone and the 6-AZS substitutions of sugar moiety are both essential to the anti-HIV-1 properties of the AZPSONs.
The anti-HIV-1 capacity of the AZPSONs seems to depend on the number and/or distribution patterns of the 6-AZN in the ONs. The DBM 2193 containing four AZNs was better than three AZN-containing DBM 2134 in its anti-HIV-1 activity, even though both of them have the same nucleotide sequence. DBM 2198, the most effective among the AZPSONs, has a random sequence and five 6-AZNs evenly distributed in 18 nucleotides. However, six (DBM 2194, anti-TAR) or seven (DBM 2199, random) AZN-containing ONs were not as effective as DBM 2193 (three AZNs, anti-TAR) or DBM 2198 (five AZNs, random), respectively, suggesting that the number of AZNs is not the sole factor for the anti-HIV-1 activity of AZPSONs. DBM 2197 and 2198, containing the same number of AZNs, but having four and three nucleotides between the AZNs, respectively, exhibited different antiviral activities. In addition, both DBM 2198 and 2240, which have the same number of AZNs (five AZNs) and spacer nucleotides but with different compositions of spacer nucleotides (CTC and CGC, respectively), showed different antiviral activities. DBM 2192 (9-mer) and 2181 (18-mer), having identical AZN composition, showed similar anti-HIV-1 activity, even though DBM 2198 has an extra nine P=S ON. These results suggest that the distribution patterns of the AZNs and the composition of the spacer nucleotides between the AZNs influence the antiviral activity of the AZPSONs. The length of the P=S backbone itself, however, may not play a crucial role in the antiviral activity of AZPSONs.
As shown in Fig. 2, AZPSONs inhibited the syncytium formation in a dose-dependent manner, but the dose-dependent responses of each AZPSON did not exhibit a clear linearity. We are not sure whether these dose-dependent response patterns are special characteristics of AZPSONs or not, but the results from our repeated experiments lead us to assume that each AZPSON has its own threshold concentration for the phenotypic inhibition of HIV-1 replication.
Among the AZPSONs, DBM 2198 was clearly the most effective with regard to its antiviral activity against, not only T-cell-tropic but also monotropic HIV-1 variants, while dextran sulfate was not effective at all against monotropic HIV-1 (Table 2). HIV-1 RT and integrase activities were not inhibited by the presence of DBM 2198 in an in vitro enzymatic assay (data not shown), suggesting that the anti-HIV-1 activity of DBM 2198 in cultures was not associated with the inhibition of intracellular viral specific enzymes in the infected cells. DBM 2198 exhibited little or no cytotoxicity in most cell types examined (Table 3), minor attenuation of its anti-HIV-1 activity in the presence of serum, and was as stable as were the P=S ONs under several nuclease-containing conditions (data not shown). These results strongly suggest that DBM 2198 can be developed into a safe and effective AIDS therapeutic drug.
dG3T4G3-s, G-quartet structure P=S ON was previously reported to be effective only on T-cell-tropic HIV-1 (11). DBM 2198, however, exhibited potent antiviral activity against a broad spectrum of HIV-1 variants, including T cell tropic (Fig. 4A) and monotropic viruses (Fig. 4B), as well as drug-resistant viruses (Fig. 4A, bottom 2 panels). Interestingly, the drug-resistant viruses were found to be more sensitive to DBM 2198 than were the laboratory-adapted variants (Fig. 4A).
According to our results, SHIV89.6 (a chimeric virus consisting of the SIV-originated gag-pol gene and the HIV-1-derived env gene) (28, 43, 44) was quite susceptible to the inhibitory effects of DBM 2198 (Fig. 4A and Table 2) while SIV was resistant to DBM 2198 (data not shown). These results imply that the anti-HIV-1 activity of DBM 2198 is reliant on the HIV-1 envelope protein, rather than on the host cell receptor. In the BLAST search for the complementary sequence of DBM 2198, no significant sequence similarity was found in the GenBank database of Eucaryota and viruses, suggesting that the sequence-specific intracellular antisense activity was not involved in the DBM 2198-mediated HIV-1 inhibition.
