Abstract
Mycoplasma pneumoniae is a major cause of community-acquired pneumonia. We evaluated the efficacy of LBM415, a novel peptide deformylase inhibitor antimicrobial agent, for the treatment of M. pneumoniae pneumonia in a mouse model. Eight-week-old BALB/c mice were intranasally inoculated once with 107 CFU of M. pneumoniae. Groups of mice were treated with LBM415 (50 mg/kg of body weight) or placebo subcutaneously daily for 13 days, starting 24 h after inoculation. Groups of mice were evaluated at the baseline; at days of treatment 1, 3, 6, and 13; and at 7 days after treatment. The MIC of LBM415 against M. pneumoniae was <0.005 μg/ml. LBM415-treated mice had significantly lower bronchoalveolar lavage fluid M. pneumoniae concentrations than placebo-treated mice on days 6 and 13 of treatment. Compared with placebo treatment, therapy with LBM415 significantly decreased lung histopathology scores at days 3, 6, and 13 of treatment and at 7 days after treatment. Airway obstruction was significantly lower in LBM415-treated mice than in placebo-treated mice on days 1, 3, and 6 of treatment and after 7 days of therapy, while airway hyperresponsiveness was significantly lower only on day 3 of therapy. The bronchoalveolar lavage fluid concentrations of tumor necrosis factor alpha, gamma interferon (IFN-γ), interleukin-6 (IL-6), IL-12, KC (functional IL-8), monocyte chemotactic protein 1, macrophage inflammatory protein 1α, monokine induced by IFN-γ, and IFN-inducible protein 10 were significantly reduced in LBM415-treated mice compared with the levels in placebo-treated mice. There were no differences in the bronchoalveolar lavage fluid concentrations of granulocyte-macrophage colony-stimulating factor, IL-1β, IL-2, IL-4, IL-5, and IL-10 between the two groups of mice. LBM415 therapy had beneficial microbiologic, histologic, respiratory, and immunologic effects on acute murine M. pneumoniae pneumonia.
Mycoplasma pneumoniae is an important cause of upper and lower respiratory tract infections in children and adults and is responsible for as many as 40% of cases of community-acquired pneumonia (7, 15, 28, 33). Tetracyclines, macrolides, ketolides, and fluoroquinolones have in vitro activities against M. pneumoniae (43, 44). Most of these agents are primarily bacteriostatic for M. pneumoniae, and only the fluoroquinolones have been shown to be bactericidal (10, 42). While M. pneumoniae can be cultured from respiratory secretions for long periods after acute infection even after appropriate antimicrobial therapy, most antibiotics produce satisfactory clinical results, with a significant reduction in the duration of symptoms compared with no treatment (30, 36). Macrolide and related antibiotics are generally considered the therapy of choice for M. pneumoniae infections in children and adults. Strains with acquired resistance to macrolides have rarely been described (29).
LBM415 (previously known as NVP PDF-713) is a novel inhibitor of the bacterial enzyme peptide deformylase (PDF) and has been documented to have in vitro activity against a wide range of respiratory pathogens, including Streptococcus pneumoniae, Streptococcus pyogenes, Moraxella catarrhalis, Haemophilus influenzae, Chlamydophila pneumoniae, and M. pneumoniae; and it is among the first PDF inhibitors to be advanced into clinical trials for the oral and parenteral treatment of community-acquired respiratory tract infections (8, 11, 24). Given the unique enzyme target of LBM415, no evidence of cross-resistance has been noted (14).
Our laboratory has previously established an experimental model of M. pneumoniae lower respiratory infection that parallels human M. pneumoniae respiratory disease (20, 21). The aim of the present study was to evaluate the efficacy of LBM415 for the treatment for acute M. pneumoniae pneumonia in a murine model and to assess its impact on the pulmonary immune response as measured by bronchoalveolar lavage (BAL) fluid cytokine and chemokine levels.
MATERIALS AND METHODS
Organism and growth conditions.
M. pneumoniae (ATCC 29342) was reconstituted in SP4 broth and subcultured after 24 to 48 h in a flask containing 20 ml of SP4 medium at 37°C. The supernatant was decanted when the broth turned an orange hue (at approximately 72 h), and 2 ml of fresh SP4 broth was added to the flask. A cell scraper was used to harvest the adherent mycoplasmas from the bottom of the flask. This achieved a M. pneumoniae concentration in the range of 108 to 109 CFU/ml. Aliquots were stored at −80°C. All SP4 media contained nystatin (50 U/ml) and ampicillin (1.0 mg/ml) to inhibit the growth of potential contaminants.
Animals and inoculation.
