Abstract
The increased incidence of human immunodeficiency virus (HIV)/AIDS disease in women aged 15 to 49 years has identified the urgent need for a female-controlled, efficacious, and safe vaginal topical microbicide. To meet this challenge, sophorolipid (SL) produced by Candida bombicola and its structural analogs have been studied in this report for their spermicidal, anti-HIV, and cytotoxic activities. The sophorolipid diacetate ethyl ester derivative is the most potent spermicidal and virucidal agent of the series of SLs studied. Its virucidal activity against HIV and sperm-immobilizing activity against human semen are similar to those of nonoxynol-9. However, it also induced enough vaginal cell toxicity to raise concerns about its applicability for long-term microbicidal contraception. Its structure-activity relationship has been established for creating new analogs with less cytotoxicity and higher activity.
According to the World Health Organization (WHO), over 100 million acts of sexual intercourse take place each day (24). They result in some 910,000 conceptions and 356,000 episodes of sexually transmitted diseases (STDs) per year, including both bacterial and viral infections (24). Family planning not only enables fertility regulation but also saves the lives of women and children. This fact has been reinforced by the interdependency of family planning and reproductive health and the deadly impact of the sexually disseminated epidemic of human immunodeficiency virus (HIV) and its consequence, AIDS. More than 40 million people are infected with HIV worldwide, and the death toll related to AIDS exceeded 3 million in 2004 (28) with women representing an increasing percentage of infected individuals (31). Many women want to control their fertility and reduce their risk of becoming infected with a STD (22). However, no currently available agent simultaneously protects against pregnancy and infection. There is a need to develop safe prophylactic agents that are spermicidal and virucidal, especially against HIV.
Nonoxynol-9 (N-9), a nonionic surfactant, is the most widely used spermicide in the world. Despite its potent virucidal activity in vitro, N-9 does not appear to reduce the risk of HIV transmission, as determined by three large clinical trials (16, 21, 29). It also seems to increase the risk of urinary tract infections, vulvovaginal candidiasis, and genital ulcers (14, 26, 32). Furthermore, because N-9 is a mixture of oligomers (35), N-9 may not meet future regulations as the health care industry moves towards using pure compounds or mixtures whose individual components have met safety standards. Several European nations have banned or restricted the use of N-9 and related compounds on the basis of health risks and potential environmental toxicity (19, 27).
Sophorolipids (SLs) are a group of microbial glycolipids produced by yeasts, such as Candida bombicola, Yarrowia lipolytica, Candida apicola, and Candida bogoriensis (4). They occur naturally as disaccharide sophoroses linked glycosidically to the hydroxyl group at the penultimate carbon of primarily C18-chain-length fatty acids (Fig. 1). First described in 1961, sophorolipids occur as mixtures of macrolactone (structures 1 to 4 [Fig. 1a]) and open ring (structures 5 to 8 [Fig. 1a]) that are acetylated to various extents at the primary hydroxyl position of the sophorose ring (Fig. 1a) (3, 11). Analytical studies revealed that at least eight structurally different sophorolipids are produced during fermentation (5). Previous publications by R. Gross’s laboratory describe chemoenzymatic transformations for the synthesis of pure compounds from the natural mixture. This resulted in a family of new SL analogs that extend and diversify structural features present in the natural compounds (3, 25). We have also shown that the natural SL mixture, selected pure isolates, and/or SL derivatives have antibacterial and antifungal activity (12, 13), function as immunomodulators for the treatment of endotoxic (septic) shock by cytokine downregulation (15), and display anticancer activity (23). Maingault (17) further proposed that sophorolipids be used to treat skin diseases. The present study documents in vitro spermicidal and anti-HIV virucidal activities for a series of SL analogs.
FIG. 1.
Structures of natural mixture sophorolipids (a) and SL analogs (b). The common names of SLs shown in the figure are as follows: mixture of compounds 1 to 8, natural mixture SL; mixture of compounds 1 to 4, lactonic SL; compound 8, open-ring nonacetylated SL; compound 9, methyl ester SL; compound 10, ethyl ester SL; compound 11, hexyl ester SL; compound 12, monoacetate ethyl ester SL; compound 13, diacetate ethyl ester SL. Ac, acetyl.
MATERIALS AND METHODS
Materials.
All chemicals and solvents were of analytical grade and were used as received unless otherwise mentioned. High-performance liquid chromatography-grade methyl alcohol, ethyl alcohol, propyl alcohol, butyl alcohol, hexyl alcohol, and silica gel were obtained from Sigma-Aldrich. All alcohols were distilled and stored over activated 4-Å molecular sieves prior to use. Analytical thin-layer chromatography was performed on precoated Merck silica gel 60F254 plates. Column chromatography was performed on 200/400 mesh, 60- Å silica gel using chloroform/methanol mixtures as the eluent. Proton (1H) and carbon (13C) nuclear magnetic resonance spectra were recorded on a Bruker 300 spectrometer at 300 MHz and 75 MHz, respectively. Microbial media were obtained from Sigma-Aldrich.
Natural mixture sophorolipid production.
