Abstract
The efficacy of telavancin, a bactericidal lipoglycopeptide, was compared to that of vancomycin and linezolid against methicillin-resistant Staphylococcus aureus (MRSA) in a murine pneumonia model. Telavancin produced greater reductions in lung bacterial titer and mortality than did vancomycin and linezolid at human doses equivalent to those described by the area under the concentration-time curve. These results suggest the potential utility of telavancin for treatment of MRSA pneumonia.
Nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus (MRSA) has been reported with increasing frequency over the past 2 decades (13). Vancomycin and linezolid, two drugs that are slowly bactericidal and bacteriostatic, respectively, are frequently used in the treatment of nosocomial pneumonia caused by Staphylococcus aureus, including MRSA. However, overall clinical cure rates attained with these two drugs are less than optimal (8).
Telavancin is a novel lipoglycopeptide that operates through multiple mechanisms to produce potent and rapid bactericidal activity against clinically relevant gram-positive bacteria, including MRSA (4, 5, 7). We have previously shown that telavancin exhibits potent antibacterial activity against a range of gram-positive bacteria, including MRSA, in two animal models of soft-tissue infection, with the area under the concentration-time curve (AUC)/MIC ratio being the pharmacodynamically linked parameter (3). In the studies described here, we assessed the efficacy of telavancin in an immunocompromised murine model of MRSA-induced pneumonia.
Telavancin for injection (250 mg/vial) was reconstituted in 5% dextrose in water. Vancomycin (Sigma-Aldrich, St. Louis, MO) and linezolid (Pharmacia, Kalamazoo, MI) were dissolved in 5% dextrose in water and hydroxypropyl-β-cyclodextrin, respectively. MRSA ATCC 33591 was obtained from the American Type Culture Collection (Manassas, VA). MICs were determined by the broth microdilution method according to protocol M7-A5 of the National Committee for Clinical Laboratory Standards (6). The MICs of telavancin, vancomycin, and linezolid against MRSA 33591 were 0.5, 1, and 1 μg/ml, respectively. The doses used in pharmacodynamic studies were chosen to approximate human doses equivalent to those described by AUCs as opposed to other pharmacokinetic parameters since the AUC/MIC ratio is believed to be the key pharmacodynamically linked parameter for all three drugs against S. aureus (1, 3).
All studies were approved by the Institutional Animal Care and Use Committee at Theravance, Inc. Female BALB/c mice (Harlan, Indianapolis, IN), weighing between 16 and 26 g, were rendered neutropenic by treatment with 250 mg/kg of cyclophosphamide intraperitoneally at 4 and 2 days prior to infection. Neutropenic animals were lightly anesthetized with isoflurane gas and then held in an upright position to be inoculated. Each animal was inoculated by placing 50 μl of inoculum containing approximately 107 CFU of MRSA onto the tip of the nares. The animals were allowed to inhale the inoculum as small droplets and were then placed back into their respective cages for recovery and observation prior to dosing them with the test compounds.
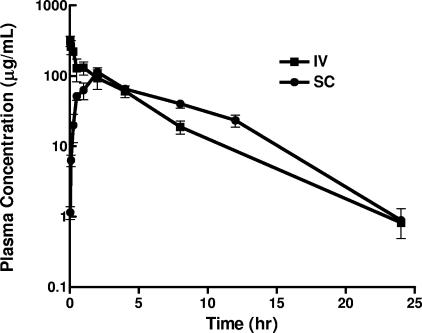
In pharmacokinetic studies, immunocompromised infected mice were dosed (24 h after inoculation) with either a single intravenous (i.v.) or subcutaneous (s.c.) injection of telavancin (40 mg/kg). Blood samples were collected via cardiac puncture under CO2 narcosis into heparinized microtainers on ice predose and at various postdose periods. Blood samples were processed to plasma by centrifugation (12,000 rpm, 4 min, 4°C) and stored at −80°C until analysis. The plasma samples were subjected to solid-phase extraction and analyzed by liquid chromatography with tandem mass spectrometry. Deuterated telavancin was used as the internal standard. Mobile phase A consisted of 0.25% formic acid in water, and mobile phase B consisted of 0.25% formic acid in acetonitrile. The limit of quantitation was 0.25 μg/ml, and the coefficient of variance for replicate samples at each level was <20%. The plasma concentration versus time profile of telavancin is summarized in Fig. 1. Following i.v. and s.c. administrations to neutropenic infected mice, telavancin (40 mg/kg) had maximum concentrations of drug in serum of 353 and 113 μg/ml, mean AUCs (0 to 24 h) of 755 and 788 μg · h/ml, clearances of 0.053 and 0.051 liter/h/kg, and half-lives of 3.3 and 2.8 h, respectively. In humans, telavancin has a longer pharmacokinetic half-life (6.9 to 9.1 h) and is well tolerated at doses of up to 15 mg/kg, i.v., once daily (10). The protein binding levels of telavancin are 93% and 96.2% in human and mouse sera, respectively. With a dosing regimen of 20 and 40 mg/kg, twice daily, the AUCfree drug (0 to 24 h) in mice (26.1 and 52.3 μg · h/ml, respectively) is expected to approximate that in humans at doses of 5 and 10 mg/kg, given once daily (22.2 and 44.3 μg · h/ml, respectively). Based on published pharmacokinetic studies of mice (9, 11), doses of vancomycin (110 mg/kg, i.v., every 12 h [q12h]) and linezolid (80 mg/kg, i.v., q12h) were chosen to target AUCs (0 to 24 h) of 440 μg · h/ml and 160 μg · h/ml, respectively, which closely approximate human exposures at doses of 1 g, q12h (2), and 600 mg, q12h (12), respectively.
