Abstract
We cloned a gene, abeM, for a multidrug efflux pump from Acinetobacter baumannii using Escherichia coli as the host. Sequence analysis revealed that AbeM is a member of the MATE family of pumps. AbeM was found to be an H+-coupled multidrug efflux pump and a unique member of the MATE family.
Acinetobacter spp. are emerging as a major cause of nosocomial infections or in outbreaks of cross-infection, particularly in intensive care units, where the use of antimicrobial agents is greatest and the host is most susceptible. Acinetobacter baumannii is one of the Acinetobacter spp. that is the most frequently isolated from human infections (3). Certain strains of A. baumannii are now resistant to many commonly prescribed antibiotics (including imipenem and fluoroquinolones), and the multiple-drug resistance is often responsible for the failure of antibiotic therapy.
Among the drug resistance mechanisms in A. baumannii, degradation of drugs seems to be a major cause because of the production of relevant enzymes in this organism (1, 13, 18, 24). Modification of drugs is also common in this organism due to the synthesis of specific modifying enzymes (19, 20). Alterations of the drug target due to mutations in gyrA and parC have been associated with high levels of resistance to fluoroquinolones (21, 22). Recently, decreased expression of the outer membrane proteins has been reported to be involved in resistance to some drugs (6, 7, 12). Studies of drug resistance mediated by an efflux pump(s) are very few; only one efflux system, AdeABC, that mediates multidrug resistance has been identified and studied in detail in this organism (9, 14). Here we report on the gene cloning and biochemical characterization of a multidrug efflux pump from A. baumannii.
Gene cloning.
A. baumannii ATCC 19606 (generously provided by Shigeo Yamamoto, Okayama University) and Escherichia coli KAM32 which lacks the major multidrug efflux pumps AcrAB and YdhE (5) were used in this study. To identify a putative multidrug efflux transporter(s) in A. baumannii, we tried to clone the gene(s) responsible for multidrug resistance. Fragments of chromosomal DNA from strain ATCC 19606 were prepared and ligated to a vector plasmid, pBR322. The resulting recombinant plasmids that were obtained (five candidates) enabled E. coli KAM32 cells to grow in the presence of 10 μg/ml of ethidium bromide. We chose one of the plasmids, pABE6, and constructed several deletion plasmids. The shortest deletion plasmid that conferred resistance to ethidium bromide, pABE618, was further analyzed. As a result of DNA sequencing, we found one open reading frame, designated abeM. Several promoter-like sequences and a Shine-Dalgarno sequence preceded the abeM gene. The deduced AbeM protein consists of 448 amino acid residues, is very rich in hydrophobic amino acid residues, and possesses 12 hydrophobic regions.
Recently, the complete genome sequence of Acinetobacter ADP1 was published (2). The AbeM protein showed sequence homology (79% identity, 94% similarity) with the sequence of ACIAD0429, which was estimated to be a NorM homolog in this organism. A search for protein sequence similarity by using the BLAST program (National Center for Biotechnology Information) showed that AbeM shared 39, 39, 37, 37, and 34% identities and 77, 76, 76, 75, and 75% similarities with PmpM of Pseudomonas aeruginosa PAO1 (8), VcmA of Vibrio cholerae non-O1 (11), YdhE of E. coli, NorM of Vibrio parahaemolyticus (17), and HmrM of Haemophilus influenzae (23), respectively. Thus, it is highly likely that AbeM is a member of the MATE family of multidrug efflux pumps (4).
Substrate specificity of AbeM.
The substrate specificity of AbeM was investigated by comparing the susceptibilities of E. coli KAM32/pABE618 (which carries abeM) and E. coli KAM32/pUC18 (control) to various antimicrobial agents. As shown in Table 1, significant increases (more than fourfold) in the MICs were observed for norfloxacin, ofloxacin, ciprofloxacin, gentamicin, 4′,6-diamino-2-phenylindol (DAPI), triclosan, acriflavine, Hoechst 33342, daunorubicin, doxorubicin, rhodamine 6G, and ethidium bromide, whereas reproducible twofold increases were observed for kanamycin, erythromycin, chloramphenicol, tetraphenylphosphonium chloride (TPPCl), and trimethoprim. The hydrophilic fluoroquinolones, such as norfloxacin and ciprofloxacin, appeared to be better substrates for the AbeM pump than the hydrophobic fluoroquinolone such as ofloxacin.
