Abstract
The present study addressed the effect of microcirculatory blood flow on the ability of ciprofloxacin to penetrate soft tissues. Twelve healthy male volunteers were enrolled in an analyst-blinded, clinical pharmacokinetic study. A single intravenous dose of 200 mg of ciprofloxacin was administered over a period of approximately 20 min. The concentrations of ciprofloxacin were measured in plasma and in the warmed and contralateral nonwarmed lower extremities. The microdialysis technique was used for the assessment of unbound ciprofloxacin concentrations in subcutaneous adipose tissue. Microcirculatory blood flow was measured by use of laser Doppler flowmetry. Warming of the extremity resulted in an increase of microcirculatory blood flow by approximately three- to fourfold compared to that at the baseline (P < 0.05) in subcutaneous adipose tissue. The ratio of the maximum concentration (Cmax) of ciprofloxacin for the warmed thigh to the Cmax for the nonwarmed thigh was 2.10 ± 0.90 (mean ± standard deviation; P < 0.05). A combined in vivo pharmacokinetic (PK)-in vitro pharmacodynamic (PD) simulation based on tissue concentration data indicated that killing of Pseudomonas aeruginosa (ATCC 27853 and two clinical isolates) was more effective by about 2 log10 CFU/ml under the warmed conditions than under the nonwarmed conditions (P < 0.05). The improvement of microcirculatory blood flow due to the warming of the extremity was paralleled by an increased ability of ciprofloxacin to penetrate soft tissue. Subsequent PK-PD simulations based on tissue PK data indicated that this increase in tissue penetration was linked to an improved antimicrobial effect at the target site.
There is growing evidence that the reduction of blood flow in soft tissues is closely associated with a significant impairment of the rate of tissue penetration of antimicrobial agents. For example, in patients with peripheral arterial occlusive disease, it was demonstrated that the peak concentrations of ciprofloxacin are significantly lower in the interstitium of ischemic soft tissue in comparison with that in healthy and well-perfused tissue (11). This confirms the theory that vasopressors have a marked effect on the tissue penetration of antimicrobial agents in critically ill patients (10, 24). Based on the concept that the number of capillaries available for drug exchange and the ratio of the capillary surface area to volume are important for the plasma-to-tissue drug exchange, we carried out the present study and tested the hypothesis that an increase in local blood flow enhances the rate of transcapillary transport of solutes.
For this purpose we measured microcirculatory blood flow in subcutaneous adipose tissue under warmed and nonwarmed conditions by use of laser Doppler flowmetry (LDF) (16). Ciprofloxacin, a fluoroquinolone antibiotic, which is frequently favored for the treatment of soft tissue infections, was selected as a model compound. Ciprofloxacin exhibits a broad antimicrobial coverage, has a low level of plasma protein binding of ∼30%, and an apparent volume of distribution of ∼250 liters (17). From these characteristics of the drug, one may expect that ciprofloxacin penetrates soft tissues excellently. The interstitial fluid concentrations of ciprofloxacin in subcutaneous adipose tissue were assessed by use of microdialysis (2).
A combined in vivo pharmacokinetic (PK)-in vitro pharmacodynamic (PD) simulation of bacterial growth was subsequently employed to clarify whether changes in tissue PKs correlate with changes in the antimicrobial effect at the target site.
MATERIALS AND METHODS
The study took place at the Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Austria. The study protocol was approved by the local ethics committee and was performed in accordance with the Declaration of Helsinki (1964) in the revised version of 2002 (26), the guidelines for good clinical practice of the European Union (8), and Austrian drug law. Healthy volunteers were informed in detail about the purpose, procedures, and risks of the study; and their written consent was obtained prior to enrollment in the study.
Subjects.
Twelve healthy, male, drug-free volunteers with a mean age of 29.5 ± 2.7 years, a mean weight of 75.6 ± 7.5 kg, and a mean height of 1.82 ± 0.08 m were included in this study. Prior to inclusion in the study, the volunteers were subjected to a screening examination comprising a medical history and a physical examination. Health was defined at the discretion of the investigator and the absence of clinically relevant abnormalities in laboratory and physical tests.
Microdialysis.
The principles of microdialysis have been described in detail previously (9, 19).