We found that acute HIV-1 infections with high MOIs (MOI > 0.1) were not completely blocked by treatment with DBM 2198, even at a final concentration of 0.5 μM (Fig. 5A). However, we were unable to detect any syncytium formation in the DBM 2198-treated cultures, even though small amounts of HIV-1 were still detected in the supernatant by RT assay. Interestingly, the RT activity in the culture supernatant decreased with time, finally diminishing to undetectable levels (data not shown). This suggests that DBM 2198 inhibits further spread of progeny viruses by blocking the secondary infections.
In our repeated experiments, numerous syncytia in severely HIV-1-infected cultures disappeared within 2 days after treatment with DBM 2198 (Fig. 5B). In a series of separate experiments, we found that DBM 2198 specifically blocks the HIV-1 Env protein without any interaction with the host cell membrane (data not shown). This means that the DBM 2198 blocks the Env protein on the plasma membrane of the preformed syncytia, resulting in inhibition of syncytium progression. Preformed syncytia rapidly collapsed within 2 days, probably due to intracellular HIV-1 replication, whereas preformed syncytia produced large amounts of progeny viruses, as mentioned above; however, the secondary infections of these free virus particles released from the primary infection were blocked by the presence of DBM 2198. We could not detect any recurrence of syncytia in further cultures, even though free virus particles were detected in the culture supernatant for a while in the presence of DBM 2198. The antiviral capacity of DBM 2198, as shown in the cell lines, was similarly repeated in experiments with PBMC against T-cell-tropic and monotropic HIV-1 (Fig. 6), suggesting that DBM 2198 can be developed as a clinically applicable AIDS therapeutic agent.
In conclusion, AZN-containing P=S ONs were much more effective than pure P=S ONs for the inhibition of HIV-1 replication, without causing any cytotoxicity at concentrations of up to 100 μM. DBM 2198, the most prominent for its anti-HIV-1 capacity among the AZPSONs, inhibits infection and/or replication of a broad spectrum of HIV-1 variants, including drug-resistant viruses, not only in the established cell lines but also in PBMC. Our results strongly suggest that DBM 2198 and some other effective AZPSONs can be developed into safe and effective AIDS-therapeutic drugs. The mechanisms underlying the anti-HIV-1 effects of DBM 2198 have been explored (manuscript submitted).
Acknowledgments
We thank J. Sodroski for the HXBc2, HXBc2/Δtat, and pSV2-tat plasmids and the Jurkat-tat strain. We are grateful to the NIH AIDS Research and Reference Reagent Program for supplying several HIV-1 strains, cell lines, and recombinant plasmids. We are also grateful to J. K. Lee for her commitment to the experiments on the cytotoxicity and efficacy of AZPSONs, C. G. Shin for his assistance with the integrase assay, and K. N. Kim for his enthusiastic support of this work.
This work was supported by Dongbu Hannong Chemical Co. and, in part, by the Korea Ministry of Science and Technology, grant M1-0016-00-0020.