TABLE 1.
Susceptibilities of transformants to antimicrobial agents
| Antimicrobial class and antimicrobial agent | MIC (μg/ml)
|
Relative resistancea | |
|---|---|---|---|
| E. coli KAM32/pUC18 | E. coli KAM32/pABE618 | ||
| Aminoglycosides | |||
| Kanamycin | 1 | 2 | 2 |
| Gentamicin | 0.25 | 1 | 4 |
| Quinolones | |||
| Norfloxacin | 0.03 | 1 | 32 |
| Ofloxacin | 0.015 | 0.06 | 4 |
| Ciprofloxacin | <0.008 | 0.125 | 16 |
| β-Lactam, imipenem | 0.25 | 0.25 | 1 |
| Erythromycin | 4 | 8 | 2 |
| Tetracycline | 0.5 | 0.5 | 1 |
| Chloramphenicol | 1 | 2 | 2 |
| Others | |||
| SDSb | >128 | >128 | 1 |
| DAPI | 0.5 | 8 | 16 |
| TPPCl | 8 | 16 | 2 |
| Triclosan | 0.5 | 2 | 4 |
| Acriflavine | 2 | 32 | 16 |
| Hoechst 33342 | 0.5 | 32 | 64 |
| Daunorubicin | 4 | 64 | 16 |
| Doxorubicin | 1 | 128 | 128 |
| Trimethoprim | 0.125 | 0.25 | 2 |
| Rhodamine 6G | 8 | 64 | 8 |
| Ethidium bromide | 4 | 128 | 32 |
Relative resistance: MIC in E. coli harboring pABE618/MIC in E. coli harboring pUC18.
SDS, sodium dodecyl sulfate.
Efflux of ethidium.
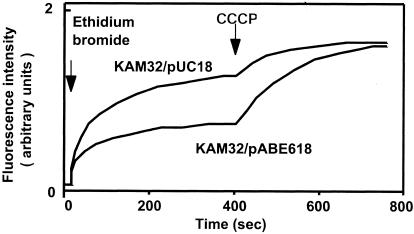
To confirm that AbeM is really a drug efflux pump, we measured the transport of drug in cells of E. coli KAM32/pABE618 and E. coli KAM32/pUC18. We measured the levels of accumulation of ethidium bromide as described previously (23) and observed that the level of accumulation of ethidium bromide in cells of E. coli KAM32/pABE618 was remarkably less than that in cells of KAM32/pUC18 under energized conditions (Fig. 1). With the addition of a protonophore, carbonyl cyanide m-chlorophenyhydrazone (CCCP), the levels of accumulation of ethidium in the cells became higher and reached a plateau. The plateau levels in the two strains were the same. These results indicate that energy-dependent ethidium efflux activity in cells of E. coli KAM32/pABE618 is much stronger than that in cells of E. coli KAM32/pUC18.
FIG. 1.
Transport of ethidium bromide via the AbeM pump. The accumulation of ethidium bromide in cells is shown. Cells of E. coli KAM32/pUC18 (control) or E. coli KAM32/pABE618 (carrying abeM) were grown in Luria-Bertani medium containing 100 μg/ml of ampicillin and suspended in modified Tanaka medium (sodium salts were replaced with potassium salts). After preincubation at 37°C for 5 min, ethidium bromide (final concentration, 10 μM) was added to initiate drug accumulation, and then a proton conductor (CCCP; final concentration, 100 μM) was added at the time point indicated by the arrow to deenergize the membrane. The increase in fluorescence indicates the accumulation of ethidium bromide in the cells.