Sophorolipids were synthesized by fermentation of Candida bombicola ATCC 22214. C. bombicola was stored in liquid nitrogen as inoculum aliquots (2 × 1011 to 4 × 1011 cells per ml, in 10:90 glycerol:phosphate buffer, pH 7.0, containing 137 mM NaCl). The culture was grown aseptically on a medium containing 100 g/liter glucose, 10 g/liter yeast extract, and 1 g/liter urea. Oleic acid (Sigma-Aldrich; 99%, used as received), 40 g per liter, was supplied to the medium prior to inoculation at the stage of fermentation. Two sequential 100-ml precultures were grown in a rotary shaker at 200 rpm in 500-ml baffled Erlenmeyer flasks at 30 °C ± 1°C. The first preculture was inoculated with 1 ml of the above inoculum (approximately 0.1 g [dry weight] biomass). The cultures were passed to the next stage at the late exponential growth phase (about 24 to 36 h) estimated by A650. Final precultures contained 33 ± 5 g (dry weight) of biomass per liter. Fermentation was performed for 8 days at 200 rpm and 30 °C ± 1°C. Subsequently, SLs were extracted thrice with ethyl acetate, the extracts were pooled, and the solvent was removed. The resulting substances were washed with hexane to remove residual fatty acids. These substances are natural mixture sophorolipids.
Synthesis of SL derivatives.
Sophorolipid derivatives were prepared and characterized exactly as was described in detail elsewhere (3, 25). The methods used are summarized below.
(i) Preparation of ring-opened acidic nonacetylated SL (structure 8 [Fig. 1a]).
Natural mixture consisting of acetylated and nonacetylated SLs was converted into the ring-opened free-acid form by alkaline hydrolysis. The natural mixture (20 g) was suspended in 50 ml of 5 M NaOH and refluxed for 1 h, and the pH was lowered to 4 with dilute HCl at 0°C. The ring-opened free-acid SL (white powder) was isolated by column chromatography using chloroform:methanol (90:10 [vol/vol]) as the eluent.
(ii) Preparation of diacetyl lactonic SL (structure 1 [Fig. 1a]).
The lactonic SL acetylated at 6′ and 6" positions was isolated from the SL natural mixture by column chromatography using chloroform:methanol (98:2 [vol/vol]) as the eluent.
(iii) Preparation of methyl, ethyl, and hexyl SL esters (structures 9 to 11 [Fig. 1b]).
Diacetyl lactonic SL was dissolved in methanol, ethanol, or hexanol that contained 0.02 N of the corresponding sodium alkaoxide. The alcoholysis reactions were conducted at 65°C for 3 h to give SL esters. Reaction mixtures were concentrated by rotoevaporation and poured with stirring into ice-cold water to precipitate the SL esters.
(iv) Selective acetylation of SL ethyl ester primary hydroxyl groups.
The SL ethyl ester (structure 10 [Fig. 1b]) was selectively acetylated with vinyl acetate at primary 6′- and 6"-hydroxyl positions using immobilized lipase B from Candida antartica (Novozym 435). The reaction was conducted in anhydrous tetrahydrofuran at 35°C for about 96 h to obtain diacetate ethyl ester SL (structure 13 [Fig. 1b]) and for 2.5 h to obtain monoacetate ethyl ester SL (structure 12 [Fig. 1b]). The reactions were terminated by filtering the enzyme, followed by evaporation of tetrahydrofuran under vacuum, gave a substance that was an oil. Silica gel chromatography using chloroform as the eluent gave a crude oil which, after column chromatography using pure chloroform as the eluent, afforded pure mono- and diacetyl SL ester derivatives.
Spermicidal activity.
The spermicidal activities of SLs were evaluated using a modification of the Sander and Cramer method (6). Semen samples were collected from healthy donors by masturbation, allowed to liquefy at room temperature, and assessed for motility. The protocol was approved by the Eastern Virginia Medical School Institutional Review Board. Stock solutions, typically at 10 mg/ml, were prepared in dimethyl sulfoxide (DMSO). Serial twofold dilutions of the test compounds in 0.9% NaCl were incubated with semen aliquots for 30 seconds. Motility was evaluated under the microscope during the first incubation and after dilution in buffer and further incubation for 1 h. Only those dilutions that immobilized 100% of the spermatozoa pass the test (22). Results were expressed as minimum effective concentrations (MECs) (also called ED100).
Sperm motility and viability tests.
Following twofold serial dilutions, the compounds were incubated with semen aliquots for 30 seconds or 0.5, 2 and 30 minutes. Subsequently, the mixtures were overdiluted with BWW medium containing 1% human serum albumin (termination of the compound's effect). Sperm were pelleted down, resuspended in fresh medium, and incubated at 37°C and 5% CO2 for 30 min (34). Progressive motility following the initial exposure and after 30-minute postdilution incubations was assessed microscopically. Results were expressed as a percentage of compound-treated motile sperm compared to control (saline-treated) sperm. To verify viability, spermatozoa were further incubated in a hypoosmotic buffer for 30 min (hypoosmotic swelling test), and the response of the tails was evaluated microscopically (coiled tails indicated conserved membrane integrity). No fewer than 200 cells were counted.