FIG. 1.
Single-dose concentration-versus-time pharmacokinetic profile of telavancin (total drug) in neutropenic mice infected in the lungs with MRSA 33591. The abscissa shows the time (h), and the ordinate shows the plasma drug concentration (μg/ml). There were three mice per group. Data are expressed as the mean ± 1 standard error of the mean.
In pharmacodynamic studies, the antibacterial effects of two to three doses of test compounds were studied under low and high pretreatment titer conditions by initiating the treatment at 12 and 24 h postinoculation, respectively. Animals were euthanized using CO2 inhalation at various time periods postinoculation, and the lungs were harvested and homogenized in saline. Serial dilutions of these homogenates were plated onto trypsin-soy agar plates containing 10 μg/ml of ampicillin, incubated overnight at 37°C, and read the following day to quantitate the numbers of CFU. Ampicillin was added to these plates to select for MRSA and reduce the chance of contamination.
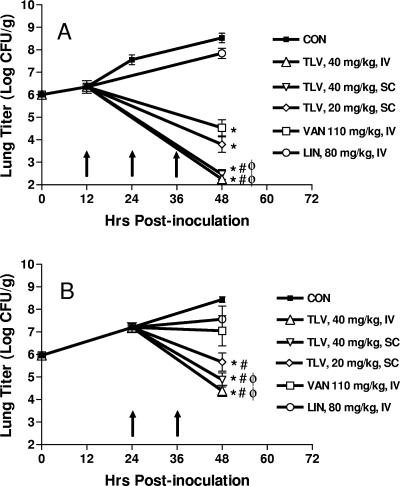
The lung bacterial titer immediately after inoculation was 6.0 ± 0.1 log CFU/g. The titer increased to 6.3 ± 0.3, 7.6 ± 0.2, and 8.5 ± 0.2 log CFU/g at 12 h, 24 h, and 48 h postinoculation, respectively, in untreated (control) animals, implying that the organisms were in logarithmic growth phase during this period. Telavancin produced dose-dependent reduction in lung bacterial titers (Fig. 2). When dosed at 12 h postinoculation, animals treated with telavancin (40 mg/kg, i.v. or s.c.) had a significantly greater reduction (from pretreatment values) in lung bacterial titer (−4.1 and −3.9 log CFU/g) than did those treated with vancomycin (−1.8 log CFU/g) or linezolid (+1.5 log CFU/g) (Fig. 2A). Similarly, when dosed at 24 h postinoculation, animals treated with telavancin (40 mg/kg, i.v. or s.c.) had a significantly greater reduction in lung bacterial titer (−2.8 and −2.3 log CFU/g) than did those treated with vancomycin (+0.14 log CFU/g) or linezolid (+0.37 log CFU/g) (Fig. 2B).
FIG. 2.
Efficacy of telavancin (TLV), vancomycin (VAN), and linezolid (LIN) in the murine pneumonia model. (A) Dosing initiated at 12 h postinoculation. Each group (at time of dosing) contained eight mice. The numbers of mice at time of harvest were two (control [CON]), seven (TLV, 40 mg/kg, i.v.), eight (TLV, 40 mg/kg, s.c.), seven (TLV, 20 mg/kg, s.c.), eight (VAN, 110 mg/kg, i.v.), and eight (LIN, 80 mg/kg, i.v.). (B) Dosing initiated at 24 h postinoculation. Each group (at time of dosing) contained eight mice. The numbers of mice at time of harvest were three (CON), eight (TLV, 40 mg/kg, i.v.), eight (TLV, 40 mg/kg, s.c.), seven (TLV, 20 mg/kg, s.c.), seven (VAN), and seven (LIN). The abscissa shows the time (h) postinoculation, and the ordinate shows the lung bacterial titer (log CFU/g). Arrows denote times of dosing. Data represent the mean ± 1 standard error of the mean. *, P < 0.05 versus pretreatment titer; #, P < 0.05 versus LIN; φ, P < 0.05 versus VAN.