In brief, microdialysis is based on sampling of analytes from the extracellular space of tissues by means of a semipermeable membrane at the tip of a microdialysis probe. The probe is constantly perfused with a physiological solution. Once the probe is implanted into the tissue, substances present in the extracellular fluid at a certain concentration (Ctissue) diffuse through the membrane into the perfusate, resulting in a concentration (Cdialysate) in the perfusion medium. For most analytes, the equilibrium between the concentration in the extracellular fluid and that in the perfusion medium is incomplete, and therefore, Ctissue is greater than Cdialysate. The calibration factor by which the concentrations are interrelated is termed “recovery.” For calibration of the microdialysis probes, in vivo recovery was assessed in each experiment by the retrodialysis method (1). The principle of this method relies on the assumption that the diffusion process is quantitatively equal in both directions along the semipermeable membrane. For probe calibration, the agent being investigated is added to the perfusate at a defined concentration. The disappearance rate (delivery) through the membrane is taken as the in vivo recovery. Retrodialysis is performed for a total period of 30 min at the beginning of each experiment. Two fractions of the dialysate are drawn at 15-min intervals, and the average concentration from these fractions is taken for the calculation of the individual recovery value. All probes are manually perfused by gently pushing the stamps of the syringes filled with the perfusate to ensure that the whole microdialysis set is free of ciprofloxacin. An extra dialysate sample is collected several minutes before intravenous administration of the agent being investigated in order to document that the microdialysis set is completely cleared from this agent. The microdialysis set is allowed to recover from this procedure at a constant flow rate of 1.5 μl/min over period of 60 min before dialysate is collected for PK assessment. In vivo recovery values for ciprofloxacin were calculated as follows: in vivo recovery (%) = 100 - [100 × (ciprofloxacin concentration in dialysate/ciprofloxacin concentration in perfusate)].
Measurement of blood flow.
Regional blood flow of the skin was measured by LDF (Moor Instruments, Devon, United Kingdom). The laser Doppler incorporates a low-power solid-state infrared laser diode as a source of coherent light. An optical fiber delivers the light to the tissue and penetrates 1 mm3 of tissue. Blood flow measurements are restricted to subcutaneous vessels and are unaffected by underlying muscle nutritive flow. The variables of the frequency and the power of the reflected photons are analyzed to yield estimates of blood volume and blood flow velocity. Laser Doppler flow probes were attached on the skin of the left and the right thighs with adhesive tape to ensure that no displacement of the probe occurred during the study. Measurements were performed continuously for both lower limbs at 20-min intervals for up to 5 h.
Study protocol.
On the study day a plastic cannula was inserted into an antecubital vein for the intravenous administration of ciprofloxacin. The right lower limb was heated by electric pads maintained at approximately 40°C, while the left lower limb was exposed to room temperature of approximately 25°C throughout the duration of the experiment. The skin temperature in the electric heating box was recorded at 60-min intervals. For microdialysis probe insertion, the skin was cleaned and disinfected. Thereafter, commercially available microdialysis probes (CMA 10; Microdialysis AB, Solna, Sweden) with a molecular cutoff of 20 kDa, an outer diameter of 0.5 mm, and a membrane length of 16 mm were inserted into the subcutaneous adipose tissue of the right and the left thighs by a previously described procedure (20). The probes were perfused with physiological solution at a flow rate of 1.5 μl/min by using a precision pump (CMA 100; Microdialysis AB). After a 30-min equilibration period, in vivo probe calibration was performed. After calibration, a single intravenous dose of 200 mg of ciprofloxacin (Bayer, Wuppertal, Germany) was administered over a period of approximately 20 min. The application was performed by using an automatic infusion apparatus (Döring Medizintechnik, Leverkusen, Germany). The infusion solution was protected from sunlight. On completion of infusion about 50 ml physiological saline solution was infused over the infusion lead to guarantee that the complete dosage had been applied. Microdialysates and venous blood samples were collected at 20-min intervals for up to 5 h. Blood samples were centrifuged, the cells were discharged, and plasma was obtained. Plasma and dialysates were frozen at −80°C until analysis.
Chemical analysis.
The ciprofloxacin concentrations in plasma and microdialysates were measured by high-performance liquid chromatography as described previously (21). The limits of quantification were defined as 0.05 mg/liter for plasma and 0.02 mg/liter for microdialysates.
Data analysis.
Interstitial space fluid ciprofloxacin concentrations were calculated according to the following equation: tissue concentration = 100 × sample concentration × in vivo recovery−1.