Mice were obtained from a commercial vendor (Charles River Laboratories, Wilmington, MA), who confirmed their mycoplasma- and murine virus-free status. The mice were housed in the animal care facility of our institution in filter-top cages in a temperature-controlled room (22°C) and were allowed to acclimate to their new environment for 1 week. The Animal Resource Center at the University of Texas Southwestern Medical Center performed quarterly health surveillance of sentinel mice housed in the mouse storage room. Sentinel mice were examined for antibodies against mouse hepatitis virus, Sendai virus, pneumonia virus of mice, reovirus 3, mouse encephalitis virus (GD-7), mouse rotavirus (EDIM), minute virus of mice, and Mycoplasma pulmonis; and the sentinel mice were also screened for pinworm and mites. They were negative for these pathogens. Methoxyflurane, an inhaled anesthetic, was used for sedation during inoculation. Two-month-old female BALB/c mice were intranasally inoculated once with 0.6 × 107 to 1.0 × 107 CFU of M. pneumoniae in 50 μl of SP4 broth. Mice from directly comparable groups received an inoculum from the same batch. Control mice were inoculated with sterile SP4 broth. All mice were housed in the same animal room and received identical daily care. Animal guidelines were in accordance with the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center at Dallas.
Administration of LBM415.
LBM415 powder (Novartis, Cambridge, MA) was dissolved in 2.5% ethanol and phosphate-buffered saline. Groups of M. pneumoniae-infected mice were treated with LBM415 at a dosage of 50 mg/kg of body weight (1.0 mg in 0.2 ml per mouse) subcutaneously once daily for 13 days, starting 24 h after inoculation. This dosage was recommended for murine studies by the manufacturer, since at the time of our study the initiation of a dosage regimen for humans had not been established. Placebo groups received the same treatment regimen with an identical solution not containing LBM415.
An aliquot of M. pneumoniae was sent to the Diagnostic Mycoplasma Laboratory (Birmingham, AL) to evaluate the in vitro activity of LBM415.
Experimental design and sample collection.
Groups of mice were evaluated at the baseline (24 h after inoculation); at days of treatment 1, 3, 6, and 13; and at 7 days after treatment. Samples were obtained from four to five mice per group at each time point; whole-body plethysmography was performed with four to eight unrestrained and nonsedated mice per group at each time point. The experiment was performed in duplicate.
Mice were anesthetized with an intraperitoneal injection of 75 mg per kg ketamine and 5 mg per kg acepromazine before cardiac puncture. The blood was centrifuged at 3,500 × g for 10 min, and the plasma was stored at −80°C. BAL fluid specimens were obtained by instilling 500 μl of SP4 broth through a 25-gauge needle into the lungs, via the trachea, followed by aspiration of this fluid into a syringe. Approximately 70 to 80% of the instilled volume was consistently retrieved. All BAL fluid samples were kept on ice until they were processed. Whole lung specimens, including the trachea and both lungs, were collected and fixed with a 10% buffered formalin solution for histological evaluation.
Culture.
Twenty-five microliters of undiluted sample and serial 10-fold dilutions of BAL fluid in SP4 broth (50 μl of undiluted sample was used for the initial dilution) were immediately cultured on SP4 agar plates at 37°C, while the remaining undiluted BAL fluid specimens were stored at −80°C. Quantification was performed by counting the colonies on plated specimens, and the quantities are expressed as log10 CFU per ml.
Histopathology.
Lung tissue was fixed in buffered formalin, and transverse sections were stained with hematoxylin and eosin. The histopathological score (HPS) was determined by a single pathologist who was unaware of the treatment status of the animals from which the specimens were taken. The HPS was based on grading of the peribronchiolar and bronchial infiltrate, bronchiolar and bronchial luminal exudate, perivascular infiltrate, and parenchymal pneumonia (neutrophilic alveolar infiltrate). This HPS system assigned values from 0 to 26: the higher the score was, the greater the inflammatory changes in the lung were (4, 19-21). The extent of variation in HPS when the same slide is scored by the same pathologist multiple times has been found to be 0 to 1.
Measurement of cytokines and chemokines in BAL fluid.
The concentrations of cytokines and chemokines in the BAL fluid specimens were assessed by using Multiplex Bead Immunoassays (BioSource International, Camarillo, CA) in conjunction with the Luminex LabMAP system, following the manufacturer's instructions. The cytokines and chemokines examined and their levels of sensitivity were as follows: tumor necrosis factor alpha (TNF-α), 5 pg/ml; interferon gamma (IFN-γ), 1 pg/ml; interleukin-1β (IL-1β), 10 pg/ml; IL-2, 15 pg/ml; IL-4, 5 pg/ml; IL-5, 10 pg/ml; IL-6, 10 pg/ml; mouse KC (functional IL-8), <15 pg/ml; IL-10, 15 pg/ml; IL-12 (p40/p70), 15 pg/ml; granulocyte-macrophage colony-stimulating factor (GM-CSF), 10 pg/ml; monocyte chemotactic protein 1 (MCP-1), <5 pg/ml; macrophage inflammatory protein 1α (MIP-1α), <15 pg/ml; monokine induced by IFN-γ (MIG), <15 pg/ml; and IFN-inducible protein 10 (IP-10), <40 pg/ml.