Cell-free HIV inactivation assay.
Pretitered concentrated virus stock (HIV type 1 [HIV-1] RF, obtained from the NIH AIDS Research and Reference Reagent Program [Rockville, MD]) was mixed with SL compounds in microtubes. At the end of the 2-minute exposure period, serial 10-fold dilutions were carried out with a multichannel pipettor so that sample dilutions were performed simultaneously. Aliquots of serial dilutions were then transferred simultaneously to another 96-well plate which had been preseeded with MT-2 cells in RPMI medium supplemented with fetal bovine serum. Four wells were used for each dilution of virus. The cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. Cultures were scored microscopically for virus-induced cytopathic effect and agent-induced cytotoxicity on days 4 and 7 of incubation, and day 7 results were reported. Cultures were routinely maintained 2 to 3 days after the initial analysis and observed visually and microscopically for abnormalities (20).
Cytotoxicity to human vaginal cells and proinflammatory cytokine production.
VK-2/E6E7 keratinocytes were grown in a culture flask until they were 70 to 80% confluent. Cells were removed from the culture flask by trypsinization followed by centrifugation. The supernatant was removed, and cells were resuspended in fresh keratinocyte-SFM medium (R&D Systems, Minneapolis, MN). Cells suspended in fresh growth medium were plated evenly on a sterile 96-well plate and incubated at 37°C in 5% CO2 until the cells were confluent. On the day of the experiment, the medium from each well was replaced with fresh medium containing the desired concentration of compound (range, 100 to 3.1 μg/ml). Experiments were run in duplicate. The cells were then incubated for 6 h at 37°C in 5% CO2. After 6 h, the supernatant of each well was removed and stored at −20°C for interleukin analysis. The 96-well plate was replenished with fresh medium, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye solution (Promega, Madison, WI) was added and incubated for 4 h at 37°C in 5% CO2. Solubilizing solution was added to each well and left overnight at 37°C in 5% CO2 for color to develop. The following day, color was quantified at 570/450 nm (8).
Cytokines were determined using culture medium supernatants of VK-2 cells incubated with compounds as stated above. The supernatants were incubated in 96-well plates for 2 h, and the plates were washed three times with washing buffer (25 mM Tris-buffered saline, pH 7.4). Anti-interleukin 1α (anti-IL-1α) or anti-IL-8 conjugate (R&D Systems, Minneapolis, MN) was added and incubated for 1 h at room temperature. The plate was then washed three times. Coloring agent was added and incubated for 30 min. The reaction was stopped, and color was quantified at 450/570 nm (8).
RESULTS
Spermicidal activity.
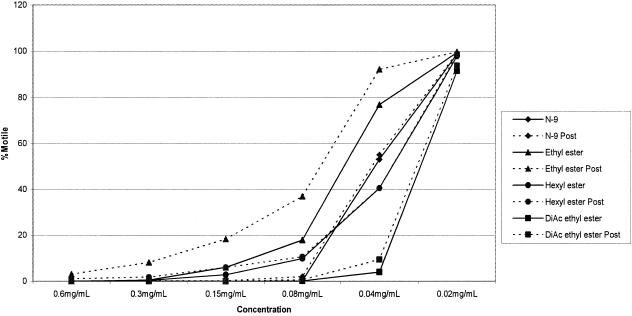
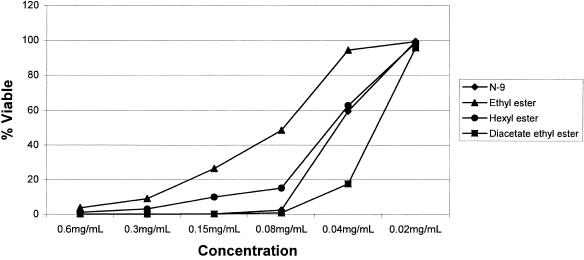
Table 1 presents data on spermicidal activity for a series of sophorolipid analogs. N-9 was used as a control. Acetylated ethyl esters were the most effective SL analogs of those tested. Diacetate ethyl ester SL showed a minimum effective concentration of 0.18 mg/ml, which was similar to that of the commercial spermicide N-9 (MEC = 0.25 mg/ml). The open-ring SL analogs that lack acetylation at the sophorose head group did not display significant spermicidal activity beyond that observed for their solvent (DMSO) control. Natural mixture SL and lactonic SL, conversely, revealed spermicidal activity with similar MECs (0.8 and 1.0 mg/ml, respectively). In dose-response studies comparing the activities of selected SLs and N-9 against sperm motility and viability, the diacetate ethyl ester SL again proved to be the most potent compound with a 50% effective concentration (EC50) of 0.030 mg/ml (Fig. 2). The parallelism of the motility and viability dose-response curves suggests that the most likely mechanism of action for these compounds is membrane perturbation and disruption (Fig. 3). This concept is reinforced by the inability of treated spermatozoa to recover motility after the compound was washed off and sperm were incubated in fresh medium (Fig. 2).
TABLE 1.