Plasma and tissue PK parameters were calculated by standard noncompartmental analysis by including all datum points without regression analysis or weighting. This was performed by using Kinetica (version 3.0) software (InnaPhase Corporation, Philadelphia, Pa.). The area under the concentration-versus-time curve (AUC) values from 0 to 5 h (AUC0-5) for plasma and the interstitium were calculated from nonfitted data by using the linear trapezoidal rule. The half-life of the terminal slope (t1/2β) was defined as ln 2 × kel−1.
AUCextra was calculated from 5 h to infinity by the equation AUCextra = Clast datum point 5 h × kel−1, whereby kel is the elimination rate constant. AUCtotal was calculated as the sum of AUC0-5 and AUCextra.
Organisms.
Pseudomonas aeruginosa strains (ATCC 27853 [MIC, 0.5 mg/liter] and two clinical isolates with MICs of 0.12 and 2 mg/liter, respectively) were chosen for the in vitro experiments. The bacteria were stored frozen in liquid nitrogen until use.
In vitro susceptibility tests.
The MICs of P. aeruginosa strains against ciprofloxacin were determined by a twofold serial Mueller-Hinton broth microdilution method according to the guidelines of CLSI (formerly NCCLS) (20). Therefore, these bacterial strains were precultured overnight on Columbia agar plates containing 5% sheep blood (Biomérieux, Marcy l'Etoile, France) and then introduced into Mueller-Hinton broth (Merck, Darmstadt, Germany) containing ciprofloxacin at an inoculum of approximately 5 × 105 CFU/ml. The lowest concentration of ciprofloxacin which inhibited visible bacterial growth after incubation for 20 h at 37°C was determined as the MIC.
Time-kill curves.
On the basis of the PK data obtained from the in vivo experiments, we simulated in vitro the concentration-versus-time profile of ciprofloxacin in the interstitial space fluid of subcutaneous adipose tissue over a period of 8 h in order to describe the antibacterial effect of ciprofloxacin on P. aeruginosa strains at the target site. For this purpose, the concentrations of ciprofloxacin from 5 to 8 h after drug administration were calculated by the equation 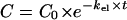 , where C represents the concentration at a defined time point, C0 is the last concentration measured in vivo (at 5 h), kel is the elimination rate constant, and t is the time between the measurement of C0 and the defined time point.
, where C represents the concentration at a defined time point, C0 is the last concentration measured in vivo (at 5 h), kel is the elimination rate constant, and t is the time between the measurement of C0 and the defined time point.
Then, 3 ml of Mueller-Hinton broth (Merck) was inoculated with the test strains in order to achieve a concentration of approximately 5 × 105 CFU/ml. The strains were then incubated at 37°C for 8 h. Hereby, the ciprofloxacin concentration-versus-time profile obtained in vivo in the interstitial fluid was simulated in vitro by changing the ciprofloxacin concentrations in broth at 20-min intervals for the first 5 h, followed by 60-min intervals. Increasing antibiotic concentrations were simulated by the addition of ciprofloxacin. Decreasing concentrations were attained by adding Mueller-Hinton broth without antibiotic at appropriate volumes, according to the equation V2 = (C1/C2) × V1, where C1 and V1 are the current ciprofloxacin concentration and the current volume in the test tube, respectively; C2 is the desired ciprofloxacin concentration; and V2 is the volume in the test tube after the addition of adequate broth. The tubes were vortexed after the addition of ciprofloxacin or pure broth. At 40-min intervals during the first 2 h, followed by 60-min intervals, the test tubes were vortexed and samples of 200 μl were drawn. The samples were serially diluted with 0.9% sodium chloride solution, and 20 μl of each dilution was plated onto Mueller-Hinton agar plates (Biomerieux). The agar plates were cultured overnight, and the bacterial counts were determined and backextrapolated to the original volume to account for the respective dilution. Each simulation was performed in triplicate. Bacterial growth control experiments were performed in culture tubes without antibiotic. Fifty CFU was considered the minimum accurately countable number of bacteria in 1 ml of broth. The detection limit, plotted in Fig. 3, rises throughout the test period due to admixture of the broth and backextrapolation to the original volume.
FIG. 3.
Time-kill curves for selected Pseudomonas aeruginosa strains with MICs of 0.12 mg/liter, 0.5 mg/liter, and 2 mg/liter. The bacteria were exposed in vitro to the PK profile determined in vivo in the nonwarmed and warmed extremities. Data are shown as means ± SDs.
Statistics.