Plethysmography.
Whole-body plethysmography (Buxco, Troy, NY) with unrestrained nonsedated mice was used to monitor the respiratory dynamics of the mice in a quantitative manner at the baseline (airway obstruction) and after methacholine exposure (airway hyperresponsiveness). Prior to methacholine exposure, the mice were allowed to acclimate to the chamber, and then plethysmography readings were recorded to establish baseline values. Next, the mice were exposed once to aerosolized methacholine (50 mg/ml); after exposure, plethysmography readings were recorded. Enhanced pause (Penh) is a dimensionless value that represents a function of the ratio of the peak expiratory flow to the peak inspiratory flow and a function of the timing of expiration. Penh correlates with pulmonary airflow resistance or obstruction. Penh, as measured by plethysmography, has previously been validated in animal models of airway hyperresponsiveness (16, 17, 37, 41).
Statistics.
Sigma Stat 2003 software (SPSS Science, San Rafael, CA) was used for all statistical analyses. The t test was used to compare the values for groups of animals treated with LBM415 versus those for animals treated with placebo at the same time point if the data were normally distributed. In the instances where the data were not normally distributed, the Mann-Whitney rank sum test was used for comparisons. The Spearman rank order test was used for correlations, as all the data taken together were not normally distributed. A comparison was considered statistically significant if the P value was <0.05.
RESULTS
In vitro data.
The MIC of LBM415 against M. pneumoniae was <0.005 μg/ml (Diagnostic Mycoplasma Laboratory).
Visual inspection.
No visual differences could be detected between the M. pneumoniae-infected mice treated with LBM415 and the mice that received placebo.
M. pneumoniae quantitative BAL fluid culture.
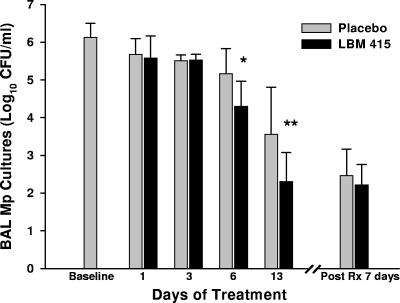
LBM415-treated mice had significantly lower BAL fluid M. pneumoniae concentrations than placebo-treated mice on days 6 and 13 of treatment (Fig. 1). The concentrations of M. pneumoniae in the BAL fluid of mice treated with LBM415 were not significantly different from those in the BAL fluid of the placebo-treated mice at 7 days after the completion of 13 days of therapy (Fig. 1). All control mice had negative BAL fluid cultures.
FIG. 1.
Quantitative M. pneumoniae (Mp) cultures of BAL fluid samples of mice inoculated with M. pneumoniae and treated (Rx) with LBM415 or placebo for 13 days (treatment began at 1 day after inoculation). Values shown are the means ± standard deviations (error bars). Bars represent results from two independent experiments, each of which included four to five mice per time point in each group. *, P < 0.05 between infected mice treated with LBM415 and infected mice treated with placebo; **, P < 0.005 between infected mice treated with LBM415 and infected mice treated with placebo.
Lung histopathology.
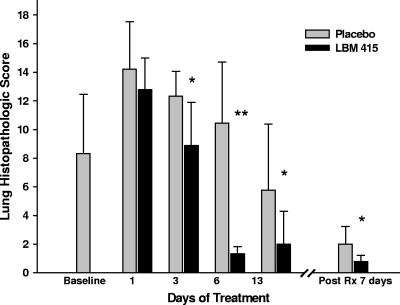
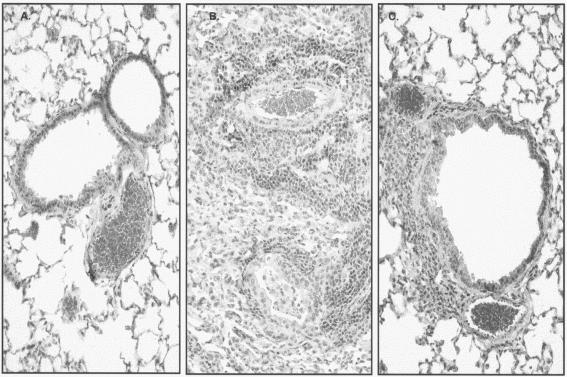
Treatment with LBM415 decreased the lung HPSs at all time points evaluated compared with the lung HPSs for the placebo-treated mice, reaching statistical significance on days 3, 6, and 13 of treatment (Fig. 2). In contrast to M. pneumoniae BAL fluid culture, the lung HPS remained significantly lower in the mice treated with LBM415 than in the mice treated with placebo 7 days after they had received LBM415 therapy for 13 days (Fig. 2). Control mice had HPSs of 0 to 1. Figure 3 presents the comparative histopathological appearance of a representative untreated control mouse lung and a representative M. pneumoniae-infected mouse lung treated with placebo or LBM415 on day 6 of therapy. The lungs from control, untreated mice demonstrated no perivascular or peribronchial inflammatory infiltrates, pneumonia, or monocytic intra-alveolar infiltrates. By contrast, the lungs of M. pneumoniae-infected mice receiving placebo demonstrated dense peribronchial and perivascular circumferential mononuclear inflammatory infiltrates (lymphocytes and histiocytes) and acute parenchymal pneumonia (neutrophilic alveolar infiltrates obliterated the air spaces). The lungs of LBM415-treated infected mice showed considerably less dense and smaller noncircumferential perivascular and peribronchial mononuclear infiltrates and no pneumonia.