REFERENCES
- 1.Agatsuma, T., K. Abe, H. Furukawa, R. Koga, M. Koizumi, H. Hotoda, and M. Kaneko. 1997. Protection of hu-PBL-SCID/beige mice from HIV-1 infection by a 6-mer modified oligonucleotide, R-95288. Antiviral Res. 34:121-130. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, L., H. H. Lu, and E. Wimmer. 1994. Polioviruses containing picornavirus type 1 and/or type 2 internal ribosomal entry site elements: genetic hybrids and the expression of a foreign gene. Proc. Natl. Acad. Sci. USA 91:1406-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arteaga, H. J., J. Hinkula, I. van Dijk-Hard, M. S. Dilber, B. Wahren, B. Christensson, A. J. Mohamed, and C. I. Smith. 2003. Choosing CCR5 or Rev. siRNA in HIV-1. Nat Biotechnol. 21:230-231. [DOI] [PubMed] [Google Scholar]
- 4.Barnor, J. S., N. Miyano-Kurosaki, K. Yamaguchi, A. Sakamoto, K. Ishikawa, Y. Inagaki, N. Yamamoto, M. Osei-Kwasi, D. Ofori-Adjei, and H. Takaku. 2004. Intracellular expression of antisense RNA transcripts complementary to the human immunodeficiency virus type-1 vif gene inhibits viral replication in infected T-lymphoblastoid cells. Biochem. Biophys. Res. Commun. 320:544-550. [DOI] [PubMed] [Google Scholar]
- 5.Berkhout, B. 2004. RNA interference as an antiviral approach: targeting HIV-1. Curr. Opin. Mol. Ther. 6:141-145. [PubMed] [Google Scholar]
- 6.Boulme, F., F. Freund, S. Moreau, P. E. Nielsen, S. Gryaznov, J. J. Toulme, and S. Litvak. 1998. Modified (PNA, 2′-O-methyl and phosphoramidate) anti-TAR antisense oligonucleotides as strong and specific inhibitors of in vitro HIV-1 reverse transcription. Nucleic Acids Res. 26:5492-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bres, V., R. E. Kiernan, L. K. Linares, C. Chable-Bessia, O. Plechakova, C. Treand, S. Emiliani, J. M. Peloponese, K. T. Jeang, O. Coux, M. Scheffner, and M. Benkirane. 2003. A non-proteolytic role for ubiquitin in Tat-mediated transactivation of the HIV-1 promoter. Nat. Cell Biol. 5:754-761. [DOI] [PubMed] [Google Scholar]
- 8.Brown, D. A., S. H. Kang, S. M. Gryaznov, L. DeDionisio, O. Heidenreich, S. Sullivan, X. Xu, and M. I. Nerenberg. 1994. Effect of phosphorothioate modification of oligodeoxynucleotides on specific protein binding. J. Biol. Chem. 269:26801-26805. [PubMed] [Google Scholar]
- 9.Buckheit, R. W., Jr., J. L. Roberson, C. Lackman-Smith, J. R. Wyatt, T. A. Vickers, and D. J. Ecker. 1994. Potent and specific inhibition of HIV envelope-mediated cell fusion and virus binding by G quartet-forming oligonucleotide (ISIS 5320). AIDS Res. Hum. Retroviruses 10:1497-1506. [DOI] [PubMed] [Google Scholar]
- 10.Campbell, J. M., T. A. Bacon, and E. Wickstrom. 1990. Oligodeoxynucleoside phosphorothioate stability in subcellular extracts, culture media, sera and cerebrospinal fluid. J. Biochem. Biophys. Methods 20:259-267. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein, F. 1986. Action of restriction endonucleases on phosphorothioate DNA. Biochem. Soc Trans. 14:204-205. [DOI] [PubMed] [Google Scholar]
- 12.Faria, M., D. G. Spiller, C. Dubertret, J. S. Nelson, M. R. White, D. Scherman, C. Helene, and C. Giovannangeli. 2001. Phosphoramidate oligonucleotides as potent antisense molecules in cells and in vivo. Nat. Biotechnol. 19:40-44. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa, H., K. Momota, T. Agatsuma, I. Yamamoto, S. Kimura, and K. Shimada. 1994. Mechanism of inhibition of HIV-1 infection in vitro by guanine-rich oligonucleotides modified at the 5′ terminal by dimethoxytrityl residue. Nucleic Acids Res. 22:5621-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gryaznov, S. M. 1999. Oligonucleotide N3′->P5′ phosphoramidates as potential therapeutic agents. Biochim. Biophys. Acta 1489:131-140. [DOI] [PubMed] [Google Scholar]
