Abstract
We previously documented the induction of Leishmania amastigote apoptosis by trivalent antimony (SbIII) and nitric oxide (NO). We demonstrate here that SbIII-resistant amastigotes were resistant to NO toxicity when delivered extracellularly by NO donors or intracellularly via macrophage activation. Shared biochemical targets for SbIII and NO resistance in Leishmania are discussed.
Leishmania infantum or Leishmania chagasi is responsible for canine and human visceral leishmaniasis in both the Old and the New Worlds. Leishmania parasites develop as flagellated promastigotes in the insect vector and reside as intracellular nonflagellated amastigotes in the mammalian host, which are responsible for the clinical disease manifestations. Basic treatment of leishmaniasis consists of the administration of pentavalent antimony (SbV) in the form of sodium stibogluconate or meglumine antimoniate. The mode of action of SbV implicates its reduction by the host cell to the trivalent form (SbIII) (8, 9, 21, 23). Besides this, successful chemotherapy in murine and canine models has been correlated with the efficiency of natural immunity (5, 26), and synergism between SbV and immunostimulant cytokines has been proven to be pertinent in the treatment of leishmaniasis (14, 18). We recently demonstrated that both nitric oxide (NO) and SbIII lead to Leishmania amastigote cell death with some characteristics of apoptosis (11, 22). Moreover, the activity of SbIII may be directly linked to the induction of reactive oxygen intermediates such as NO (24). In order to clarify more precisely the potency of NO and SbIII, we investigated the cross-resistance of SbIII-resistant parasites to NO.
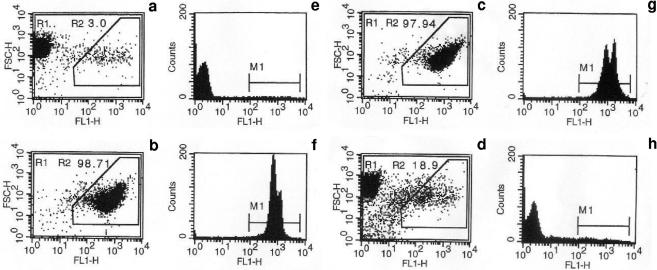
SbIII-resistant amastigotes previously described (20) were used in all experiments. The susceptibility to NO of wild-type (WT) amastigotes and amastigotes resistant to 120 μg/ml potassium antimonyl tartrate (LiSbIIIR120) was ascertained using either acidified sodium nitrite (NaNO2) or the NO donors SNAP (S-nitroso-N-acetylpenicillamine) and DETA-NONOate {(Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate}. For NaNO2, 107 amastigotes per ml were incubated for 2 h at 37 ± 1°C in the dark in 0.01 M phosphate-buffered saline (PBS), pH 4.5, in the presence of 0 to 10 mM NaNO2. After a wash in PBS, parasites were seeded in 96-well microplates at 2 × 105/well in 100 μl of medium for axenic amastigotes (MAA) (13). To estimate parasite survival after NO treatment, amastigotes were cultured in the presence of 2.5 μCi of [3H]thymidine for 24 h and then filtered through GF/C filters (Whatman International) for radioactivity determination. The relative inhibition of [3H]thymidine incorporation by 50% (IC50) was calculated. The NO donors SNAP and DETA-NONOate were added directly in the culture medium at concentrations ranging from 0 to 500 μM, immediately after inoculation of 2 × 105 amastigotes/100 μl of MAA in 96-well microplates. After 72 h of incubation, the effective concentrations of NO donors inhibiting WT and LiSbIIIR120 amastigote growth by 50% (IC50) were estimated by MTT test (21). IC50 for WT and LiSbIIIR120 amastigotes are indicated in Table 1. LiSbIIIR120 amastigotes were 21.5-, 3.2-, and 3.6-fold more resistant than the WT to NO delivered by NaNO2, SNAP, and DETA-NONOate. NO-mediated Leishmania amastigote killing was also determined using impermeable DNA intercalatant YOPRO-1 staining analyzed by a FACScan flow cytometer (Becton Dickinson, Ivry, France) (11, 22). Green cell fluorescence could selectively differentiate viable (Fig. 1a and e) and apoptotic (Fig. 1b and f) amastigotes by using the combined analysis of their forward scatter (FSC-H) and FL1-H (525 ± 10 nm band pass filter) patterns. Both WT and LiSbIIIR120 amastigotes incubated in MAA alone displayed a homogeneous population of living cells (data not shown). When amastigotes were treated with 500 μM NaNO2, a new cell population with a high FL1 fluorescence corresponding to apoptotic cells was detected for the WT (Fig. 1c and g), whereas more than 80% living cells were recorded for LiSbIIIR120 amastigotes (Fig. 1d and h).