Spermicidal activities of sophorolipid analogs
| Sophorolipid analog or compound | Highest spermicidal dilution (l/X)a | MEC (mg/ml) |
|---|---|---|
| Natural mixture SL | 13.6 ± 1.2 | 0.8 ± 0.09 |
| Lactonic SL | 12.0 ± 2.3 | 1.0 ± 0.1 |
| Open-ring SL | 3.0 ± 0.3 | 3.8 ± 0.4 |
| Methyl ester SL | 3.8 ± 0.2 | 2.8 ± 0.2 |
| Ethyl ester SL | 3.4 ± 0.3 | 3.3 ± 0.4 |
| Hexyl ester SL | 2.7 ± 0.3 | 4.5 ± 0.7 |
| Monoacetate ethyl ester SL | 19.2 ± 2.0 | 0.5 ± 0.04 |
| Diacetate ethyl ester SL | 94.4 ± 20.8 | 0.18 ± 0.05 |
| Nonoxynol-9 | 44.8 ± 5.0 | 0.25 ± 0.02 |
Sperm-immobilizing activity was determined in a Sander-Cramer assay using 10 different semen samples. Results are expressed as means ± standard errors. Stock solutions were prepared at 10 mg/ml in DMSO. The DMSO solvent displayed spermicidal activity. Its highest spermicidal dilution for DSMO was 1:4.
FIG. 2.
Sperm motility dose-response curves. Sperm motility was assessed after a short 30-s incubation with multiple concentrations of the compounds and after a 30-min incubation in fresh medium deprived of the compound (Post). Lines connecting data points do not represent mathematical fitting of the data. Standard deviations were low, ranging from 0 to 23%. DiAc, diacetate.
FIG. 3.
Sperm viability dose-response curves. Sperm membrane integrity was assessed after the cells had been exposed to various compound concentrations for 30 s and incubated in fresh medium for 30 min. Lines connecting data points do not represent mathematical fitting of the data. Standard deviations were low, ranging from 0 to 21%.
In a time-response study, the diacetate ethyl ester derivative again proved to be the most efficacious, immobilizing almost all spermatozoa exposed to 20 μg/ml of compound in less than 120 seconds (Table 2). As expected, sperm immobilization occurred faster than sperm death. The action kinetics of lactonic and hexyl ester SLs was similar, inducing 50% sperm immobilization in about 2 min.
TABLE 2.
Sperm motility and viability time responsea
| Compound | Initial solvent | Time | Concn (mg/ml) | Real-time motility (%) | Postdilution motility (%) | Postdilution viability (%) |
|---|---|---|---|---|---|---|
| Lactonic SL | DMSO | 30 s | 0.02 | 98.31 ± 0.78 | 98.15 ± 1.06 | 99.50 ± 0.50 |
| 2 min | 0.02 | 50.83 ± 5.40 | 15.28 ± 6.48 | 38.68 ± 4.68 | ||
| 30 min | 0.02 | 30.18 ± 4.38 | 4.04 ± 0.86 | 31.29 ± 3.88 | ||
| Ethyl ester SL | DMSO | 30 s | 0.02 | 99.66 ± 1.05 | 99.49 ± 0.88 | 99.33 ± 0.29 |
| 2 min | 0.02 | 98.32 ± 0.28 | 99.16 ± 0.60 | 99.17 ± 0.76 | ||
| 30 min | 0.02 | 96.35 ± 0.98 | 98.31 ± 1.18 | 99.16 ± 0.77 | ||
| Hexyl ester SL | DMSO | 30 s | 0.02 | 98.13 ± 1.80 | 98.16 ± 1.16 | 99.83 ± 0.29 |
| 2 min | 0.02 | 50.17 ± 2.03 | 22.58 ± 1.80 | 31.16 ± 3.14 | ||
| 30 min | 0.02 | 33.40 ± 0.68 | 10.19 ± 8.41 | 19.20 ± 8.92 | ||
| Diacetate ethyl ester SL | DMSO | 30 s | 0.02 | 93.27 ± 1.48 | 94.30 ± 1.76 | 96.52 ± 2.26 |
| 2 min | 0.02 | 4.89 ± 0.82 | 5.69 ± 1.69 | 37.34 ± 2.02 | ||
| 30 min | 0.02 | 2.70 ± 0.76 | 3.37 ± 1.80 | 26.00 ± 2.18 | ||
| N-9 | DMSO | 30 s | 0.02 | 98.66 ± 1.90 | 99.00 ± 1.32 | 99.84 ± 0.29 |
| 2 min | 0.02 | 98.16 ± 2.23 | 98.18 ± 1.03 | 98.84 ± 1.15 | ||
| 30 min | 0.02 | 96.63 ± 1.03 | 97.99 ± 0.99 | 98.67 ± 0.30 | ||
| DMSO (1:100) | 30 s | 99.33 ± 0.31 | 99.49 ± 0.51 | 99.50 ± 0.00 | ||
| 2 min | 99.32 ± 0.59 | 99.66 ± 0.77 | 99.51 ± 0.85 | |||
| 30 min | 99.16 ± 0.30 | 99.82 ± 0.58 | 99.50 ± 0.00 |
Sperm were exposed to 20 μg/ml of compound for 0.5, 2, and 30 minutes. Motility was assessed immediately after their exposure times ("Real-time motility") and following a 30-min additional incubation in fresh medium ("Postdilution motility"). Sperm viability was assessed only after the latter 30-min incubation ("Postdilution viability"). A 1:100 dilution of DMSO, containing the same amount of DMSO as the test concentration of the compounds, did not show sperm-immobilizing or spermicidal activity. Values are expressed as means ± standard deviations.