- 15.Hamma, T., and P. S. Miller. 1999. Syntheses of alternating oligo-2′-O-methylribonucleoside methylphosphonates and their interactions with HIV TAR RNA. Biochemistry 38:15333-15342. [DOI] [PubMed] [Google Scholar]
- 16.Hotoda, H., M. Koizumi, R. Koga, M. Kaneko, K. Momota, T. Ohmine, H. Furukawa, T. Agatsuma, T. Nishigaki, J. Sone, S. Tsutsumi, T. Kosaka, K. Abe, S. Kimura, and K. Shimada. 1998. Biologically active oligodeoxyribonucleotides. 5. 5′-End-substituted d(TGGGAG) possesses anti-human immunodeficiency virus type 1 activity by forming a G-quadruplex structure. J. Med. Chem. 41:3655-3663. [DOI] [PubMed] [Google Scholar]
- 17.Humeau, L. M., G. K. Binder, X. Lu, V. Slepushkin, R. Merling, P. Echeagaray, M. Pereira, T. Slepushkina, S. Barnett, L. K. Dropulic, R. Carroll, B. L. Levine, C. H. June, and B. Dropulic. 2004. Efficient lentiviral vector-mediated control of HIV-1 replication in CD4 lymphocytes from diverse HIV+ infected patients grouped according to CD4 count and viral load. Mol. Ther. 9:902-913. [DOI] [PubMed] [Google Scholar]
- 18.Jagodzinski, P. P., and W. H. Trzeciak. 1999. High molecular weight dextran sulfate increases the activity of NF-kappaB-regulated promoter in monocyte-derived macrophages. Microbiol. Immunol. 43:187-189. [DOI] [PubMed] [Google Scholar]
- 19.Jagodzinski, P. P., A. Wierzbicki, J. Wustner, Y. Kaneko, and D. Kozbor. 1999. Enhanced human immunodeficiency virus infection in macrophages by high-molecular-weight dextran sulfate is associated with conformational changes of gp120 and expression of the CCR5 receptor. Viral Immunol. 12:23-33. [DOI] [PubMed] [Google Scholar]
- 20.Jeong, L. S., J. H. Lee, K. E. Jung, H. R. Moon, K. Kim, and H. Lim. 1999. Synthesis and hybridization property of sugar and phosphate linkage modified oligonucleotides. Bioorg. Med. Chem. 7:1467-1473. [DOI] [PubMed] [Google Scholar]
- 21.Jing, N., E. De Clercq, R. F. Rando, L. Pallansch, C. Lackman-Smith, S. Lee, and M. E. Hogan. 2000. Stability-activity relationships of a family of G-tetrad forming oligonucleotides as potent HIV inhibitors. A basis for anti-HIV drug design. J. Biol. Chem. 275:3421-3430. [DOI] [PubMed] [Google Scholar]
- 22.Jing, N., and M. E. Hogan. 1998. Structure-activity of tetrad-forming oligonucleotides as a potent anti-HIV therapeutic drug. J. Biol. Chem. 273:34992-34999. [DOI] [PubMed] [Google Scholar]
- 23.Jing, N., C. Marchand, J. Liu, R. Mitra, M. E. Hogan, and Y. Pommier. 2000. Mechanism of inhibition of HIV-1 integrase by G-tetrad-forming oligonucleotides in vitro. J. Biol. Chem. 275:21460-21467. [DOI] [PubMed] [Google Scholar]
- 24.Jung, K. E., K. Kim, M. Yang, K. Lee, and H. Lim. 1999. Synthesis and hybridization properties of oligonucleotides containing 6-membered azasugar nucleotides. Bioorg. Med. Chem. Lett. 9:3407-3410. [DOI] [PubMed] [Google Scholar]
- 25.Liang, H., C. F. Reich, D. S. Pisetsky, and P. E. Lipsky. 2000. The role of cell surface receptors in the activation of human B cells by phosphorothioate oligonucleotides. J. Immunol. 165:1438-1445. [DOI] [PubMed] [Google Scholar]
