Abstract
The in vitro activities of DA-7867, a novel oxazolidinone, and garenoxacin (BMS-284756) were compared to those of linezolid in 67 susceptible and drug-resistant clinical isolates of Mycobacterium tuberculosis. DA-7867 was the most active drug with an MIC90 of 0.125 μg/ml, compared to the MIC90s of 4 μg/ml of garenoxacin and 2 μg/ml of linezolid.
Tuberculosis is still a very common infectious disease worldwide, particularly in developing countries, with about 8 million new cases and 3 million deaths every year (7). The infection can be treated properly if an early diagnosis is made and the appropriate therapy is applied. However, in places where drugs are not easily available or delivered, multidrug-resistant strains of Mycobacterium tuberculosis arise and, in some countries, are the predominant strains, with a frequency as high as 14% (5). To date, the therapeutic choices for this disease are scarce, and new drugs are urgently needed. In the present study, we report the susceptibility of M. tuberculosis clinical isolates to garenoxacin (BMS-284756), a newly developed quinolone, and DA-7867, a novel oxazolidinone.
Garenoxacin was kindly donated by Bristol-Myers Squibb; DA-7867 was obtained from Dong-A Pharmaceutical Company, Ltd., Yongin, Korea. Stock solutions of garenoxacin and DA-7867 were prepared in 100% dimethyl sulfoxide and 7H9GC (4.7 g of Middlebrook 7H9 broth base [Difco, Detroit, Mich.], 20 ml of 10% [vol/vol] glycerol, 1 g of Bacto Casitone [Difco], 880 ml of distilled water, 100 ml of oleic acid-albumin-dextrose-catalase [Becton Dickinson, Maryland]) (6) broth, respectively. In order to compare our results, we also ran susceptibility assays for linezolid, a drug previously tested in vitro against M. tuberculosis with good results. The latter was obtained from Pharmacia and Upjohn (Kalamazoo, Mich.), and the stock was prepared in 7H9GC broth. All working solutions were diluted in 7H9GC broth to a 2× concentration prior to their addition to the microplates.
Sixty-seven clinical isolates of M. tuberculosis obtained from the Laboratorio Estatal de los Servicios de Salud de Nuevo León were subcultured on Lowenstein-Jensen medium. Twenty-one of the isolates were determined to be resistant to isoniazid and rifampin by the proportion method. In order to determine the susceptibility to garenoxacin, DA-7867, and linezolid, the broth microdilution method with Alamar Blue was utilized (6). In brief, mycobacterial suspensions were prepared in 0.04% (vol/vol) Tween 80-0.2% bovine serum albumin so that their turbidities matched that of the McFarland no. 1 turbidity standard. Suspensions were further diluted 1:25 in 7H9GC broth.
Two hundred microliters of sterile deionized water was added to all outer-perimeter wells of sterile 96-well plates (Falcon 3072; Becton Dickinson, Lincoln Park, N.J.) to minimize evaporation of the medium in the test wells during incubation. One hundred microliters of 2× drug solutions were added to the wells in rows B to G. Final drug concentration ranges were as follows: for linezolid and garenoxacin, we utilized 0.0625 to 16 μg/ml, and for DA-7867, it was necessary to decrease the lower drug concentration to 0.0078 μg/ml.
One hundred microliters of M. tuberculosis inoculum was added to the wells in rows B to G in columns 2 to 11 by using an Eppendorf repeating pipette (yielding a final volume of 200 μl per well). The plates were sealed with Parafilm and incubated at 37°C for 5 days. Fifty microliters of a freshly prepared 1:1 mixture of 10× Alamar Blue (Accumed International, Westlake, Ohio) reagent and 10% Tween 80 was added to the wells containing only 7H9GC broth plus inoculum. The plates were reincubated at 37°C for 24 h. If the positive controls turned pink, the reagent mixture was added to all other wells in the microplate. The microplates were resealed with Parafilm and incubated for an additional 24 h at 37°C, and the colors of all wells were recorded. A blue color was interpreted as no growth, and a pink color was scored as positive growth. The MIC was defined as the lowest drug concentration which prevented a color change from blue to pink. M. tuberculosis H37Rv was run as a control of a susceptible strain.
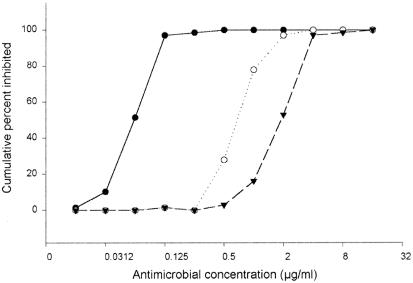
The range of MICs and the MIC50s and MIC90s are shown in Table 1. The MIC90 of linezolid was 2 μg/ml, similar to the value reported previously that was obtained by using the proportion method (MIC90 of 0.5 μg/ml) (1). The M. tuberculosis isolates were more susceptible to another oxazolidinone utilized, DA-7867, with an MIC90 of 0.125 μg/ml. Most of the isolates were susceptible to 4 μg/ml of garenoxacin, although two of the isolates, one susceptible to isoniazid and rifampin and one resistant to both, presented MICs of 8 and 16 μg/ml, respectively (data not shown). The DA-7867 MIC for these isolates was 0.0625. When the susceptibilities of the 21 M. tuberculosis isolates resistant to both isoniazid and rifampin were analyzed, the MICs of DA-7867 and garenoxacin were exactly the same; in the case of linezolid, a lower value was observed (an MIC90 of 1 μg/ml, compared to 2 μg/ml for the susceptible group). When the cumulative inhibition percentages of the three drugs were compared (Fig. 1), a remarkable difference in susceptibility was observed with DA-7867, which inhibited the mycobacterial growth at much lower concentrations than linezolid. This effect has also been observed with Nocardia spp. and other bacterial species (9, 10)
TABLE 1.