FIG. 2.
Effect of treatment (Rx) with LBM415 on lung HPS of mice inoculated with M. pneumoniae (Mp). Infected mice were treated with LBM415 or placebo daily for 13 days starting at 1 day after inoculation. Values shown are the means ± standard deviations (error bars). Bars represent results from two independent experiments, each of which included four to five mice per time point in each group. *, P < 0.05 between the HPS of infected mice treated with LBM415 and infected mice treated with placebo; **, P < 0.001 between the HPS of infected mice treated with LBM415 and infected mice treated with placebo.
FIG. 3.
Comparative histopathological appearance of untreated control mouse lung (A) and M. pneumoniae-infected lungs of mice treated with placebo (B) or LBM415 (C) on day 6 of therapy. Magnifications, ×20.
Plethysmography.
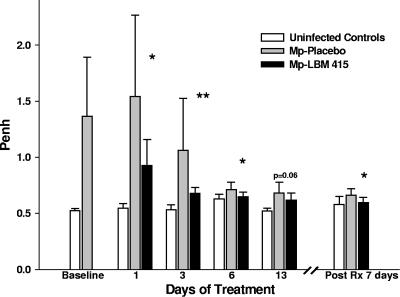
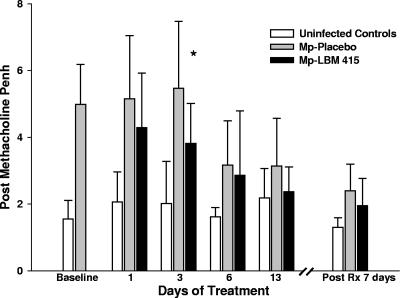
Therapy with LBM415 significantly reduced airway obstruction, as measured by Penh, compared with treatment with placebo (Fig. 4). The difference in airway obstruction between the LBM415 treatment group and the placebo group was significant after only 1 day of LBM415 therapy, and it was sustained 7 days after the completion of 13 days of therapy (Fig. 4). Airway hyperresponsiveness, as measured by Penh after standardized methacholine challenge, was lower in LBM415-treated mice than in the placebo-treated mice at all time points, but the difference reached statistical significance only on day 3 of treatment (Fig. 5).
FIG. 4.
Effect of treatment (Rx) with LBM415 on airway obstruction of mice inoculated with M. pneumoniae (Mp). Infected mice were treated with LBM415 or placebo daily for 13 days starting at 1 day after inoculation. Untreated controls were inoculated with sterile SP4. Airway obstruction was assessed by whole-body plethysmography by measuring Penh. Values shown are the means ± standard deviations (error bars). *, P < 0.05 005 between infected mice treated with LBM415 (n = 12) and infected mice treated with placebo (n = 12); **, P < 0.005 between infected mice treated with LBM415 (n = 12) and infected mice treated with placebo (n = 12). Values represent results from two independent experiments.
FIG. 5.
Effect of treatment (Rx) with LBM415 on airway hyperresponsiveness of mice inoculated with M. pneumoniae (Mp). Infected mice were treated with LBM415 or placebo daily for 13 days starting at 1 day after inoculation. Untreated controls were inoculated with sterile SP4. Airway hyperresponsiveness was assessed by whole-body plethysmography by measuring Penh after methacholine challenge. Values shown are the means ± standard deviations (error bars). *, P < 0.05 between infected mice treated with LBM415 (n = 12) and infected mice treated with placebo (n = 12). Values represent the results from two independent experiments.
BAL fluid cytokines and chemokines.