It seemed that AbeM is a member of the MATE family of transporters. An electrochemical potential of Na+ is the driving force for the MATE family of transporters, such as NorM, YdhE, VmrA, VcmA, VcrM, and HmrM (5, 10, 11, 16, 17, 23). On the other hand, a recent study in our laboratory revealed that a multidrug efflux pump, PmpM, from Pseudomonas aeruginosa belongs to the MATE family and is an H+-coupled efflux pump (8). Thus, we investigated whether AbeM is either an Na+-coupled pump or an H+-coupled pump. First, we tested the effects of NaCl, LiCl, and KCl (control) on ethidium efflux. However, addition of NaCl or LiCl to the assay mixture at 1 to 100 mM did not give any significant effect (data not shown). These results suggest that AbeM is not an Na+-driven pump but is an H+-driven drug efflux pump.
We also examined whether drug influx could elicit Na+ efflux via the AbeM pump using an Na+ electrode (16). However, we were unable to detect Na+ efflux (data not shown). Thus, we conclude that AbeM is not an Na+-coupled pump.
H+-drug antiport mediated by AbeM.
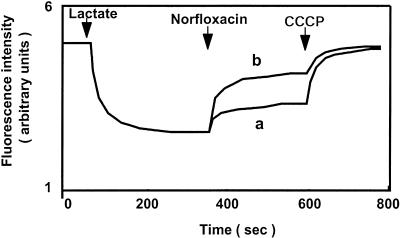
If the coupling cation in AbeM is an H+, then it must be an H+-drug antiporter. One convenient method that can be used to test for this possibility is to measure the flux of H+ caused by the addition of a substrate of the pump. We measured H+ flux by measuring the changes in fluorescence quenching in everted membrane vesicles, as described previously (15). Addition of norfloxacin to the assay mixture containing vesicles from E. coli KAM32/pABE618 elicited a much larger efflux of H+ (dequenching of the fluorescence) compared with that from vesicles from E. coli KAM32/pUC18 (Fig. 2). This indicates that H+-norfloxacin antiport via AbeM took place. Thus, we conclude that the coupling ion in the AbeM pump is H+ and not Na+. It should be pointed out that some H+-norfloxacin antiport activity is present in vesicles of E. coli KAM32/pUC18.
FIG. 2.
H+-norfloxacin antiport via AbeM. H+-norfloxacin antiport was measured in everted membrane vesicles prepared from cells of E. coli KAM32/pUC18 (control) (curve a) and E. coli KAM32/pABE618 (carrying abeM) (curve b). The formation and dissipation of the H+ gradient across vesicle membrane, which reflect the influx and the efflux of H+, respectively, were monitored by measuring the changes in fluorescence intensity of quinacrine. At the time points indicated by arrows, potassium d,l-lactate (final concentration, 5 mM) was added to initiate respiration. After the fluorescence quenching reached a steady-state level, norfloxacin (final concentration, 5 μM) was added to the assay mixture, and then CCCP (final 50 μM) was added to collapse the H+ gradient.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession number AB204810.
Acknowledgments
We thank M. Varela of Eastern New Mexico University for critical reading of the manuscript prior to submission.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Afzal-Shah, M., N. Woodford, and D. M. Livermore. 2001. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D beta-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 45:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbe, V., D. Vallenet, N. Fonknechten, A. Kreimeyer, S. Oztas, L. Labarre, S. Cruveiller, C. Robert, S. Duprat, P. Wincker, L. N. Ornston, J. Weissenbach, P. Marliere, G. N. Cohen, and C. Medigue. 2004. Unique features revealed by the genome sequence of Acinetobacter sp. ADP1, a versatile and naturally transformation competent bacterium. Nucleic Acids Res. 32:5766-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, M. H., I. T. Paulsen, and R. A. Skurray. 1999. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 31:394-395. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J., Y. Morita, M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2002. VmrA, a member of a novel class of Na+-coupled multidrug efflux pumps from Vibrio parahaemolyticus. J. Bacteriol. 184:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, R. B. 1996. Imipenem resistance among Acinetobacter baumannii: association with reduced expression of a 33-36 kDa outer membrane protein. J. Antimicrob. Chemother. 38:245-251. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Cuenca, F., L. Martinez-Martinez, M. C. Conejo, J. A. Ayala, E. J. Perea, and A. Pascual. 2003. Relationship between beta-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 51:565-574. [DOI] [PubMed] [Google Scholar]