TABLE 1.
Cytotoxic effects of NO donors on WT amastigotes of L. infantum and amastigotes resistant to 120 μg/ml potassium antimonyl tartrate LiSbIIIR120a
| NO donor | IC50 (μM) for:
|
|
|---|---|---|
| WT amastigotes | LiSbIIIR120 resistant amastigotes | |
| NaNO2 | 315.9 ± 5.3 | 6,785.9 ± 1133 |
| SNAP | 72 ± 0.8 | 229.1 ± 15.9 |
| DETA/NONOate | 37.7 ± 4.25 | 134.2 ± 2.3 |
The IC50s of NO donors for WT and LiSbIIIR120-resistant amastigotes were determined by [3H]thymidine incorporation for NaNO2 (24,482 ± 2,441 cpm in controls) and by the MTT-based microassay for SNAP and DETA/NONOate (21).
FIG. 1.
Analysis of NO-mediated apoptosis in WT amastigotes of L. infantum and amastigotes resistant to 120 μg/ml potassium antimonyl tartrate LiSbIIIR120. NO-induced apoptosis of WT and LiSbIIIR120 amastigotes was analyzed using a cytofluorometry YOPRO-1 differential staining technique. Viable control (a and e), LiSbIIIR120 amastigotes cultured for 24 h in culture medium alone; apoptotic control (b and f), LiSbIIIR120 amastigotes treated for 24 h with 2 mg/ml Geneticin; c and g, WT amastigotes incubated for 2 h at 37 ± 1°C in the dark in 0.01 M PBS, pH 4.5, in the presence of 500 μM NaNO2; d and h, LiSbIIIR120 amastigotes incubated for 2 h at 37 ± 1°C in the dark in 0.01 M PBS, pH 4.5, in the presence of 500 μM NaNO2. The apoptotic cell percentage (R2), corresponding to both reduced forward scatter and high fluorescence intensity, is indicated for each experimental condition (a, b, c, and d). M1 shows the peak fluorescence intensity (e, f, g, and h). Experiments were done three times in duplicate.
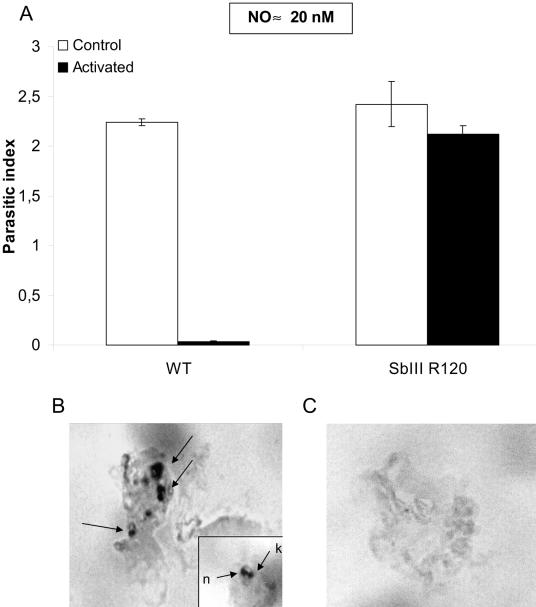
LiSbIIIR120 amastigotes were also able to resist NO-mediated cell death in an intracellular position. A human leukemia monocyte cell line (THP-1 cells) was differentiated in 16-well LabTek tissue culture slides (Nalge Nunc International) (25), infected with stationary-phase L. infantum extracellular amastigotes (parasite-macrophage ratio of 3:1 for 2 h at 37°C with 5% CO2), and activated by lipopolysaccharide (10 ng/ml) and gamma interferon (100 U/ml). Culture supernatants were collected 48 h later for nitrate and nitrite measurement (19), and macrophages were fixed with methanol and stained with Giemsa for parasite counts or processed for apoptosis determination (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling [TUNEL] technique, in situ colorimetric apoptosis detection system; Promega, Madison, Wis.). The parasitic index (PI) was calculated as follows: PI = mean number of amastigotes per macrophage × percentage of infected macrophages × 100. A PI reduction of more than 95%, correlated with a nitrate-nitrite accumulation indicative of NO production, was observed for WT amastigotes inside activated THP-1 macrophages (Fig. 2A). When THP-1 cells were infected with LiSbIIIR120 amastigotes, no such PI reduction was noticed (Fig. 2A). The in situ TUNEL analysis revealed that apoptosis of intracellular amastigotes only occurred in WT-infected activated THP-1 macrophages (Fig. 2B and C).
FIG. 2.
