Abstract
Melioidosis (infection caused by Burkholderia pseudomallei) requires a prolonged course of oral antibiotics following initial intravenous therapy to reduce the risk of relapse after cessation of treatment. The current recommendation is a four-drug regimen (trimethoprim [TMP], sulfamethoxazole [SMX], doxycycline, and chloramphenicol) and a total treatment time of 12 to 20 weeks. Drug side effects are common; the aim of this study was to compare the efficacy and tolerance of the four-drug regimen with a three-drug regimen (TMP-SMX and doxycycline). An open-label, randomized trial was conducted in northeast Thailand. A total of 180 adult Thai patients were enrolled, of which 91 were allocated to the four-drug regimen and 89 to the three-drug regimen. The trial was terminated early due to poor drug tolerance, particularly of the four-drug regimen. The culture-confirmed relapse rates at 1 year were 6.6% and 5.6% for the four- and three-drug regimens, respectively (P = 0.79). The three-drug regimen was better tolerated than the four-drug regimen; 36% of patients receiving four drugs and 19% of patients receiving three drugs required a switch in therapy due to side effects (P = 0.01). The duration of oral therapy was significantly associated with relapse; after adjustment for confounders, patients receiving less than 12 weeks of oral therapy had a 5.7-fold increase of relapse or death. A combination of TMP-SMX and doxycycline is as effective as and better tolerated than the conventional four-drug regimen for the oral treatment phase of melioidosis.
Melioidosis, the infection caused by Burkholderia pseudomallei, is endemic to southeast Asia and northern Australia. Large numbers of cases are seen in northeast Thailand, where it constitutes almost 20% of community-acquired bacteremia (2). An important feature of disease is that recurrence is common after completion of antibiotic treatment and apparent cure, occurring in 3 to 25% of patients. Molecular typing indicates that most cases of recurrence are due to recrudescence of the original infecting strain (relapse) (3, 8). Prolonged therapy has been demonstrated to reduce relapse. Current treatment recommendations include administration of intravenous antibiotics (ceftazidime or a carbapenem) for at least 10 days, followed by oral antibiotics for at least 12 weeks.
Our current oral regimen is a four-drug combination of trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, and chloramphenicol. This regimen is associated with high rates of adverse events, including gastrointestinal intolerance, anemia, and allergic reactions. In other centers, the use of a three-drug regimen or even TMP-SMX alone has been associated with low relapse rates (6, 7). Higher relapse rates have been found during clinical trials of amoxicillin-clavulanate with supplemental amoxicillin, doxycycline monotherapy, and a combination of azithromycin and ciprofloxacin (5, 6, 11). The aim of this study was to examine the efficacy and side effects associated with the four-drug (TMP-SMX, doxycycline, and chloramphenicol) regimen versus the three-drug (TMP-SMX and doxycycline) regimen.
MATERIALS AND METHODS
An open-label, randomized study was conducted at Sappasithiprasong Hospital, Ubon Ratchathani, northeast Thailand. The study protocol was approved by the Ethical Committee of the Ministry of Public Health, Royal Government of Thailand. Recruitment was undertaken between July 1998 and October 2002 during twice-daily rounds of the medical, surgical, and intensive care wards. Patients with suspected melioidosis were examined, and microbiological samples (blood, sputum, throat swab, urine, and pus) were obtained for bacterial culture. Patients were enrolled into the study if they were 15 years of age or older, had completed an intravenous course of appropriate antibiotics for culture-confirmed melioidosis, had provided written informed consent to participate, and agreed to outpatient follow-up for at least 20 weeks. Patients with mild disease who did not require intravenous antibiotics were also eligible. Exclusion criteria were being pregnant or lactating; known intolerance or allergy to TMP-SMX, doxycycline, or chloramphenicol; and infection with a strain of B. pseudomallei with in vitro resistance to doxycycline or chloramphenicol as determined by disk diffusion. Diabetes was defined by contemporary definitions (1). Renal impairment was defined as a creatinine level greater than 2.0 mg/dl prior to admission.