In separate studies intended to investigate effects of treatment on survival, animals were given, beginning at 24 h postinfection, four doses (q12h) of vancomycin (110 mg/kg, i.v.), linezolid (80 mg/kg, i.v.), or telavancin (40 mg/kg, s.c.). The cessation of antibacterial treatments coincided with the recovery of the immune system (data not shown). Animals were observed twice daily, and deaths were recorded over a 14-day period. The proportions of survivors in the control, telavancin, vancomycin, and linezolid groups were 3/22 (14%), 19/22 (86%), 16/22 (73%), and 11/22 (50%), respectively. Telavancin- and vancomycin-treated groups showed significantly greater improvement in survival than did control and linezolid-treated groups. The lungs from a subset of surviving untreated and telavancin-treated animals were excised, inflated with 10% neutral buffered formalin, processed for paraffin embedding, sectioned at 0.6 mm, and stained with hematoxylin and eosin. Lungs from the untreated groups were characterized by the presence of bacterial colonies surrounded by a zone of necrosis with minimal to moderate inflammatory cell infiltrates (data not shown). In contrast, the lungs of animals treated with telavancin (14 days postinoculation) contained minimal multifocal inflammatory cell infiltrates, and bacterial colonies were absent (data not shown).
The superior efficacy of telavancin in the current studies could be explained, in part, by the rapid bactericidal activity against MRSA which potentially emanates from its dual mechanism of action (4). We have reported previously on the differential bactericidal properties of linezolid, vancomycin, and telavancin against MRSA 33591 when they are tested at equal multiples (8×) of their MICs (7). Linezolid behaved as a bacteriostatic drug, whereas vancomycin was slowly bactericidal, requiring up to 24 h to achieve a >3-log decrease in number of bacteria. In contrast, telavancin produced a >3-log decrease in less than 8 h. An alternate explanation for the findings of the present study could be differences in the degree of penetration of the drugs into the lung compartments, including the epithelial lining fluid, alveolar cells, and resident macrophages. A caveat of the present studies is that a single strain of MRSA was studied, and it remains to be determined whether the findings of this investigation extend to other strains as well.
In summary, the data from the present study demonstrate the efficacy of telavancin in reducing lung bacterial titers and improving survival in an MRSA model of pneumonia in neutropenic mice. These findings support further development of telavancin for MRSA pneumonia.
REFERENCES
- 1.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17:479-501. [DOI] [PubMed] [Google Scholar]
- 2.Healy, D. P., R. E. Polk, M. L. Garson, D. T. Rock, and T. J. Comstock. 1987. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob. Agents Chemother. 31:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegde, S. S., N. Reyes, T. Wiens, N. Vanasse, R. Skinner, J. McCullough, K. Kaniga, J. Pace, R. Thomas, J.-P. Shaw, G. Obedencio, and J. K. Judice. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King, A., I. Phillips, and K. Kaniga. 2004. Comparative in vitro activity of telavancin (TD-6424), a rapidly bactericidal, concentration-dependent anti-infective with multiple mechanisms of action against Gram-positive bacteria. J. Antimicrob. Chemother. 53:797-803. [DOI] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 7.Pace, J. L., K. Krause, D. Johnston, D. Debabov, T. Wu, L. Farrington, C. Lane, D. L. Higgins, B. Christensen, J. K. Judice, and K. Kaniga. 2003. In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3602-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinstein, E., S. Cammarata, T. Oliphant, and R. Wunderink. 2001. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin. Infect. Dis. 32:402-412. [DOI] [PubMed] [Google Scholar]
- 9.Shaw J.-P., S. M. Adams, D. N. Li, J. D. Seroogy, and S. Jaw-Tsai. 2002. Preclinical pharmacokinetics and allometric scaling of vancomycin. AAPS Pharm Sci 4:T3252.
- 10.Shaw, J.-P., J. Seroogy, K. Kaniga, D. L. Higgins, M. Kitt, and S. Barriere. 2004. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob. Agents Chemother. 49:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slatter, J. G., L. A. Adams, E. C. Bush, K. Chiba, P. T. Daley-Yates, K. L. Feenstra, S. Koike, N. Ozawa, G. W. Peng, J. P. Sams, M. R. Schuette, and S. Yamazaki. 2002. Pharmacokinetics, toxicokinetics, distribution, metabolism and excretion of linezolid in mouse, rat and dog. Xenobiotica 32:907-924. [DOI] [PubMed] [Google Scholar]
- 12.Stalker, D. J., G. L. Jungbluth, N. K. Hopkins, and D. H. Batts. 2003. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J. Antimicrob. Chemother. 51:1239-1246. [DOI] [PubMed] [Google Scholar]
- 13.Vincent, J. L., D. J. Bihari, P. M. Suter, H. A. Bruining, J. White, M. H. Nicolas-Chanoin, M. Wolff, R. C. Spencer, and M. Hemmer. 1995. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 274:639-644. [PubMed] [Google Scholar]