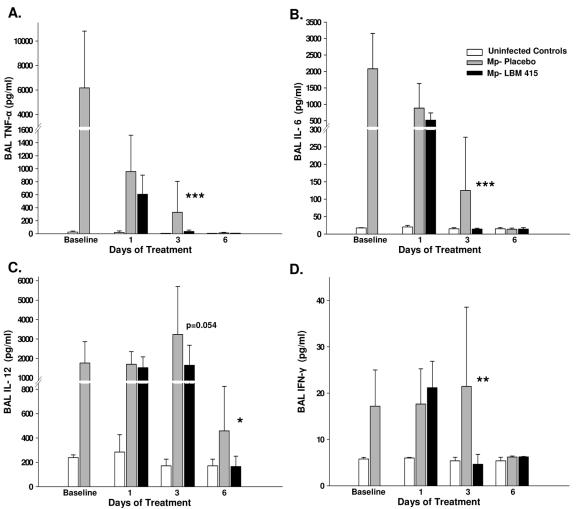
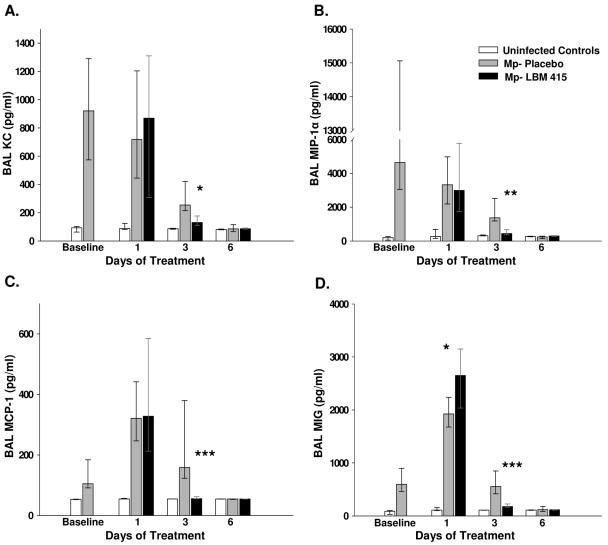
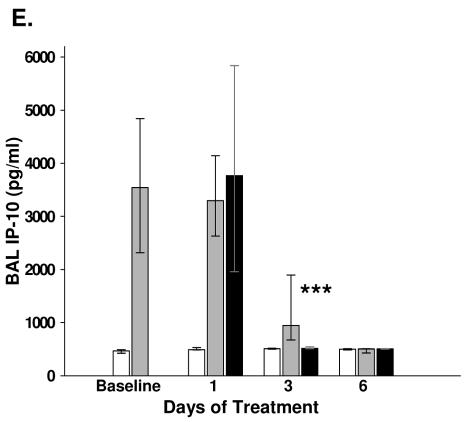
The concentrations of TNF-α, IFN-γ, IL-6, IL-12, KC, MCP-1, MIP-1α, MIG, and IP-10 were significantly reduced in the BAL fluid of mice treated with LBM415 compared with the levels in the placebo-treated mice (Fig. 6 and 7). There were no differences in the BAL fluid concentrations of IL-1β, GM-CSF, IL-2, IL-4, IL-5, and IL-10 between the LBM415-treated mice and the placebo-treated mice.
FIG. 6.
Cytokine concentrations in BAL fluid specimens from mice inoculated with M. pneumoniae (Mp) and treated with LBM415 versus placebo for 13 days starting 1 day after inoculation. Untreated controls were inoculated with sterile SP4. Values shown are the means ± standard deviations (error bars). Bars represent results from two independent experiments, each of which included four to five mice per time point in each group. *, P < 0.05 between infected mice treated with LBM415 and infected mice treated with placebo; **, P < 0.005 between infected mice treated with LBM415 and infected mice treated with placebo; ***, P < 0.001 between infected mice treated with LBM415 and infected mice treated with placebo.
FIG. 7.
Chemokine concentrations in BAL fluid specimens from mice inoculated with M. pneumoniae (Mp) and treated with LBM415 or placebo for 13 days starting 1 day after inoculation. Untreated controls were inoculated with sterile SP4. Values shown are the medians and the 25th to 75th percentiles (error bars). The bars represent the results from two independent experiments, each of which included four to five mice per time point in each group. *, P < 0.05 between infected mice treated with LBM415 and infected mice treated with placebo; **, P < 0.005 between infected mice treated with LBM415 and infected mice treated with placebo; ***, P < 0.001 between infected mice treated with LBM415 and infected mice treated with placebo.
Correlations.
The correlations among markers of disease severity, BAL fluid cytokine and chemokine levels, and therapy with LBM415 on day 3 of treatment are shown in Table 1. Therapy with LBM415 demonstrated significant inverse correlations with airway obstruction, airway hyperresponsiveness, and lung HPS on day 3 of therapy (Table 2). On day 6 of treatment, significant inverse correlations between LBM415 therapy and BAL fluid M. pneumoniae culture results, airway obstruction, and HPS were found (Table 3).