Randomization was performed by a computer-generated sequence and was balanced after each block of 10 patients. Sealed envelopes were prepared by a member of the research unit who was not involved in enrollment. These were opened by a member of the study team following a decision to start oral treatment. Patients were randomly allocated to receive TMP-SMX (8 mg TMP and 40 mg SMX/kg of body weight daily; maximum dose, 160 mg TMP and 800 mg SMX twice daily), doxycycline (4 mg/kg daily; maximum dose, 100 mg twice daily), and chloramphenicol (40 mg/kg daily; maximum dose, 500 mg four times daily for the first 4 weeks of oral therapy) or TMP-SMX and doxycycline without chloramphenicol at the same doses. All study drugs were manufactured either by Thai pharmaceutical companies or the Government Pharmaceutical Organization and were provided free to study patients. Minimum duration of oral treatment was 12 weeks, but total duration was based on clinical history and the course of illness. Patients that had evidence of deep-seated infection (such as undrained visceral abscesses or infections involving bones or joints) or those with more severe disease on presentation tended to receive longer courses of antibiotics. Conversely, patients with mild disease, such as infections involving skin and soft tissue, or patients with parotid abscesses tended to receive shorter courses of antibiotics. Duration of antibiotic treatment was determined by a single clinician with extensive experience in the clinical management of melioidosis (W. Chaowagul), who undertook outpatient review within 4 weeks after discharge and periodically thereafter based on clinical progress.
Follow-up was continued until July 2004. Patients were primarily monitored through the outpatient department. Nonattendees were sent letters inquiring about their subsequent clinical course and visited at home by the study team if necessary.
Patients who failed to attend clinic appointments were deemed to have ceased their medications when last reviewed in the outpatient department. Recurrence of disease was treated as for the primary episode, with readmission for investigation and intensive intravenous therapy if required, followed by the four-drug conventional combination. Amoxicillin-clavulanate with supplemental amoxicillin was used for patients who developed suspected drug allergies.
The primary outcome measure was culture-confirmed relapse or time to death attributable to melioidosis. “Clinical relapse” was also determined; this was defined as possible relapse characterized by clinical features compatible with melioidosis that was treated with antibiotics active against B. pseudomallei but was culture negative. Mortality was categorized as attributable to primary infection or relapse or attributable to other causes.
Time to outcome was measured as the start of oral treatment to relapse or attributable death. Censoring events were death from causes other than culture-confirmed or clinical relapse and loss to follow-up. A comparison of adverse drug events was made between each group.
The original study design called for 226 patients to achieve an 80% power to detect a difference in relapse rates of 25% and 10% at the 0.05 significance level. All analyses were done on an intention-to-treat basis and then repeated per protocol. In the per-protocol analyses, patients who switched treatment to the other study arm were reallocated according to the final treatment they received. In this analysis, patients who changed treatment regimens to antibiotics that did not include a study regimen were not reallocated.
Statistical tests were performed using the statistical program STATA/SE, version 8.0 (StataCorp LP, College Station, Tex.). Comparisons of continuous data were performed using a Student's t test or the Mann Whitney U test where appropriate. Proportions were compared using Fisher's exact test. Time-to-event endpoints were compared using the log-rank test or Wilcoxon test as appropriate and were depicted graphically using a Kaplan-Meier graph. A Cox proportional hazards model was used to adjust the treatment effect for potential confounding factors; the following variables were considered in the forward-variable selection procedure: age, distribution of infection, initial use of ceftazidime, history of chronic renal failure, diabetes mellitus, and failure to complete at least 12 weeks of therapy for analysis of relapse after cessation of oral treatment. The final Cox proportional hazards models were reanalyzed with TMP-SMX resistance as a variable to explore its effect.