Anti-HIV activity.
At 3 mg/ml, all the SL derivatives displayed anti-HIV virucidal activity, inactivating the virus in less than 2 min and reducing its titer by more than 2 log units. Comparatively, the diacetate ethyl ester SL was the most active derivative, slightly less potent than N-9 (Table 3). Comparing the lactonic to ring-opened SL free acid, the latter compound was clearly more virucidal.
TABLE 3.
In vitro assay for inactivation of cell-free HIV showing the effect of concentration on the reduction of virus infectivitya
| Compound | Log reduction in virus titer at the following compound concn (mg/ml):
|
|||
|---|---|---|---|---|
| 0.33 | 0.09 | 0.03 | 0.009 | |
| Natural mixture SL | ≥4.2 | 2.0 | 1.7 | 1.7 |
| Lactonic SL | 2.7 | 0.7 | 0.4 | 0.0 |
| Open-ring SL | ≥4.2 | ≥4.2 | 1.9 | 1.0 |
| Methyl ester SL | NAb | NA | NA | NA |
| Ethyl ester SL | >5.2 | 2.7 | 1.0 | 0.0 |
| Hexyl ester SL | 3.5 | 2.4 | 0.2 | 0.0 |
| Mono acetate ethyl ester SL | NA | NA | NA | NA |
| Di acetate ethyl ester SL | ≥4.2 | ≥5.2 | 3.2 | 0.2 |
| N-9 | NA | ≥4.5 | ≥5.0 | 0.5 |
HIV-1 (RF) (control titer = 6.7 −log10/0.1 ml) was incubated with multiple half-log compound concentrations for 2 minutes and then serially diluted (10-fold) to terminate the compound effect. Resulting dilutions were then incubated with preseeded MT-2 cells. Virus-induced cytopathicity was assessed microscopically on day 7. The concentration of solvent (DMSO) in the highest compound concentration did not have any significant effect on viral titer.
NA, not assayed.
Epithelial cytotoxicity and secretion of proinflammatory cytokines.
To study the cytotoxicity of SLs against human vaginal cells, a VK-2/E6E7 cell line that closely resembles the characteristics of its tissue of origin was used (8). The epithelial cell toxicity dose responses ranked the ring-opened (nonlactonic) SL free acid as the least cytotoxic compound (EC50, >100 μg/ml), while the lactonic and SL natural mixture were the most cytotoxic (Fig. 4). In comparison to N-9 (EC50 = 0.020 mg/ml), ethyl, methyl, and hexyl esters were less toxic, whereas the diacetate ethyl ester displayed similar or slightly less cytotoxicity (EC50 = 0.022 to 0.043 mg/ml).
FIG. 4.
Vaginal cell cytotoxicity. Vaginal epithelial cells (VK-2/E6E7) were incubated with multiple concentrations of the compounds for 6 h at 37°C and 5% CO2. The concentrations (in micrograms per milliliter) of the compounds are shown on the x axis. After the supernatants were removed, the cells were replenished with culture medium and incubated with MTT dye solution. After color developed, it was quantified in a colorimeter at 570/450 nm. Lines connecting data points do not represent mathematical fitting of the data. Standard deviations were low, ranging from 0 to 29%.
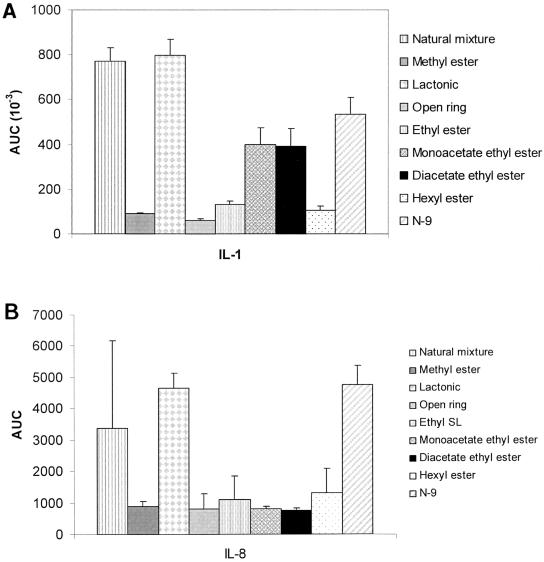
Induction of proinflammatory cytokines IL-1α and IL-8 by vaginal epithelial cells was measured by incubating multiple concentrations of SLs (3 to 100 μg/ml) with VK-2 cells for 6 h. Cell culture supernatants were then assessed for interleukin concentrations. As seen in Fig. 5, the lactonic SL induced the greatest amount of IL-1 and IL-8. The smallest effect was seen with the open-ring SLs; however, alkylation or acetylation increased cytokine production of these derivatives.