TABLE 1.
Correlations on day 3 of treatment of M. pneumoniae culture, histopathological score, airway obstruction, and airway hyperresponsiveness with cytokine and chemokine concentrations in BAL fluid samples of mice inoculated with M. pneumoniae and treated with LBM415 or placeboa
| Cytokine or chemokine | Mp culture
|
HPS
|
AO
|
AHR
|
Therapy with LBM415
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | n | r | P | n | r | P | n | r | P | n | r | P | n | |
| TNF-α | 0.19 | 0.441 | 18 | 0.72 | <0.001 | 18 | 0.86 | <0.001 | 16 | 0.45 | 0.073 | 16 | −0.80 | <0.001 | 18 |
| IFN-γ | 0.12 | 0.615 | 18 | 0.81 | <0.001 | 18 | 0.83 | <0.001 | 16 | 0.79 | <0.001 | 16 | −0.71 | <0.001 | 18 |
| IL-6 | 0.02 | 0.915 | 18 | 0.71 | <0.001 | 18 | 0.90 | <0.001 | 16 | 0.73 | <0.001 | 16 | −0.80 | <0.001 | 18 |
| IL-12 | 0.13 | 0.574 | 18 | 0.57 | 0.011 | 18 | 0.74 | <0.001 | 16 | 0.46 | 0.067 | 16 | −0.50 | 0.033 | 18 |
| KC | 0.28 | 0.258 | 17 | 0.46 | 0.059 | 17 | 0.59 | 0.019 | 15 | 0.22 | 0.410 | 15 | −0.62 | 0.007 | 17 |
| MIP-1α | 0.25 | 0.307 | 17 | 0.67 | 0.002 | 17 | 0.80 | <0.001 | 15 | 0.52 | 0.046 | 15 | −0.79 | <0.001 | 17 |
| MCP-1 | 0.23 | 0.361 | 17 | 0.59 | 0.012 | 17 | 0.77 | <0.001 | 15 | 0.51 | 0.046 | 15 | −0.84 | <0.001 | 17 |
| MIG | 0.08 | 0.737 | 17 | 0.67 | 0.002 | 17 | 0.83 | <0.001 | 15 | 0.52 | 0.044 | 15 | −0.84 | <0.001 | 17 |
| IP-10 | 0.06 | 0.797 | 16 | 0.59 | 0.015 | 16 | 0.77 | <0.001 | 14 | 0.56 | 0.033 | 14 | −0.83 | <0.001 | 16 |
Abbreviations: Mp, M. pneumoniae; AO, airway obstruction; AHR, airway hyperresponsiveness; n, number of mice. Boldface indicates statistically significant values.
TABLE 2.
Correlations on day 3 of treatment among outcome variables in mice inoculated with M. pneumoniae and treated with LBM415 and those in inoculated mice treated with placeboa
| Variable | Mp culture
|
AO
|
AHR
|
HPS
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | n | r | P | n | r | P | n | r | P | n | |
| Therapy with LBM415 | 0.13 | 0.574 | 18 | −0.86 | <0.001 | 16 | −0.56 | 0.020 | 16 | −0.62 | 0.005 | 18 |
| Mp culture | −0.14 | 0.578 | 16 | −0.11 | 0.664 | 16 | 0.25 | 0.309 | 18 | |||
| AO | 0.65 | 0.005 | 16 | 0.63 | 0.008 | 16 | ||||||
| AHR | 0.47 | 0.061 | 16 | |||||||||
Abbreviations: Mp, M. pneumoniae; AO, airway obstruction; AHR, airway hyperresponsiveness; n, number of mice. Boldface indicates statistically significant values.
TABLE 3.
Correlations on day 6 of treatment among outcome variables in mice inoculated with M. pneumoniae and treated with LBM415 and those in inoculated mice treated with placeboa
| Variable | Mp culture
|
AO
|
AHR
|
HPS
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | n | r | P | n | r | P | n | r | P | n | |
| Therapy with LBM415 | −0.58 | 0.010 | 18 | −0.65 | 0.006 | 16 | −0.24 | 0.354 | 16 | −0.88 | <0.001 | 18 |
| Mp culture | 0.48 | 0.053 | 16 | 0.31 | 0.233 | 16 | 0.73 | <0.001 | 18 | |||
| AO | 0.51 | 0.040 | 16 | 0.53 | 0.034 | 16 | ||||||
| AHR | 0.24 | 0.336 | 16 | |||||||||
Abbreviations: Mp, M. pneumoniae; AO, airway obstruction; AHR, airway hyperresponsiveness; n, number of mice. Boldface indicates statistically significant values.