Susceptibility to ceftazidime, imipenem, chloramphenicol, doxycycline, and amoxicillin-clavulanate was performed by the Kirby-Bauer disk diffusion test. As TMP-SMX susceptibility testing by this method is not reliable and testing for MICs was not in routine use at this time (10), test results were not reported to clinicians during this trial. Susceptibility testing using the Etest (AB Biodisk) was retrospectively performed on isolates stored at −70°C in trypticase soy broth with 15% glycerol using NCCLS methodology and breakpoints for B. pseudomallei (susceptible, ≤2/38 μg trimethoprim-sulfamethoxazole; resistant, >4/76 μg) (9, 12).
RESULTS
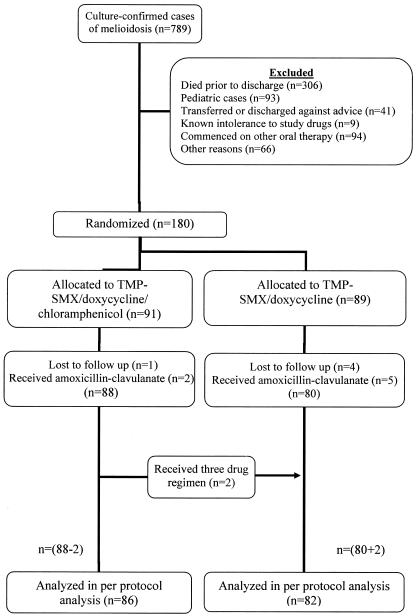
A total of 789 patients presented to Sappasithiprasong Hospital with culture-confirmed melioidosis between July 1998 and October 2002. The number of patients included at each stage of the study is illustrated in Fig. 1. The decision was taken to terminate the trial early after enrollment of 180 patients, since the level of drug intolerance, particularly to the four-drug regimen, was very high. A post hoc power calculation suggested that the power to detect a difference in relapse rates of 25% and 10% at the 0.05 significance level was reduced to 69%. Baseline characteristics of the study patients are shown in Table 1. These were similar, and most of the patients were treated with ceftazidime as initial intravenous therapy. Two patients in the three-drug group had mild disease and did not receive intravenous therapy.
FIG. 1.
Flow chart illustrating patients included at each stage of the study.
TABLE 1.
Admission details at randomization
| Variable | No. of patients positive with the four-drug regimen (n = 91) | No. of patients positive with the three-drug regimen (n = 89) | Significance |
|---|---|---|---|
| Male | 53 (58.2%) | 58 (65.2%) | 0.34 |
| Age (yr) (median, range) | 47 (15-70) | 47 (16-74) | 0.79 |
| Rice farmer | 77 (84.6%) | 72 (80.9%) | 0.51 |
| Diabetes mellitus | 40 (44.0%) | 35 (39.3%) | 0.53 |
| Chronic renal impairment | 2 (2.2%) | 5 (5.6%) | 0.28 |
| Blood culture positive | 41 (45.1%) | 41 (46.1%) | 0.89 |
| Distribution of infectiona | 0.87 | ||
| Disseminated | 20 (22.0%) | 21 (23.6%) | |
| Septicemic | 21 (23.1%) | 19 (21.3%) | |
| Multifocal | 9 (9.9%) | 12 (13.5%) | |
| Localized | 41 (45.1%) | 37 (41.6%) | |
| First intravenous drug received | 0.12 | ||
| Ceftazidime | 86 (94.5%) | 81 (91.0%) | |
| Imipenem | 1 (1.1%) | 2 (2.2%) | |
| Amoxicillin-clavulanate | 4 (4.4%) | 4 (4.5%) | |
| None | 0 | 2 (2.2%) | |
| Duration of intravenous therapy (days, range) | 13 (1-50) | 13 (0-56) | 0.79 |
Disseminated disease was defined as blood culture positive plus ≥2 organs involved; septicemic, blood culture positive +1 or no organ involved; multifocal, ≥2 organs involved but blood culture negative; localized, 1 organ involved and blood culture negative.
The median duration of follow-up of patients without relapse was 45 weeks, with a total duration of follow-up of 11,691 patient weeks. Similar proportions of patients in both groups were lost to follow-up before the end of the study. The median duration of the allocated oral treatment in patients was longer in the three-drug group (10.1 weeks versus 3.7 weeks; P = 0.046). The total duration of oral treatment including the subsequent regimens after cessation of the allocated regimen was similar (19 versus 17 weeks; P = 0.21) (Table 2).