NO-dependent cytotoxicity for trivalent-antimony-resistant L. infantum amastigotes in a human leukemia monocyte cell line (THP-1 cells). THP-1 macrophages were infected with either WT L. infantum amastigotes or amastigotes resistant to 120 μg/ml potassium antimonyl tartrate LiSbIIIR120 at a parasite cell ratio of 3:1 in RPMI 1640 medium supplemented with 10% fetal calf serum and treated with lipopolysaccharide and gamma interferon for activation. NO production and PIs (73.3% ± 2.6% infected macrophages and 3.05 ± 0.8 amastigotes/macrophage in the controls) (A) were determined 48 h later, and in situ NO-mediated apoptosis of WT (B) or LiSbIIIR120 (C) amastigotes was determined using the TUNEL technique and analyzed under a microscope at ×1,000 magnification. A magnification of ×1,600 was used to identify labeled amastigotes (B, inset: n, nucleus; k, kinetoplast). Results are representative of two similar experiments done in duplicate.
Our observation that in vitro-selected, SbIII-resistant amastigotes were cross-resistant to NO raised the questions of the existence of shared biochemical targets for SbIII and NO resistance in Leishmania and of the occurrence of such phenomenon in areas of endemicity.
Among the mechanisms responsible for antimony resistance in vitro, implication of trypanothione and an As-thiol pump is well documented (1, 6, 17). Interestingly, thiol groups of proteins are currently considered as targets for NO (4, 16). Thus, one explanation for NO resistance in SbIII-resistant amastigotes should be an increased content of trypanothione and thiol-related compounds (3). Extrusion of nitrosothiols by an AS-thiol pump could participate in reducing the detrimental action of NO. A recent finding showed that SbIII was able to stimulate the host immune system, leading to potential synergistic effects between the chemical and natural microbicidal molecules (24). The authors suggest that antimonial drug treatment induced the production of nitrogen species by macrophages and can cause apoptosis of intracellular amastigotes. The involvement of NO in the cytotoxic effect of SbIII could explain the lower susceptibility of SbIII-resistant amastigotes to NO action and could greatly help in the understanding of mechanism underlying antimony resistance.
Increasing cases of SbV treatment failure have been described (2, 7, 10, 12, 15), suggesting that antimony-resistant parasites could be transmitted even in hosts developing an efficient immune response. These data strongly suggest that NO-SbIII cross-resistance could have a dreadful implication for the spread of chemoresistance in the field. In this view, it will be of interest to determine whether or not SbIII-resistant strains isolated from relapsed patients were NO cross resistant.
Acknowledgments
This work was supported by grants from the Institut de Recherche pour le développement (IRD Institute) and the Fondation pour la Recherche Médicale (FRM Foundation).
REFERENCES
- 1.Brochu, C., J. Wang, G. Roy, N. Messier, X. Y. Wang, N. G. Saravia, and M. Ouellette. 2003. Antimony uptake systems in the protozoan parasite Leishmania and accumulation differences in antimony-resistant parasites. Antimicrob. Agents Chemother. 47:3073-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrio, J., and C. Portus. 2002. In vitro susceptibility to pentavalent antimony in Leishmania infantum strains is not modified during in vitro or in vivo passages but is modified after host treatment with meglumine antimoniate. BMC Pharmacol. 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter, K. C., S. Sundar, C. Spickett, O. C. Pereira, and A. B. Mullen. 2003. The in vivo susceptibility of Leishmania donovani to sodium stibogluconate is drug specific and can be reversed by inhibiting glutathione biosynthesis. Antimicrob. Agents Chemother. 47:1529-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colasanti, M., L. Gradoni, M. Mattu, T. Persichini, L. Salvati, G. Venturini, and P. Ascenzi. 2002. Molecular bases for the anti-parasitic effect of NO. Int. J. Mol. Med. 9:131-134. [PubMed] [Google Scholar]
- 5.Das, L., N. Datta, S. Bandyopadhyay, and P. K. Das. 2001. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves up-regulation of nitric oxide and a favorable T cell response. J. Immunol. 166:4020-4028. [DOI] [PubMed] [Google Scholar]