DISCUSSION
In the present study, therapy with LBM415 significantly improved the manifestations of experimental acute pulmonary M. pneumoniae infection by reducing the levels of the organism in quantitative cultures, pulmonary inflammation, airway obstruction, and airway hyperreactivity.
PDF is an essential bacterial metalloenzyme that deformylates the N-formylmethionine of newly synthesized polypeptides (6, 22). Its essential role in bacterial protein synthesis and maturation, but not in eukaryote cytoplasmic protein production, makes it a promising target in the development of new antimicrobial agents (1). LBM415 has in vitro activity against a wide spectrum of pathogens, including Streptococcus pneumoniae, group A and B streptococci, staphylococci, enterococci, M. pneumoniae, Chlamydophila pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Neisseria meningitidis (8, 11, 24). LBM415 retains activity against many antimicrobial-resistant gram-positive organisms, including methicillin-resistant Staphylococcus aureus, penicillin-nonsusceptible S. pneumoniae, and vancomycin- and linezolid-resistant enterococci (25). Cross-resistance with other classes of antimicrobials has not been identified.
In a study by Reddy et al., LBM415 demonstrated excellent in vitro activity against 100 M. pneumoniae strains collected from a broad geographical area over a 10-year period from the respiratory tracts of individuals with proven respiratory disease (MIC90 = 0.001 μg/ml). The minimum bactericidal concentration and time-kill assays indicated that LBM415 is bacteriostatic against M. pneumoniae (N. B. Reddy, D. M. Crabb, L. B. Duffy, and K. B. Waites, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-2050, 2004), an observation that is consistent with what has been reported for the activity of LBM415 against other pathogens, such as Staphylococcus aureus.
The pharmacokinetic profile of LBM415 in rodents was recently reported (D. Chen, V. Tembe, J. Cramer, L. Ramos, B. Press, G. Withers, R. Jain, A. Sundaram, S. Alvarez, K. Bracken, K. Dean, B. Weidmann, D. Patel, R. White, and Z. Yuan, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1965, 2004). In that study, mice were given an intravenous dose of 4.9 mg/kg and an oral dose of 14.9 mg/kg. LBM415 was rapidly absorbed after oral administration, with the time to the maximum concentration in plasma occurring within 0.5 h; the mean maximum concentration in plasma was 0.76 mg/liter, and the oral bioavailability ranged from 22 to 101%. LBM415 was rapidly distributed to all tissues, with twofold higher levels of drug exposure in the lungs than in serum. In vivo pharmacodynamic studies with mice suggest that LBM415 has a prolonged postantibiotic effect and that the 24-h area under the concentration-time curve/MIC ratio is the parameter that best correlates with in vivo efficacy (W. A. Craig and D. R. Andes, Abstr. 14th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. P921 and P922, 2004).
Eradication of M. pneumoniae from the respiratory tract has proven difficult in some patients, which underscores the fact that the organism is inhibited but not killed by the antibiotics most commonly used for treatment of this infection (13, 38, 45). In animal models of M. pneumoniae pneumonia, treatment with quinolones, ketolides, clarithromycin, and azithromycin has failed to eradicate this organism from the respiratory tract (2, 21, 35, 39,; A. M. Rios, M. Fonseca-Aten, A. Mejias, S. Chavez-Bueno, A. M. Gomez, O. Ramilo, G. H. McCracken, and R. D. Hardy, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1672, 2003). In agreement with these reports, the present study demonstrated that although LBM415 therapy reduced the levels of the organism in quantitative M. pneumoniae cultures, it did not result in elimination of the organism. The ability of M. pneumoniae to reside and replicate in human cells has recently been described (9, 31). The possibility that M. pneumoniae might move transiently into an intracellular reservoir could explain the common finding of the prolonged recovery of this organism from respiratory tract secretions even after clinically successful therapy.
In the present investigation, therapy with LBM415 had a more pronounced effect on pulmonary histologic inflammation than on quantitative M. pneumoniae cultures. The lung HPS was significantly reduced in LBM415-treated mice after only 3 days of treatment, a time at which no significant differences in BAL fluid M. pneumoniae concentrations were found. Furthermore, a significant difference in the lung HPS was still present 7 days after the completion of therapy with LBM415, while no difference in the levels of the organism in quantitative M. pneumoniae cultures was observed at this time. A similar dissociation between histologic and microbiologic improvement in response to antimicrobial therapy has previously been demonstrated in studies that evaluated clarithromycin, cethromycin (a ketolide), and azithromycin for the treatment of experimental M. pneumoniae pneumonia (21, 35; Rios et al., 43rd ICAAC).