TABLE 2.
Patient outcomes
| Variable | No. of patients positive with the four-drug regimen (n = 91) | No. of patients positive with the three-drug regimen (n = 89) | P value |
|---|---|---|---|
| Relapse to end of follow-up period | |||
| Culture confirmed | 9 (9.9%) | 7 (7.9%) | 0.63 |
| Clinical | 9 (9.9%) | 7 (7.9%) | 0.63 |
| Overall | 18 (19.8%) | 14 (15.7%) | 0.48 |
| Died | 10 (11.0%) | 12 (13.5%) | 0.61 |
| Attributable to primary or relapse infection | 9 (9.9%) | 9 (10.1%) | 0.96 |
| Other cause | 0 | 3a (3.4%) | 0.12 |
| Unknown | 1 (1.1%) | 0 | 1.00 |
| Switch in treatment | 48 (52.7%) | 22 (24.7%) | <0.001 |
| Due to adverse drug event | 33 (36.3%) | 17 (19.1%) | 0.01 |
| Due to other reasons | 15 (16.5%) | 5 (5.6%) | 0.02 |
| Follow-up of less than 3 months | 14 (15.4%) | 22 (24.7%) | 0.12 |
| Duration of allocated regimen, median (IQR)b | 3.7 (1.9-18.1) | 10.1 (2.3-19.6) | 0.046 |
| ≤12 weeks | 43 (47.3%) | 47 (52.8%) | 0.46 |
| 12 to 20 weeks | 36 (39.6%) | 28 (31.5%) | 0.26 |
| >20 weeks | 12 (13.2%) | 14 (15.7%) | 0.63 |
| Duration of total oral treatment, median (IQR) | 19 (13.3-20.3) | 17 (4.0-20.6) | 0.17 |
| ≤12 weeks | 23 (25.3%) | 33 (37.1%) | 0.09 |
| 12 to 20 weeks | 41 (45.1%) | 32 (36.0%) | 0.21 |
| >20 weeks | 27 (29.7%) | 24 (27.0%) | 0.69 |
Death due to human immunodeficiency virus coinfection (1), heart disease (1), and non-melioidosis postoperative wound infection (1).
IQR, interquartile range.
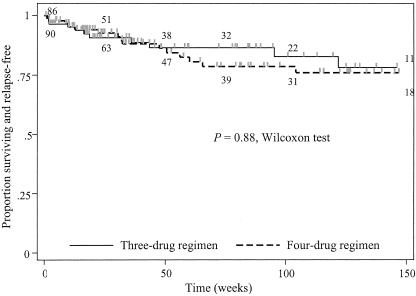
Relapse was analyzed from the start of therapy to take account of cases that developed early relapse following an initial response to treatment. Results of patient outcome are shown in Table 2. The culture-confirmed relapse rates at 1 year were 6.6% and 5.6% for the four- and three-drug regimens, respectively (P = 0.79). Case fatality for the three-drug and four-drug groups was 14% and 11%, respectively; this was equal when considering mortality attributable to infection (10.1% versus 9%; P = 1.00). A Kaplan-Meier graph for time to relapse and/or death from melioidosis during follow-up is shown in Fig. 2. There were no differences between the three-drug group and the four-drug group at 1 year (P = 0.96, Wilcoxon test), at 1.5 years (P = 0.79, Wilcoxon test), or at 2 years (P = 0.86, Wilcoxon test), respectively.
FIG. 2.
Kaplan-Meier plot analysis illustrating time to death or culture-confirmed relapse after start of oral therapy for the four-drug versus three-drug groups. Numbers in brackets represent the number of patients at risk at 0.5, 1, 1.5, 2, and 3 years from left to right, respectively (the upper row shows the three-drug group and the lower row shows the four-drug group).