FIG. 5.
Proinflammatory cytokine induction. (A) IL-1; (B) IL-8. Vaginal epithelial cells were incubated with multiple concentrations of the compounds for 6 h. Cell culture supernatants were assessed for interleukin levels by enzyme-linked immunosorbent assays. Results represent the area under the curve (AUC) covering the production of interleukin throughout the tested concentrations (3.1 to 100 μg/ml).
DISCUSSION
A diverse family of microbial glycolipids was successfully prepared by fermentative production, isolation, purification, and chemoenzymatic modification of SLs. Our findings show that the SL diacetate ethyl ester derivative is the most potent spermicidal and virucidal agent of the series of SLs thus far studied. Its virucidal activity against HIV and sperm-immobilizing activity against human semen are similar to those of N-9, a commercial spermicide that has been tested as an HIV transmission-preventative agent (30).
Structure-activity relationship (SAR) analysis reveals that the chain length of fatty acids influences the spermicidal activity. On the basis of dose- and time-response data, the SL ethyl ester with a 20-carbon chain length displayed lower spermicidal activity than the SL hexyl ester with a 24-carbon chain length. This phenomenon has been reported for two other series of spermicidal lipids, albeit studying longer chains (22, 33). Analysis of the ring-opened SL free acid (18 carbons) and the alkyl ester (19 to 24 carbons) structures shows that alkylation also increases cytotoxicity while decreasing antiviral activity.
Acetylation of the sophorose head group has a drastic influence on compound activity. By adding acetate groups to the sophorose head group, the potency of the derivatives is increased. Diacetate ethyl ester is the most potent spermicidal agent followed by monoacetate and nonacetylated ethyl esters. A similar trend is seen for anti-HIV activity. It is worth noting, however, that the open-ring free-acid SL displays good antiviral activity and is the least cytotoxic of the compounds tested.
The ability of molecules to form micelles has been associated with their spermicidal and virucidal potencies (33). Physicochemical studies on SL derivatives that we performed showed that the critical micelle concentration (CMC) of SLs decreased with increased SL ester chain length. All concentrations of SLs used to interrogate their biological activity were well above the CMC values of these compounds. Nevertheless, SL esters of longer carbon chain length with a higher tendency to self-associate (lower CMC) have increased spermicidal activity and cytotoxicity. This finding reinforces the relationship between the self-associating capacity of a compound and its spermicidal activity. The relationship between chain length, at least within the range tested in this study, and virucidal activity would appear to be inverse. Thus, it may be that shorter-carbon-chain SLs have higher extents of self-association within viral envelopes, resulting in higher potency as virucidal agents. However, additional work will be needed to verify this hypothesis.
Sophorolipids appear to act as membrane-perturbing agents. Other membrane-perturbing agents have been shown to display spermicidal and virucidal properties (18). The membrane partitioning of these compounds is critically involved in their microbicidal activity. However, this property does not strictly follow a simple model (1). The lipid composition of cell membranes plays a crucial role in the membrane-perturbing activity of compounds. The presence and abundance of cholesterol, for instance, has the greatest influence (2). In this regard, in spite of their general similarity, sperm, microbial, and somatic cell membranes have several distinguishing features which provide the basis for design of a selective microbicidal spermicide.
Interestingly, natural SLs and analogs thereof showed some degree of selectivity in their spermicidal, virucidal, and cytotoxic effects. For instance, the lactonic SL displayed high spermicidal, cytotoxic, and proinflammatory activities but only low virucidal activity. Conversely, open-ring SLs were weak spermicides but potent virucides. The diacetate ethyl ester SL displayed the highest spermicidal and anti-HIV activities. Unfortunately, it also induced enough vaginal cell toxicity to raise concerns about its applicability for long-term microbicidal contraception. As mentioned earlier, frequent use of an N-9-containing vaginal product has been associated with a possible increase in the rate of HIV transmission (30) and genital ulcers (32). Women treated with daily intravaginal doses of N-9 showed an increase in the levels of proinflammatory cytokines in their vaginal fluids (9). In vitro, these fluids proved to enhance HIV replication in latently infected monocytic cells. Furthermore, in a rabbit model, N-9 and other detergents induced increased levels of IL-1, IL-6, and IL-8 in vaginal fluids, which correlated well with mucosal irritation and the presence of activated leukocytes in vaginal tissues (7, 8).
In theory, however, strategies to selectively decrease irritation potential while maintaining specific microbicidal or spermicidal activity exist. The irritation potential of N-9 was reduced when it was coprecipitated with polyvinylpyrrolidone (PVP) (10). N-9/PVP coprecipitation altered self-associating properties and reduced N-9's irritating properties. Indeed, work is currently under way in our laboratories to develop new SL agents that have some combination of enhanced microbicidal or spermicidal activity and decreased irritating properties. Among the strategies under study are the covalent or physical association of SLs with nonactive polymeric carriers, conjugation of SLs to other compounds with known microbicidal or spermicidal activity, and exploration of other modified SL analogs, such as those with sulfation that might enhance the ratio of contraceptive and antiviral efficacy to irritating properties.