- 26.Loke, S. L., C. A. Stein, X. H. Zhang, K. Mori, M. Nakanishi, C. Subasinghe, J. S. Cohen, and L. M. Neckers. 1989. Characterization of oligonucleotide transport into living cells. Proc. Natl. Acad. Sci. USA 86:3474-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, X., Q. Yu, G. K. Binder, Z. Chen, T. Slepushkina, J. Rossi, and B. Dropulic. 2004. Antisense-mediated inhibition of human immunodeficiency virus (HIV) replication by use of an HIV type 1-based vector results in severely attenuated mutants incapable of developing resistance. J. Virol. 78:7079-7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu, Y., P. Brosio, M. Lafaile, J. Li, R. G. Collman, J. Sodroski, and C. J. Miller. 1996. Vaginal transmission of chimeric simian/human immunodeficiency viruses in rhesus macaques. J. Virol. 70:3045-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majumdar, C., C. A. Stein, J. S. Cohen, S. Broder, and S. H. Wilson. 1989. Stepwise mechanism of HIV reverse transcriptase: primer function of phosphorothioate oligodeoxynucleotide. Biochemistry 28:1340-1346. [DOI] [PubMed] [Google Scholar]
- 30.Marshall, W. S., G. Beaton, C. A. Stein, M. Matsukura, and M. H. Caruthers. 1992. Inhibition of human immunodeficiency virus activity by phosphorodithioate oligodeoxycytidine. Proc. Natl. Acad. Sci. USA 89:6265-6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall, W. S., and M. H. Caruthers. 1993. Phosphorodithioate DNA as a potential therapeutic drug. Science 259:1564-1570. [DOI] [PubMed] [Google Scholar]
- 32.Miller, P. S., R. A. Cassidy, T. Hamma, and N. S. Kondo. 2000. Studies on anti-human immunodeficiency virus oligonucleotides that have alternating methylphosphonate/phosphodiester linkages. Pharmacol. Ther. 85:159-163. [DOI] [PubMed] [Google Scholar]
- 33.Miyano-Kurosaki, N., J. S. Barnor, H. Takeuchi, T. Owada, H. Nakashima, N. Yamamoto, T. Matsuzaki, F. Shimada, and H. Takaku. 2004. In vitro and in vivo transport and delivery of phosphorothioate oligonucleotides with cationic liposomes. Antivir. Chem. Chemother. 15:93-100. [DOI] [PubMed] [Google Scholar]
- 34.Morris, K. V., and J. J. Rossi. 2004. Anti-HIV-1 gene expressing lentiviral vectors as an adjunctive therapy for HIV-1 infection. Curr. HIV Res. 2:185-191. [DOI] [PubMed] [Google Scholar]
- 35.Novina, C. D., M. F. Murray, D. M. Dykxhoorn, P. J. Beresford, J. Riess, S. K. Lee, R. G. Collman, J. Lieberman, P. Shankar, and P. A. Sharp. 2002. siRNA-directed inhibition of HIV-1 infection. Nat. Med. 8:681-686. [DOI] [PubMed] [Google Scholar]
- 36.Ojwang, J. O., R. W. Buckheit, Y. Pommier, A. Mazumder, K. De Vreese, J. A. Este, D. Reymen, L. A. Pallansch, C. Lackman-Smith, T. L. Wallace, and et al. 1995. T30177, an oligonucleotide stabilized by an intramolecular guanosine octet, is a potent inhibitor of laboratory strains and clinical isolates of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 39:2426-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 38.Pusch, O., D. Boden, R. Silbermann, F. Lee, L. Tucker, and B. Ramratnam. 2003. Nucleotide sequence homology requirements of HIV-1-specific short hairpin RNA. Nucleic Acids Res. 31:6444-6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin, X. F., D. S. An, I. S. Chen, and D. Baltimore. 2003. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. USA 100:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabin, L., M. Hincenbergs, M. B. Moreno, S. Warren, V. Linquist, R. Datema, B. Charpiot, J. Seifert, H. Kaneshima, and J. M. McCune. 1996. Use of standardized SCID-hu Thy/Liv mouse model for preclinical efficacy testing of anti-human immunodeficiency virus type 1 compounds. Antimicrob. Agents Chemother. 40:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rando, R. F. 1998. Oligonucleotides as inhibitors of the human immunodeficiency virus type 1. Curr. Res. Mol. Ther. 1:67-75. [Google Scholar]