In a Cox regression analysis, age was found to be significantly associated with the risk of treatment failure (hazard ratio [HR] = 1.05; 95% confidence interval [CI], 1.01 and 1.08; P = 0.01). In the final model, treatment allocation was not significantly associated with outcome (HR = 1.11; 95% CI, 0.50 and 2.47; P = 0.80 for the four-drug group compared to the three-drug group).
The culture-confirmed relapse rate for 124 patients who received a total of at least 12 weeks of oral therapy was 11.8% in the four-drug group and 5.4% in the three-drug group (P = 0.296, log-rank test). In a multiple-variable Cox proportional hazards model, failure to complete at least 12 weeks of therapy was associated with a shorter time to relapse or death (HR = 5.70; 95% CI, 2.61 and 12.45; P < 0.001). Adjusting for these predictors, the allocated treatment arm was not associated with outcome (HR = 1.28; 95% CI, 0.58 and 2.85; P = 0.54).
There were nine protocol violations, as follows. Amoxicillin-clavulanate with supplemental amoxicillin was substituted for the allocated treatment in seven patients (two patients allocated to four drugs and five patients to three drugs). Two patients allocated to receive four-drug therapy received the three-drug regimen; these were included in the analysis. In a per-protocol analysis, culture-confirmed relapse was not significantly different between the two groups (8.1% in the four-drug group versus 9.8% in the three-drug group; P = 0.47, log-rank test).
A total of 169 B. pseudomallei isolates were available for susceptibility testing. Of these, 9 out of 82 (11%) in the four-drug group and 7 out of 87 (8.1%) in the three-drug group were resistant to TMP-SMX by Etest (P = 0.52). In a multiple-variable Cox proportional hazards model adjusting for allocated regimen, age, and failure to complete 12 weeks of therapy, TMP-SMX resistance was not statistically associated with shorter relapse-free duration from the commencement of oral therapy (HR = 2.05; 95% CI, 0.44 and 9.62; P = 0.36) or the cessation of oral therapy (HR = 1.62; 95% CI, 0.20 and 13.02; P = 0.65).
In total, 63 patients required a change in treatment, of which 48 (53%) were in the four-drug group and 22 (25%) in the three-drug group (P < 0.001) (Table 2). Patients randomized to receive four drugs switched treatment to amoxicillin-clavulanate (n = 23) or three-drug therapy (n = 25). Patients randomized to the three-drug therapy switched treatment to amoxicillin-clavulanate (n = 17), TMP-SMX (n = 4), or doxycycline (n = 1). A higher proportion of patients reported adverse drug events in the four-drug group (Table 3) and switched therapy within the first four weeks: 42 out of 48 (87.5% in the four-drug group) versus 13 out of 22 (59.1% in the three-drug group; P = 0.007). Gastrointestinal intolerance and anemia were reported at lower rates in the three-drug group. Allergic phenomena were similar in both groups. In the four-drug group, chloramphenicol was ceased prior to 4 weeks in 41 patients (45%).
TABLE 3.
Reported adverse events
| Event | No. (%) with four-drug regimen (n = 91) | No. (%) with three-drug regimen (n = 89) | Significance |
|---|---|---|---|
| Nausea, vomiting, or abdominal pain | 14 (15.4%) | 6 (6.7%) | 0.07 |
| Rash or other allergic reaction | 9 (9.9%) | 8 (9.0%) | 0.84 |
| Photosensitivity | 6 (6.6%) | 3 (3.4%) | 0.50 |
| Anemia | 7 (7.7%) | 1 (1.1%) | 0.06 |
| Angular stomatitis | 3 (3.3%) | 0 | 0.25 |
| Anorexia | 3 (3.3%) | 0 | 0.25 |
| Chest discomfort | 1 (1.1%) | 0 | 1.00 |
| Dry mouth | 1 (1.1%) | 0 | 1.00 |
| Seizure | 1 (1.1%) | 0 | 1.00 |
| Azotemia | 0 | 1 (1.1%) | 0.49 |
| Other complications | 4 (4.4%) | 3 (3.4%) | 1.00 |
| Any adverse event | 37 (40.7%) | 20 (22.5%) | 0.009 |
DISCUSSION
This six-year trial is the largest study of eradication therapy for melioidosis reported to date. The data presented suggest that the 4-week chloramphenicol component of the four-drug regimen is associated with significant intolerance. The inability to adhere to this regimen limited our ability to draw conclusions regarding the relative efficacy of four drugs versus three drugs. Given these limitations, it is not surprising that relapse rates were similar in both groups.