This study clearly demonstrates that certain sophorolipids and modified forms thereof have excellent spermicidal and anti-HIV activity. SAR analysis showed that the acetylation of SL sophorose head groups is a key structural parameter that determines the potency of these molecules. Esterifying the carboxyl groups of SL fatty acids had a similar impact on specific activity. The ability to manipulate the biological properties of SLs over such a broad range with the small number of natural and modified SL structures thus far studied indicates that a much broader SAR analysis for this family of compounds would be of great value. For instance, the importance of acetylating SL sophorose head groups demonstrated in this work leads one to question what would be the effect of substituting acetate with propionate, butyrate, or charged moieties, such as sulfate or phosphate groups. Furthermore, only a few simple n-alkyl groups have thus far been esterified to the carboxyl group of SL fatty acids. The results of this study suggest that expanding the range of SL-related structures and assessing their biological properties will provide a deeper knowledge of SAR. Through these activities there is good reason to believe that SL-based compounds with significantly enhanced microbicidal, spermicidal, or other desirable biological attributes but with lower irritating properties will result.
Acknowledgments
We are grateful to the Polytechnic University and CONRAD for providing critical financial support for this research through a technology development seed grant and intramural funds, respectively.
The views of the authors do not necessarily reflect those of the funding agencies and institutions.
We are also grateful to Christine Farrigan for her assistance on the preparation of the manuscript.
REFERENCES
- 1.Apel-Paz, M., G. F. Doncel, and T. K. Vanderlick. 2003. Membrane perturbation by surfactant candidates for STD prevention. Langmuir 9:591-597. [Google Scholar]
- 2.Apel-Paz, M., T. K. Vanderlick, N. Chandra, and G. F. Doncel. 2003. A hierarchy of lipid constructs for the sperm plasma membrane. Biochem. Biophys. Res. Commun. 309:724-732. [DOI] [PubMed] [Google Scholar]
- 3.Bisht, K. S., R. A. Gross, and D. L. Kaplan. 1999. Enzyme-mediated regioselective acylations of sophorolipids. J. Org. Chem. 64:780-789. [DOI] [PubMed] [Google Scholar]
- 4.Cameotra, S. S., and R. S. Makkar. 2004. Recent applications of biosurfactants as biological and immunological molecules. Curr. Opin. Microbiol. 7:262-266. [DOI] [PubMed] [Google Scholar]
- 5.Davila, A. M., R. Marchal, and J. P. Vandecasteele. 1992. Kinetics and balance of fermentation free from product inhibition: sophorose lipid production by Candida bombicola. Appl. Microbiol. Biotechnol. 38:6-11. [Google Scholar]
- 6.D'Cruz, O. J., M.-J. Shih, S. H. Yiv, C.-L. Chen, and F. M. Uckun. 1999. Synthesis, characterization and preclinical formulation of a dual-action phenyl phosphate derivative of bromo-methoxy zidovudine (compound WHI-07) with potent anti-HIV and spermicidal activities. Mol. Hum. Reprod. 5:421-432. [DOI] [PubMed] [Google Scholar]
- 7.Doncel, G. F., N. Chandra, and R. N. Fichorova. 2004. Preclinical assessment of proinflammatory potential of microbicide candidates. J. Acquir. Immune Defic. Syndr. 37:S174-S180. [PubMed] [Google Scholar]
- 8.Fichorova, R. N., M. Bajpai, N. Chandra, J. G. Hsiu, M. Spangler, V. Ratnam, and G. F. Doncel. 2004. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol. Reprod. 71:761-769. [DOI] [PubMed] [Google Scholar]
- 9.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 10.Fowler, P. T., G. F. Doncel, P. M. Bummer, and G. A. Digenis. 2003. Coprecipitation of nonoxynol-9 with polyvinylpyrrolidone to decrease vaginal irritation potential while maintaining spermicidal potency. AAPS Pharm. Sci. Tech. 4:E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorin, A. P. J., J. F. T. Spencer, and A. P. Tulloch. 1961. Hydroxy fatty acid glycosides of sophorose from Torulopsis magnoliae. Can. J. Chem. 39:846-895. [Google Scholar]
- 12.Gross, R., and V. Shah. Antifungal activity of various forms of sophorolipids. U.S. patent application filed. Application no. 11/020683.
- 13.Gross, R., and V. Shah. Antibacterial properties of various forms of sophorolipids. U.S. patent application filed. Application no. PCT/US2003/035871.