- 42.Rando, R. F., J. Ojwang, A. Elbaggari, G. R. Reyes, R. Tinder, M. S. McGrath, and M. E. Hogan. 1995. Suppression of human immunodeficiency virus type 1 activity in vitro by oligonucleotides which form intramolecular tetrads. J. Biol. Chem. 270:1754-1760. [DOI] [PubMed] [Google Scholar]
- 43.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reimann, K. A., J. T. Li, G. Voss, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Parritz, Y. Lu, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 70:3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rockwell, P., W. J. O'Connor, K. King, N. I. Goldstein, L. M. Zhang, and C. A. Stein. 1997. Cell-surface perturbations of the epidermal growth factor and vascular endothelial growth factor receptors by phosphorothioate oligodeoxynucleotides. Proc. Natl. Acad. Sci. USA 94:6523-6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarin, P. S., S. Agrawal, M. P. Civeira, J. Goodchild, T. Ikeuchi, and P. C. Zamecnik. 1988. Inhibition of acquired immunodeficiency syndrome virus by oligodeoxynucleoside methylphosphonates. Proc. Natl. Acad. Sci. USA 85:7448-7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein, C. A., A. M. Cleary, L. Yakubov, and S. Lederman. 1993. Phosphorothioate oligodeoxynucleotides bind to the third variable loop domain (v3) of human immunodeficiency virus type 1 gp120. Antisense Res. Dev. 3:19-31. [DOI] [PubMed] [Google Scholar]
- 48.Stein, C. A., C. Subasinghe, K. Shinozuka, and J. S. Cohen. 1988. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 16:3209-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevenson, M. 2003. Dissecting HIV-1 through RNA interference. Nat. Rev. Immunol. 3:851-858. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki, J., N. Miyano-Kurosaki, T. Kuwasaki, H. Takeuchi, G. Kawai, and H. Takaku. 2002. Inhibition of human immunodeficiency virus type 1 activity in vitro by a new self-stabilized oligonucleotide with guanosine-thymidine quadruplex motifs. J. Virol. 76:3015-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki, J., N. Miyano-Kurosaki, H. Takeuchi, Y. Tamura, G. Kawai, K. Takai, Y. Tanaka, R. Tanaka, N. Yamamoto, and H. Takaku. 1999. Phosphorothioate G3T4G3 motifs inhibits the early stage of HIV-1 infection. Nucleic Acids Symp. Ser. 42:227-228. [DOI] [PubMed] [Google Scholar]
- 52.Vodicka, M. A., W. C. Goh, L. I. Wu, M. E. Rogel, S. R. Bartz, V. L. Schweickart, C. J. Raport, and M. Emerman. 1997. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology 233:193-198. [DOI] [PubMed] [Google Scholar]
- 53.Wyatt, J. R., T. A. Vickers, J. L. Roberson, R. W. Buckheit, Jr., T. Klimkait, E. DeBaets, P. W. Davis, B. Rayner, J. L. Imbach, and D. J. Ecker. 1994. Combinatorially selected guanosine-quartet structure is a potent inhibitor of human immunodeficiency virus envelope-mediated cell fusion. Proc. Natl. Acad. Sci. USA 91:1356-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]