For statistical comparison of PK parameters Mann-Whitney U tests and Wilcoxon tests were employed, as the data were nonnormally distributed. Statistical tests were performed by use of commercially available software (Statistica, version 5.0, 1997 edition; Statsoft, Statsoft Inc., Tulsa, Okla.). Repetitive testing for significance was adjusted according to Bonferroni. All data are presented as means ± standard deviations (SDs). A two-sided P value of <0.05 was considered significant.
RESULTS
Safety and tolerability.
Study drug administration was well tolerated by all subjects. No drug-related adverse effects were detected throughout the study period.
In vivo recovery.
The mean in vivo recovery values for ciprofloxacin in warmed and reference tissues were 16.0% ± 5.0% and 16.2% ± 5.0%, respectively. Individual in vivo recovery values were used to calculate the absolute concentrations of ciprofloxacin in subcutaneous adipose tissue. One microdialysis probe provided inaccurate volumes of dialysate in a warmed thigh of a volunteer. After removal of the probe visible damage of the semipermeable membrane was detected, and the PK data derived from this probe were abandoned. Thus, only 11 complete data sets for the warmed tissue were available for PK calculations.
Tissue and plasma pharmacokinetics.
The subjects received a single intravenous dose of 200 mg of ciprofloxacin over a period of approximately 20 min. The concentration-versus-time profiles of ciprofloxacin for the interstitial space fluid of subcutaneous adipose tissue (warmed and reference) are shown in Fig. 1. The values of the main PK parameters of ciprofloxacin are presented in Table 1.
FIG. 1.
Time-concentration profiles of ciprofloxacin for the interstitial space fluid of subcutaneous adipose tissue of warmed (up triangles) and reference (down triangles) tissue following administration of a single intravenous dose of 200 mg over a period of 20 min (n = 12). The shaded horizontal bar indicates the duration of infusion. Results are presented as means ± SDs.
TABLE 1.
Values of PK parameters for ciprofloxacin in plasma and interstitium of subcutaneous adipose tissuea
| PK parameter | Plasma total | Tissue free
|
|
|---|---|---|---|
| Warmedb | Reference | ||
| AUC0-5 (mg · h /liter) | 2.81 ± 0.44 | 3.06 ± 1.37 | 2.17 ± 0.66 |
| Cmax (mg/liter) | 2.49 ± 0.73 | 1.97 ± 0.92c | 1.06 ± 0.41 |
| Tmax (h) | 0.33 ± 0.0 | 0.76 ± 0.16 | 0.75 ± 0.29 |
| t1/2β (h) | 2.88 ± 0.53 | 1.93 ± 0.49 | 1.82 ± 0.41 |
| AUC0-∞ (mg · h /liter) | 3.67 ± 0.69 | 3.64 ± 1.74 | 2.56 ± 0.81 |
| AUCextra (%) | 22.7 ± 6.1 | 14.4 ± 5.7 | 14.4 ± 6.2 |
Results are presented as means ± SDs. The area under the concentration-time curve (AUC), maximal concentrations (Cmax), the time to reach the maximal concentrations (Tmaxs) and elimination half-life (t1/2β) for ciprofloxacin in subcutaneous tissue and in plasma following administration of a single dose of 200 mg over 20 min to 12 healthy male volunteers were determined. AUCextra was expressed as a percentage of AUCtotal.
Calculated from 11 subjects.
P < 0.05 compared with the results for reference tissue.
Microcirculatory blood flow.
Laser Doppler flow measurements were not significantly different (P > 0.05) between tissues at baseline. After local warming of the extremity, microcirculatory blood flow increased approximately three- to fourfold compared with that at the baseline (P < 0.05) within 40 min and remained constant over the whole period of 5 h. In contrast, blood flow remained unaffected compared with that at the baseline (P > 0.05) in the reference tissue. The relative changes in LDF measurements compared with those at the baseline are depicted in Fig. 2.
FIG. 2.
Relative change in laser Doppler flowmetry measurements after the start of heating of the lower limbs versus that at the baseline. Data are shown as means ± SDs.
Temperature.
The skin temperatures of the reference and warmed tissue were not significantly different at the baseline, with values of 31.8 ± 1.1°C and 32.0 ± 0.9°C (P > 0.05), respectively. After the start of warming, the temperature of the warmed thigh increased significantly to a mean value of 39.0 ± 0.9°C compared with that of the reference thigh (P < 0.05), which was kept constant at a mean temperature of 31.2 ± 1.4°C.