- 14.Jones, B. M., A. Eley, D. A. Hicks, R. Patel, and J. M. Wordsworth. 1994. Comparison of the influence of spermicidal and non-spermicidal contraception on bacterial vaginosis, candidal infection and inflammation of the vagina—a preliminary study. Int. J. STD AIDS 5:362-364. [DOI] [PubMed] [Google Scholar]
- 15.Kandil, E., H. Zhang, R. Schulze, L. Dresner, M. Nowakowski, R. Gross, and M. E. Zenilman. 2003. Sophorolipids block lethal effects of septic shock. J. Am. Coll. Surg. 197:S40-S41. [DOI] [PubMed] [Google Scholar]
- 16.Kreiss, J., E. Ngugi, K. Holmes, J. Ndinya-Achola, P. Waiyaki, P. L. Roberts, I. Ruminjo, R. Sajabi, J. Kimata, and T. R. Fleming. 1992. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA 268:477-482. [PubMed] [Google Scholar]
- 17.Maingault, M. December1997. Pharmaceutical and cosmetic compositions containing sophorolipids. Canada patent CAN 126,242,874.
- 18.Mauck, C., and G. Doncel. 2001. An update on vaginal microbicides. Curr. Infect. Dis. Rep. 3:561-568. [DOI] [PubMed] [Google Scholar]
- 19.Renner, R. 1997. European bans on surfactant trigger transatlantic debate. Environ. Sci. Technol. 31:A316-A320. [DOI] [PubMed] [Google Scholar]
- 20.Resnick, L., M. E. Busso, and R. C. Duncan. 1990. Anti-HIV screening technology, p. 311-325. In Heterosexual transmission of AIDS. Alan R. Liss, Inc., New York, N.Y.
- 21.Roddy, R. E., L. Zekeng, K. A. Ryan, U. Tamoufe, S. S. Weir, and E. L. Wong. 1998. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N. Engl. J. Med. 339:504-510. [DOI] [PubMed] [Google Scholar]
- 22.Savle, P. S., G. F. Doncel, S. D. Bryant, M. P. Hubieki, R. G. Robinette, and R. D. Gandour. 1999. Acylcarnitine analogues as topical, microbicidal spermicides. Bioorg. Med. Chem. Lett. 9:2545-2548. [DOI] [PubMed] [Google Scholar]
- 23.Scholz, C., S. Mehta, K. Bisht, V. Guilmanov, D. Kaplan, R. Nicolosi, and R. Gross. 1998. Bioactivity of extracellular glycolipids: investigation of potential anti-cancer activity of sophorolipids and sophorolipid-derivatives. Proc. Am. Chem. Soc. Polymer Preprints 39:168-169. [Google Scholar]
- 24.Senanayake, P. 1994. Contraception by the end of 20th century—the role of voluntary organizations. Hum. Reprod. 9(Suppl. 2):133-144. [DOI] [PubMed] [Google Scholar]
- 25.Singh, S. K., A. P. Felse, A. Nunez, T. A. Foglia, and R. A. Gross. 2003. Regioselective enzyme-catalyzed synthesis of sophorolipid esters, amides, and multifunctional monomers. J. Org. Chem. 68:5466-5477. [DOI] [PubMed] [Google Scholar]
- 26.Steiner, M. J., and W. Cates, Jr. 1997. Condoms and urinary tract infections: is nonoxynol-9 the problem or the solution? Epidemiology 8:612-614. [DOI] [PubMed] [Google Scholar]
- 27.Thiele, B., K. Gunther, and M. J. Schwuger. 1997. Alkylphenol ethoxylates: trace analysis and environmental behavior. Chem. Rev. 97:3247-3272. [DOI] [PubMed] [Google Scholar]
- 28.United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization. 2004. AIDS epidemic update 2004. The Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization. [Online] www.unaids.org.
- 29.Van Damme, L., V. Chandeyingm, G. Ramjeem, H. Rees, P. Sirivongrangson, M. Laga, J. Perriens, and the COL-1492 Phase II Study Group. 2000. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. AIDS 14:85-88. [DOI] [PubMed] [Google Scholar]
- 30.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, S. S. Karim, B. Masse, J. Perriens, M. Laga, and the COL-1492 Study Group. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 31.Voelker, R. 2005. Women shoulder growing HIV/AIDS burden. JAMA 293:281-282. [DOI] [PubMed] [Google Scholar]
- 32.Weir, S. S., R. E. Roddy, L. Zekeng, and P. J. Feldblum. 1995. Nonoxynol-9 use, genital ulcers, and HIV infection in a cohort of sex workers. Genitourin. Med. 71:78-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong, Y. L., M. P. Hubieki, C. L. Curfman, G. F. Doncel, T. C. Dudding, P. S. Savle, and R. D. Gandour. 2002. A structure-activity study of spermicidal and anti-HIV properties of hydroxylated cationic surfactants. Bioorg. Med. Chem. 10:3599-3608. [DOI] [PubMed] [Google Scholar]
- 34.Wood, B. L., G. F. Doncel, P. R. Reddy, and D. C. Sokal. 2003. Effect of diltiazem and methylene blue on human sperm motility, viability and cervical mucus penetration: potential use as vas irrigants at the time of vasectomy. Contraception 67:241-245. [DOI] [PubMed] [Google Scholar]
- 35.Yu, K., and Y. W. Chien. 1995. Spermicidal activity-structure relations of nonoxynol oligomers: physicochemical basis. Int. J. Pharmacol. 125:81-90. [Google Scholar]