Other alternatives to the four-drug regimen have been explored previously. A ciprofloxacin-azithromycin regimen and doxycycline monotherapy have both been associated with relapse rates exceeding 20% in clinical trials in Thailand (5, 6). In one of these trials the comparator arm was the three-drug regimen in which only one relapse was recorded in 33 patients that received this regimen (relapse rate, 3%; 95% CI, 0 and 16%) (6). Quinolone monotherapy was associated with a relapse rate of 29% in an uncontrolled study (4). Amoxicillin-clavulanate with supplemental amoxicillin was associated with a higher relapse rate than the four-drug conventional therapy in 101 patients, although poor adherence to the 20-week course of therapy appeared to be a more important factor than antibiotic regimen (11). Factors previously demonstrated to be associated with relapse include eradication regimens of less than 12 weeks, severity of disease, and the use of amoxicillin-clavulanate in place of the intensive intravenous phase (3).
Higher rates of culture-positive relapse were seen by the end of this trial compared with previous studies. This may be explained in part by the longer follow-up (up to 5.5 years) and larger sample size (two to three times) in this study compared to those reported previously, our active surveillance for follow-up, and the poor tolerance to the regimens generally. When the analysis time was limited to 52 weeks, the overall relapse rate was similar to that of previous trials (6% compared to 2 to 4%) (5, 6, 11). These findings reinforce the need for ongoing follow-up as relapse may occur many years after initial infection (8).
A high rate of TMP-SMX resistance in clinical isolates was demonstrated in this study. Testing for TMP-SMX has been problematic; testing by the Kirby Bauer disk diffusion technique has tended to overestimate the rates of resistance (10). We have recently reported the results of TMP-SMX susceptibility testing by Etest for a large number of isolates stored at our center, including those from patients in this study (12). The high rate of resistance to TMP-SMX has clear implications for a potential future trial of TMP-SMX alone in this region. No major differences were seen in patients infected with TMP-SMX-resistant isolates, but this study was underpowered to examine the effect of resistance on relapse rates reflected in the wide confidence intervals in the estimate.
There are several limitations to this open-label trial. Awareness of the treatment received may have influenced reporting of adverse events, although it is unlikely to have influenced the more objective endpoint of culture-confirmed relapse. A significant proportion of patients were lost to follow-up, highlighting the difficulty in monitoring long-term outcome in this primarily rural population. The high rate of switching of drug regimens reflects the poor tolerance of both regimens. Adherence to therapy was not formally assessed by pill counts or drug levels.
This study suggests that the three-drug regimen is better tolerated and is as effective as the four-drug regimen for oral eradication therapy of melioidosis in adults. However, both regimens in this study were associated with significant rates of intolerance. As tolerance is a key factor in promoting adherence to prolonged antibiotic therapy, it is likely that the use of simpler therapies are likely to improve outcomes. TMP-SMX alone is used for eradication therapy in Australia (7); a prospective, multicenter randomized trial is now planned to compare the efficacy and side effect profile of the three-drug regimen versus TMP-SMX alone.
Acknowledgments
We thank the director of Sappasithiprasong Hospital, the medical and nursing staff of the Medical Department, and the outpatient department and the staff of the Microbiology Laboratory, especially Nittaya Teerawattanasook. We also thank Nongluk Getchalarat, Premjit Amornchai, Gumphol Wongsuvan, Sayan Langla, and Jintana Suwannapruk for their continued support. Arjen Dondorp, Anna Checkley, and Tihana Bicanic also participated in the enrollment of patients into this study.