In vivo PK-in vitro PD simulation.
The time-kill curves of selected P. aeruginosa strains (MICs of 0.12 mg/liter, 0.5 mg/liter, and 2.0 mg/liter) are presented in Fig. 3 for the warmed and the nonwarmed thighs. The P. aeruginosa strain with the lowest MIC of 0.12 mg/liter was completely eradicated after exposure to the PK profile determined in vivo in the warmed and reference tissue. Inhibition of bacterial growth of bacterial strains with MICs of 0.5 mg/liter and 2 mg/liter was more effective by simulating the PK profile of the warmed extremity in comparison to that of the reference tissue. Growth curves for P. aeruginosa, which were determined in broth without ciprofloxacin, served as controls and showed an approximately 2.5 log10 increase in the numbers of CFU/ml over a period of 8 h. Each time-kill curve analysis was performed in triplicate.
DISCUSSION
The impairment of local or systemic blood flow was speculated to account for a hampered rate of penetration of antimicrobial agents from the plasma compartment to peripheral sites in patients with arterial occlusive disease (11) and sepsis (12), respectively. This, among other factors, has been suggested to be responsible for therapeutic failure in the antimicrobial management of diabetic ulcers and the still high mortality rate in patients with sepsis, despite substantial improvements in overall medical care (10). Thus, reduced blood flow coincides with lower peak tissue concentrations of anti-infectives, and from this it is tempting to hypothesize that an increase in local blood flow may reverse this phenomenon.
In the present study, we tested this hypothesis and selected ciprofloxacin as a model drug for the class of fluoroquinolones, although ciprofloxacin is not the therapy of first choice for the treatment of complicated and severe soft tissue infections. The dose of 200 mg of ciprofloxacin was chosen for administration to healthy volunteers because we aimed to expose the subjects to low drug dosages while keeping the overall clinical setting as appropriate as possible for the testing of our hypothesis.
The main finding of our study was that interstitial space fluid concentrations of ciprofloxacin in subcutaneous adipose tissue were significantly higher after local warming compared with those in the reference tissue (P < 0.05). The mean ratio of the maximum concentration (Cmax) for the warmed tissue to the Cmax for the reference tissue was 2.10 ± 0.90. The times to reach Cmax were identical for both conditions (P > 0.05). The AUC0-5 for ciprofloxacin was descriptively higher for the warmed thigh (Table 1) but did not reach the level of significance (P > 0.05), probably because of the relatively small sample size. This descriptive difference, however, was present only for AUC0-5 but declined by looking at the AUC from time zero to infinity (AUC0-∞), confirming the current knowledge that blood flow influences the rate of drug absorption, while the overall extent of drug absorption remains unaffected (Table 1; P > 0.05).
As expected, microcirculatory blood flow increased approximately three- to fourfold compared with that at the baseline (P < 0.05) by warming the dermis. No change versus that at the baseline was detected for the reference tissue over the observation period of 5 h (Fig. 2).
The finding of higher Cmax values in warmed tissue might be of particular clinical interest given that previous studies revealed that the antimicrobial activities of the fluoroquinolones are concentration dependent. Conceivably, an increase in the antibiotic concentration at the site of infection is of high relevance for less susceptible pathogens and could theoretically help prevent the development of resistant bacterial strains.
To underline our PK observations, we have employed a previously established in vivo PK-in vitro PD method (7), which simulates the inhibition of growth of selected bacteria at the target site (Fig. 3). The results confirm that the inhibition of P. aeruginosa growth (MICs, 0.5 mg/liter and 2.0 mg/liter) was approximately 2.0 log10 CFU/ml more effective for the warmed extremity in comparison to that for the reference thigh (Fig. 3). As blood flow may be considered comparable between skin and skeletal muscle under resting conditions, the data presented can be also regarded as representative for muscle tissue, although a thorough study on the effects of blood flow on the interstitial space fluid concentrations of antibiotics in skeletal muscle is currently not available in literature.
As an effect of local warming of the skin, vasodilatation of underlying arterioles is induced, and initially this may be caused by a reduced effectiveness of α-adrenergic vasoconstriction responsiveness (5, 25). While this mechanism has been reported to contribute not more than 10% of the maximal skin vasodilatation (22), endothelial factors such as nitric oxide and acetylcholine are suggested to be more actively involved in vasodilatation after prolonged local warming (4, 14, 23). The dilatation of arterioles increases the number of recruited and well-perfused capillaries. This leads to an increase in microcirculatory blood flow, which, in turn, may cause an active increase in the surface area-to-volume ratio of capillaries and causes higher transcapillary exchange rates of solutes.