- 6.Dey, S., M. Ouellette, J. Lightbody, B. Papadopoulou, and B. P. Rosen. 1996. An ATP-dependent As(III)-glutathione transport system in membrane vesicles of Leishmania tarentolae. Proc. Natl. Acad. Sci. USA 93:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faraut-Gambarelli, F., R. Piarroux, M. Deniau, B. Giusiano, P. Marty, G. Michel, B. Faugere, and H. Dumon. 1997. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob. Agents Chemother. 41:827-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frezard, F., C. Demicheli, C. S. Ferreira, and M. A. Costa. 2001. Glutathione-induced conversion of pentavalent antimony to trivalent antimony in meglumine antimoniate. Antimicrob. Agents Chemother. 45:913-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin, L. G. 1995. Pentostam (sodium stibogluconate); a 50-year personal reminiscence. Trans. R. Soc. Trop. Med. Hyg. 89:339-341. [DOI] [PubMed] [Google Scholar]
- 10.Gradoni, L., M. Gramiccia, and A. Scalone. 2003. Visceral leishmaniasis treatment, Italy. Emerg. Infect. Dis. 9:1617-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzmuller, P., D. Sereno, M. Cavaleyra, I. Mangot, S. Daulouede, P. Vincendeau, and J. L. Lemesre. 2002. Nitric oxide-mediated proteasome-dependent oligonucleosomal DNA fragmentation in Leishmania amazonensis amastigotes. Infect. Immun. 70:3727-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn, S. D., V. Yardley, F. Vega-Lopez, J. Watson, and D. N. Lockwood. 2003. New World cutaneous leishmaniasis in returned travellers: treatment failures using intravenous sodium stibogluconate. Trans. R. Soc. Trop. Med. Hyg. 97:443-445. [DOI] [PubMed] [Google Scholar]
- 13.Lemesre, J. L., M. P. Blanc, P. Grebaut, V. Zilberfarb, and V. Carrière. 1994. Culture continue des formes amastigotes de leishmanies en condition axénique. Réalisation du cycle évolutif in vitro. Med. Armees 22:99-100. [Google Scholar]
- 14.Li, J., S. Sutterwala, and J. P. Farrell. 1997. Successful therapy of chronic, nonhealing murine cutaneous leishmaniasis with sodium stibogluconate and gamma interferon depends on continued interleukin-12 production. Infect. Immun. 65:3225-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marovich, M. A., R. Lira, M. Shepard, G. H. Fuchs, R. Kruetzer, T. B. Nutman, and F. A. Neva. 2001. Leishmaniasis recidivans recurrence after 43 years: a clinical and immunologic report after successful treatment. Clin. Infect. Dis. 33:1076-1079. [DOI] [PubMed] [Google Scholar]
- 16.Mnaimneh, S., M. Geffard, B. Veyret, and P. Vincendeau. 1997. Albumin nitrosylated by activated macrophages possesses antiparasitic effects neutralized by anti-NO-acetylated-cysteine antibodies. J. Immunol. 158:308-314. [PubMed] [Google Scholar]
- 17.Mukhopadhyay, R., S. Dey, N. Xu, D. Gage, J. Lightbody, M. Ouellette, and B. P. Rosen. 1996. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc. Natl. Acad. Sci. USA 93:10383-10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray, H. W., and S. Delph-Etienne. 2000. Roles of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect. Immun. 68:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy, J. B., and R. G. Wilkerson. 1984. Fallibility of Griess (nitrite) test. Urology. 23:270-271. [PubMed] [Google Scholar]
- 20.Sereno, D., E. Guilvard, S. Maquaire, M. Cavaleyra, P. Holzmuller, A. Ouaissi, and J. L. Lemesre. 2001. Experimental studies on the evolution of antimony-resistant phenotype during the in vitro life cycle of Leishmania infantum: implications for the spread of chemoresistance in endemic areas. Act. Trop. 80:195-205. [DOI] [PubMed] [Google Scholar]
- 21.Sereno, D., and J. L. Lemesre. 1997. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob. Agents Chemother. 41:972-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sereno, D., P. Holzmuller, I. Mangot, G. Cuny, A. Ouaissi, and J. L. Lemesre. 2001. Antimonial-mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob. Agents Chemother. 45:2064-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaked-Mishan, P., N. Ulrich, M. Ephros, and D. Zilberstein. 2001. Novel intracellular SbV reducing activity correlates with antimony susceptibility in Leishmania donovani. J. Biol. Chem. 276:3971-3976. [DOI] [PubMed] [Google Scholar]
- 24.Sudhandiran, G., and C. Saha. 2003. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J. Biol. Chem. 278:25120-25132. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya, S., Y. Kobayashi, Y. Goto, H. Okumura, S. Nakae, T. Konno, and K. Tada. 1982. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 42:1530-1536. [PubMed] [Google Scholar]
- 26.Vouldoukis, I., J. C. Drapier, A. K. Nussler, Y. Tselentis, O. A. Da Silva, M. Gentilini, D. M. Mossalayi, L. Monjour, and B. Dugas. 1996. Canine visceral leishmaniasis: successful chemotherapy induces macrophage antileishmanial activity via the l-arginine nitric oxide pathway. Antimicrob. Agents Chemother. 40:253-256. [DOI] [PMC free article] [PubMed] [Google Scholar]