S.J.P. is supported by a Wellcome Trust Career Development Award in Clinical Tropical Medicine, and A.C. was supported by an Australian National Health and Medical Research Council Training Scholarship. This study was part of the Wellcome Trust-Mahidol University-Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of Great Britain.
REFERENCES
- 1.Anonymous. 1997. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183-1197. [DOI] [PubMed] [Google Scholar]
- 2.Chaowagul, W., N. J. White, D. A. Dance, Y. Wattanagoon, P. Naigowit, T. M. Davis, S. Looareesuwan, and N. Pitakwatchara. 1989. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J. Infect. Dis. 159:890-899. [DOI] [PubMed] [Google Scholar]
- 3.Chaowagul, W., Y. Suputtamongkol, D. A. Dance, A. Rajchanuvong, J. Pattara-Arechachai, and N. J. White. 1993. Relapse in melioidosis: incidence and risk factors. J. Infect. Dis. 168:1181-1185. [PubMed] [Google Scholar]
- 4.Chaowagul, W., Y. Suputtamongkul, M. D. Smith, and N. J. White. 1997. Oral fluoroquinolones for maintenance treatment of melioidosis. Trans. R Soc. Trop. Med. Hyg. 91:599-601. [DOI] [PubMed] [Google Scholar]
- 5.Chaowagul, W., A. J. Simpson, Y. Suputtamongkol, M. D. Smith, B. J. Angus, and N. J. White. 1999. A comparison of chloramphenicol, trimethoprim-sulfamethoxazole, and doxycycline with doxycycline alone as maintenance therapy for melioidosis. Clin. Infect. Dis. 29:375-380. [DOI] [PubMed] [Google Scholar]
- 6.Chetchotisakd, P., W. Chaowagul, P. Mootsikapun, D. Budhsarawong, and B. Thinkamrop. 2001. Maintenance therapy of melioidosis with ciprofloxacin plus azithromycin compared with cotrimoxazole plus doxycycline. Am. J. Trop. Med. Hyg. 64:24-27. [DOI] [PubMed] [Google Scholar]
- 7.Currie, B. J., D. A. Fisher, D. M. Howard, J. N. Burrow, D. Lo, S. Selva-Nayagam, N. M. Anstey, S. E. Huffam, P. L. Snelling, P. J. Marks, D. P. Stephens, G. D. Lum, S. P. Jacups, and V. L. Krause. 2000. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin. Infect. Dis. 31:981-986. [DOI] [PubMed] [Google Scholar]
- 8.Currie, B. J., D. A. Fisher, N. M. Anstey, and S. P. Jacups. 2000. Melioidosis: acute and chronic disease, relapse and re-activation. Trans. R Soc. Trop. Med. Hyg. 94:301-304. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing; 14th informational supplement. NCCLS document M100-S14. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Piliouras, P., G. C. Ulett, C. Ashhurst-Smith, R. G. Hirst, and R. E. Norton. 2002. A comparison of antibiotic susceptibility testing methods for cotrimoxazole with Burkholderia pseudomallei. Int. J. Antimicrob. Agents 19:427-429. [DOI] [PubMed] [Google Scholar]
- 11.Rajchanuvong, A., W. Chaowagul, Y. Suputtamongkol, M. D. Smith, D. A. Dance, and N. J. White. 1995. A prospective comparison of co-amoxiclav and the combination of chloramphenicol, doxycycline, and co-trimoxazole for the oral maintenance treatment of melioidosis. Trans. R Soc. Trop. Med. Hyg. 89:546-549. [DOI] [PubMed] [Google Scholar]
- 12.Wuthiekanun, V., A. C. Cheng, W. Chierakul, P. Amornchai, D. Limmathurotsakul, W. Chaowagul, A. J. Simpson, J. M. Short, G. Wongsuvan, B. Maharjan, N. J. White, and S. J. Peacock. 2005. Trimethoprim/sulfamethoxazole resistance in clinical isolates of Burkholderia pseudomallei. J. Antimicrob. Chemother. 55:1029-1031. [DOI] [PubMed] [Google Scholar]