- 8.He, G. X., T. Kuroda, T. Mima, Y. Morita, T. Mizushima, and T. Tsuchiya. 2004. An H+-coupled multidrug efflux pump, PmpM, a member of the MATE family of transporters, from Pseudomonas aeruginosa. J. Bacteriol. 186:262-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins, P. G., H. Wisplinghoff, D. Stefanik, and H. Seifert. 2004. Selection of topoisomerase mutations and overexpression of adeB mRNA transcripts during an outbreak of Acinetobacter baumannii. J. Antimicrob. Chemother. 54:821-823. [DOI] [PubMed] [Google Scholar]
- 10.Huda, M. N., J. Chen, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Gene cloning and characterization of VcrM, a Na+-coupled multidrug efflux pump, from Vibrio cholerae non-O1. Microbiol. Immunol. 47:419-427. [DOI] [PubMed] [Google Scholar]
- 11.Huda, M. N., Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2001. Na+-driven multidrug efflux pump VcmA from Vibrio cholerae non-O1, a non-halophilic bacterium. FEMS Microbiol. Lett. 203:235-239. [DOI] [PubMed] [Google Scholar]
- 12.Limansky, A. S., M. A. Mussi, and A. M. Viale. 2002. Loss of a 29-kilodalton outer membrane protein in Acinetobacter baumannii is associated with imipenem resistance. J. Clin. Microbiol. 40:4776-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Otsoa, F., L. Gallego, K. J. Towner, L. Tysall, N. Woodford, and D. M. Livermore. 2002. Endemic carbapenem resistance associated with OXA-40 carbapenemase among Acinetobacter baumannii isolates from a hospital in northern Spain. J. Clin. Microbiol. 40:4741-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnet, S., P. Courvalin, and T. Lambert. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45:3375-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mine, T., Y. Morita, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1998. Evidence for chloramphenicol/H+ antiport in Cmr (MdfA) system of Escherichia coli and properties of the antiporter. J. Biochem. (Tokyo) 124:187-193. [DOI] [PubMed] [Google Scholar]
- 16.Morita, Y., A. Kataoka, S. Shiota, T. Mizushima, and T. Tsuchiya. 2000. NorM of Vibrio parahaemolyticus is an Na+-driven multidrug efflux pump. J. Bacteriol. 182:6694-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita, Y., K. Kodama, S. Shiota, T. Mine, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1998. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 42:1778-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poirel, L., O. Menuteau, N. Agoli, C. Cattoen, and P. Nordmann. 2003. Outbreak of extended-spectrum beta-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 41:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seward, R. J., T. Lambert, and K. J. Towner. 1998. Molecular epidemiology of aminoglycoside resistance in Acinetobacter spp. J. Med. Microbiol. 47:455-462. [DOI] [PubMed] [Google Scholar]
- 20.Vila, J., A. Marcos, F. Marco, S. Abdalla, Y. Vergara, R. Reig, R. Gomez-Lus, and T. Jimenez de Anta. 1993. In vitro antimicrobial production of beta-lactamases, aminoglycoside-modifying enzymes, and chloramphenicol acetyltransferase by and susceptibility of clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 37:138-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vila, J., J. Ruiz, P. Goni, and T. Jimenez de Anta. 1997. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J. Antimicrob. Chemother. 39:757-762. [DOI] [PubMed] [Google Scholar]
- 22.Wisplinghoff, H., M. Decker, C. Haefs, O. Krut, G. Plum, and H. Seifert. 2003. Mutations in gyrA and parC associated with resistance to fluoroquinolones in epidemiologically defined clinical strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 51:177-180. [DOI] [PubMed] [Google Scholar]
- 23.Xu, X. J., X. Z. Su, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Molecular cloning and characterization of the HmrM multidrug efflux pump from Haemophilus influenzae Rd. Microbiol. Immunol. 47:937-943. [DOI] [PubMed] [Google Scholar]
- 24.Yum, J. H., K. Yi, H. Lee, D. Yong, K. Lee, J. M. Kim, G. M. Rossolini, and Y. Chong. 2002. Molecular characterization of metallo-beta-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the blaVIM-2 gene cassettes. J. Antimicrob. Chemother. 49:837-840. [DOI] [PubMed] [Google Scholar]