From this, it might be assumed that inflammation is linked to higher drug concentrations in hyperemic, inflamed lesions, as inflammation is generally associated with local redness, tumors, and warming. However, the available data on the tissue concentrations of antimicrobial agents in inflammatory conditions are conflicting. Several clinical studies have found significantly lower concentrations in inflammatory sites than in healthy tissue (3, 10, 12), while others were not able to detect any difference in drug penetration rates between affected and healthy tissues (15, 18, 19). A reasonable explanation may be that the study populations had a variety of different diseases with different stages of severity of inflammation, resulting in high interindividual variability of local blood flow in tissues. In addition, the development of substantial pathophysiologic changes like upregulation or activation of active transport pumps, such as P-glycoprotein, fibrosis and sclerosis, or edema at the site of infection may contribute considerably to the low concentrations of antibiotics in acute or chronic infectious diseases.
In the interpretation of the present data, it is necessary to consider that plasma protein binding is relevant to the tissue penetration characteristics of antimicrobials. Plasma protein binding may vary extensively within patients, but it is fairly constant in healthy volunteers (6, 13). In addition, the direct measurement of individual plasma protein binding is not decisive for the present study, because it is indirectly taken into consideration by the use of microdialysis, as this method exclusively allows the assessment of the unbound drug fraction in the interstitial space fluid. Thus, the description of the concentration-versus-time course of ciprofloxacin in adipose tissue by use of microdialysis already implies intersubject variability in plasma protein binding.
In conclusion, in the present study, we demonstrated that the concentrations of ciprofloxacin in subcutaneous adipose tissue increase significantly by means of local warming of the dermis. However, it needs to be noted that this finding was observed in healthy volunteers and does not necessarily mirror the situation in patients with underlying vascular disease. In addition, the conclusions drawn by the use of optimized models, such as the present in vivo PK-in vitro PD approach, remain to be verified in subsequent prospective clinical trials.
Acknowledgments
We are indebted to our study nurse, Petra Zeleny, for her essential contribution to this study.
REFERENCES
- 1.Bouw, M. R., and M. Hammarlund-Udenaes. 1998. Methodological aspects of the use of a calibrator in in vivo microdialysis—further development of the retrodialysis method. Pharm. Res. 15:1673-1679. [DOI] [PubMed] [Google Scholar]
- 2.Brunner, M., U. Hollenstein, S. Delacher, D. Jager, R. Schmid, E. Lackner, A. Georgopoulos, H. G. Eichler, and M. Muller. 1999. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob. Agents Chemother. 43:1307-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunner, M., T. Pernerstorfer, B. X. Mayer, H. G. Eichler, and M. Muller. 2000. Surgery and intensive care procedures affect the target site distribution of piperacillin. Crit. Care Med. 28:1754-1759. [DOI] [PubMed] [Google Scholar]
- 4.Charkoudian, N. 2003. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin. Proc. 78:603-612. [DOI] [PubMed] [Google Scholar]
- 5.Cooke, J. P., J. T. Shepherd, and P. M. Vanhoutte. 1984. The effect of warming on adrenergic neurotransmission in canine cutaneous vein. Circ. Res. 54:547-553. [DOI] [PubMed] [Google Scholar]
- 6.Dehghanyar, P., C. Burger, M. Zeitlinger, F. Islinger, F. Kovar, M. Muller, C. Kloft, and C. Joukhadar. 2005. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob. Agents Chemother. 49:2367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delacher, S., H. Derendorf, U. Hollenstein, M. Brunner, C. Joukhadar, S. Hofmann, A. Georgopoulos, H. G. Eichler, and M. Muller. 2000. A combined in vivo pharmacokinetic-in vitro pharmacodynamic approach to simulate target site pharmacodynamics of antibiotics in humans. J. Antimicrob. Chemother. 46:733-739. [DOI] [PubMed] [Google Scholar]
- 8.European Agency for the Evaluation of Medicinal Products. 2002. Guideline for good clinical practice. ICH topic E6. European Agency for the Evaluation of Medicinal Products [Online.] http://www.emea.eu.int/pdfs/human/ich/013595en.pdf.