MICs of garenoxacin, linezolid, and DA-7867 for 67 clinical isolates of M. tuberculosis and reference strain H37Rv
| Drug | MICa(μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Garenoxacin | 0.125-16 | 2 | 4 |
| Linezolid | 0.25-4 | 1 | 2 |
| DA-7867 | 0.0312-0.5 | 0.0625 | 0.125 |
50% and 90%, the antimicrobial concentrations at which 50% and 90% of the M. tuberculosis isolates are inhibited, respectively
FIG. 1.
Susceptibility of the M. tuberculosis isolates to the antimicrobials tested. The graph shows the cumulative percentages of M. tuberculosis isolates inhibited by DA-7867 (•), linezolid (○), and garenoxacin (▾).
Although effective if applied properly, antituberculous therapy with first-line drugs has several disadvantages, including potential hepatotoxicity, long-term duration and the development of resistance. Desfluoroquinolones, including levofloxacin, sparfloxacin, and moxifloxacin, either alone or in combination with other antimicrobials have also shown promising results, both in vitro and in vivo (8). However, their effect on human cases of tuberculosis is not known yet. Garenoxacin is a new class of quinolone for which activity on M. tuberculosis isolates has not been assayed, with the exception of a report using only M. tuberculosis H37Rv by the proportion method (3). Although the latter technique and the broth microdilution method are not 100% agreeable, the MIC90 found by us in the 67 M. tuberculosis isolates is quite similar (4 μg/ml) to that reported by these authors (2 μg/ml). Although there are no breakpoint concentrations for garenoxacin, we found a couple of strains with high MICs (8 and 16 μg/ml), which may represent a population of isolates resistant to this drug, produced by cross-resistance with other quinolones.
DA-7867 (Dong-A Pharmaceutical Co., Yongin, Korea) is a new hetero-ring-substituted pyridine-containing oxazolidinone, (S)-[N-3-(4-(2-(1-methyl-5-tetrazolyl)-pyridin-5-yl)-3-fluorophenyl)- 2-oxo-5-oxazolidinyl]methyl acetamide, which has been proven to be more active than linezolid against several gram-positive and gram-negative microorganisms, including Nocardia brasiliensis, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, penicillin-resistant Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis (9, 10). In our work, we also observed that DA-7867 is more active in vitro against M. tuberculosis isolates than linezolid, with an MIC range of 0.03 to 0.5 μg/ml. Similar ranges, although with only six susceptible M. tuberculosis isolates, have been observed with a newly developed drug, an ATP synthase inhibitor, R207910 (2).
With the exception of DA-7867, linezolid and other oxazolidinones, such as eperozolid, have been proven to be active in vitro and in vivo (4). The animal assays with linezolid have been done by utilizing CD-1 mice infected with the M. tuberculosis Erdman strain with an MIC of 0.5 μg/ml. In those assays, linezolid was less active to stop the growth in mouse tissues than isoniazid (4). Even if linezolid and DA-7867 are structurally quite similar, they have different pharmacokinetics in mice (data not shown). After 4 h, the levels of the first are very low, and that can explain the low level of activity in the mouse model. On the other hand, mouse plasma levels of DA-7867 remain higher than those of linezolid and R207910, which makes this drug a good candidate to be tested in animal models.
REFERENCES
- 1.Alcala, L., M. J. Ruiz-Serrano, C. Perez-Fernandez Turegano, D. Garcia De Viedma, M. Diaz-Infantes, M. Marin-Arriaza, and E. Bouza. 2003. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob. Agents Chemother. 47:416-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andries, K., P. Verhasselt, J. Guillemont, H. W. Gohlmann, J. M. Neefs, H. Winkler, J. Van Gestel, P. Timmerman, M. Zhu, E. Lee, P. Williams, D. de Chaffoy, E. Huitric, S. Hoffner, E. Cambau, C. Truffot-Pernot, N. Lounis, and V. Jarlier. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223-227. [DOI] [PubMed] [Google Scholar]
- 3.Aubry, A., X. S. Pan, L. M. Fisher, V. Jarlier, and E. Cambau. 2004. Mycobacterium tuberculosis DNA gyrase: interaction with quinolones and correlation with antimycobacterial drug activity. Antimicrob. Agents Chemother. 48:1281-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cynamon, M. H., S. P. Klemens, C. A. Sharpe, and S. Chase. 1999. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 43:1189-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinal, M. A. 2003. The global situation of MDR-TB. Tuberculosis (Edinburgh) 83:44-51. [DOI] [PubMed] [Google Scholar]
- 6.Franzblau, S. G., R. S. Witzig, J. C. McLaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B. Cook, V. K. Quenzer, R. M. Ferguson, and R. H. Gilman. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 36:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raviglione, M. C. 2003. The TB epidemic from 1992 to 2002. Tuberculosis 83:4-14. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez, J. C., M. Ruiz, M. Lopez, and G. Royo. 2002. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 20:464-467. [DOI] [PubMed] [Google Scholar]
- 9.Vera-Cabrera, L., E. Gonzalez, S. H. Choi, and O. Welsh. 2004. In vitro activities of new antimicrobials against Nocardia brasiliensis. Antimicrob. Agents Chemother. 48:602-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yong, D., J. H. Yum, K. Lee, Y. Chong, S. H. Choi, and J. K. Rhee. 2004. In vitro activities of DA-7867, a novel oxazolidinone, against recent clinical isolates of aerobic and anaerobic bacteria. Antimicrob. Agents Chemother. 48:352-357. [DOI] [PMC free article] [PubMed] [Google Scholar]