- 9.Joukhadar, C., H. Derendorf, and M. Muller. 2001. Microdialysis. A novel tool for clinical studies of anti-infective agents. Eur. J. Clin. Pharmacol. 57:211-219. [DOI] [PubMed] [Google Scholar]
- 10.Joukhadar, C., M. Frossard, B. X. Mayer, M. Brunner, N. Klein, P. Siostrzonek, H. G. Eichler, and M. Muller. 2001. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 29:385-391. [DOI] [PubMed] [Google Scholar]
- 11.Joukhadar, C., N. Klein, M. Frossard, E. Minar, H. Stass, E. Lackner, M. Herrmann, E. Riedmuller, and M. Muller. 2001. Angioplasty increases target site concentrations of ciprofloxacin in patients with peripheral arterial occlusive disease. Clin. Pharmacol. Ther. 70:532-539. [DOI] [PubMed] [Google Scholar]
- 12.Joukhadar, C., N. Klein, B. X. Mayer, N. Kreischitz, G. Delle-Karth, P. Palkovits, G. Heinz, and M. Muller. 2002. Plasma and tissue pharmacokinetics of cefpirome in patients with sepsis. Crit. Care Med. 30:1478-1482. [DOI] [PubMed] [Google Scholar]
- 13.Joynt, G. M., J. Lipman, C. D. Gomersall, R. J. Young, E. L. Wong, and T. Gin. 2001. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 47:421-429. [DOI] [PubMed] [Google Scholar]
- 14.Kellogg, D. L., Jr., Y. Liu, I. F. Kosiba, and D. O'Donnell. 1999. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J. Appl. Physiol. 86:1185-1190. [DOI] [PubMed] [Google Scholar]
- 15.Legat, F. J., A. Maier, P. Dittrich, P. Zenahlik, T. Kern, S. Nuhsbaumer, M. Frossard, W. Salmhofer, H. Kerl, and M. Muller. 2003. Penetration of fosfomycin into inflammatory lesions in patients with cellulitis or diabetic foot syndrome. Antimicrob. Agents Chemother. 47:371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leslie, S. J., J. Affolter, M. A. Denvir, and D. J. Webb. 2003. Validation of laser Doppler flowmetry coupled with intra-dermal injection for investigating effects of vasoactive agents on the skin microcirculation in man. Eur. J. Clin. Pharmacol. 59:99-102. [DOI] [PubMed] [Google Scholar]
- 17.Ljungberg, B., and I. Nilsson-Ehle. 1988. Pharmacokinetics of intravenous ciprofloxacin at three different doses. J. Antimicrob. Chemother. 22:715-720. [DOI] [PubMed] [Google Scholar]
- 18.Muller, M., M. Brunner, U. Hollenstein, C. Joukhadar, R. Schmid, E. Minar, H. Ehringer, and H. G. Eichler. 1999. Penetration of ciprofloxacin into the interstitial space of inflamed foot lesions in non-insulin-dependent diabetes mellitus patients. Antimicrob. Agents Chemother. 43:2056-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, H. Pehamberger, E. Agneter, and H. G. Eichler. 1996. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Neckel, U., C. Joukhadar, M. Frossard, W. Jäger, M. Muller, and B. X. Mayer. 2002. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography. Anal. Chim. Acta 463:199-206. [Google Scholar]
- 22.Pergola, P. E., D. L. Kellogg, Jr., J. M. Johnson, W. A. Kosiba, and D. E. Solomon. 1993. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am. J. Physiol. 265:H785-H792. [DOI] [PubMed] [Google Scholar]
- 23.Shibasaki, M., T. E. Wilson, J. Cui, and C. G. Crandall. 2002. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J. Appl. Physiol. 93:1947-1951. [DOI] [PubMed] [Google Scholar]
- 24.Steiner, I. M., H. Langenberger, C. Marsik, B. X. Mayer, M. Fischer, A. Georgopoulos, M. Muller, G. Heinz, and C. Joukhadar. 2004. Effect of norepinephrine on cefpirome tissue concentrations in healthy subjects. J. Antimicrob. Chemother. 53:506-511. [DOI] [PubMed] [Google Scholar]
- 25.Wilson, T. E., J. Cui, and C. G. Crandall. 2002. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton. Neurosci. 97:122-128. [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association. 6October2002. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. [Online.] http://www.wma.net/e/policy/b3.htm. [DOI] [PubMed]