Previous investigations with M. pneumoniae-infected BALB/c mice have shown that the concentrations of TNF-α, IFN-γ, IL-6, IL-12, KC, MIP-1α, and MCP-1 in BAL fluid are significantly reduced by antibiotic therapy effective against M. pneumoniae, while the BAL fluid concentrations of GM-CSF, IL-2, IL-4, and IL-10 are not affected by antibiotic therapy (21, 35). In addition, the reductions in pulmonary cytokines and chemokines observed were associated with improvement in pulmonary histological inflammation and airway obstruction. These findings are consistent with the results of the present study and suggest that the cytokines and chemokines affected by antimicrobial therapy have a role in orchestrating pulmonary inflammation and function after M. pneumoniae infection. Furthermore, evidence from human and animal studies also suggests that the more vigorous the immune response and cytokine stimulation in response to M. pneumoniae infection is, the more severe the pulmonary injury and clinical illness are (5, 23, 34, 40).
Recent studies have demonstrated a pathogenic role for the chemokine receptor CXCR3 and its ligands in human inflammatory diseases (3, 18, 26). IP-10-CXC chemokine ligand (CXCL)10 and MIG-CXCL9 are among the major CXCR3 ligands. These chemokines are induced by IFN-γ, are potent chemoattractants for mononuclear cells, and are thought to promote Th1 immune responses (12, 27, 32). We measured for the first time the BAL fluid concentrations of IP-10 and MIG in our model of experimental M. pneumoniae pneumonia. The M. pneumoniae-infected mice had higher BAL fluid concentrations of IP-10 and MIG than the noninfected controls. Additionally, we found that therapy with LBM415 significantly reduced the BAL fluid concentrations of these chemokines. These findings are consistent with those of our previous studies and suggest that Th1 cytokines and chemokines are associated with disease severity in our model.
The mechanism for the significant improvement in markers of disease severity (pulmonary HPS, airway obstruction, and BAL fluid cytokine and chemokine concentrations) associated with LBM415 therapy, in spite of its modest antimicrobiological effect, is unknown. Although M. pneumoniae is not known to produce any exotoxins (45), we speculate that the beneficial effect of LBM415 treatment is related at least in part to inhibition of protein synthesis, with the resultant suppression of mycoplasma virulence factors.
In the present study, therapy with LBM415 significantly improved the manifestations of acute pulmonary M. pneumoniae infection. Future studies are needed to determine whether the dissociation between the microbiologic and the inflammatory responses is related to suppression of mycoplasma virulence factors or a host immunomodulatory effect.
Acknowledgments
This study was funded in part by a grant from Novartis Pharmaceuticals.
REFERENCES
- 1.Apfel, C. M., H. Locher, S. Evers, B. Takacs, C. Hubschwerlen, W. Pirson, M. G. Page, and W. Keck. 2001. Peptide deformylase as an antibacterial drug target: target validation and resistance development. Antimicrob. Agents Chemother. 45:1058-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai, S., Y. Gohara, A. Akashi, K. Kuwano, M. Nishimoto, T. Yano, K. Oizumi, K. Takeda, and T. Yamaguchi. 1993. Effects of new quinolones on Mycoplasma pneumoniae-infected hamsters. Antimicrob. Agents Chemother. 37:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belperio, J. A., M. P. Keane, M. D. Burdick, J. P. Lynch III, Y. Y. Xue, K. Li, D. J. Ross, and R. M. Strieter. 2002. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J. Immunol. 169:1037-1049. [DOI] [PubMed] [Google Scholar]
- 4.Cimolai, N., G. P. Taylor, D. Mah, and B. J. Morrison. 1992. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 36:465-478. [DOI] [PubMed] [Google Scholar]
- 5.Cimolai, N., D. Wensley, M. Seear, and E. T. Thomas. 1995. Mycoplasma pneumoniae as a cofactor in severe respiratory infections. Clin. Infect. Dis. 21:1182-1185. [DOI] [PubMed] [Google Scholar]
- 6.Clements, J. M., R. P. Beckett, A. Brown, G. Catlin, M. Lobell, S. Palan, W. Thomas, M. Whittaker, S. Wood, S. Salama, P. J. Baker, H. F. Rodgers, V. Barynin, D. W. Rice, and M. G. Hunter. 2001. Antibiotic activity and characterization of BB-3497, a novel peptide deformylase inhibitor. Antimicrob. Agents Chemother. 45:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clyde, W. A., Jr. 1983. Mycoplasma pneumoniae respiratory disease symposium: summation and significance. Yale J. Biol. Med. 56:523-527. [PMC free article] [PubMed] [Google Scholar]
- 8.Credito, K., G. Lin, L. M. Ednie, and P. C. Appelbaum. 2004. Antistaphylococcal activity of LBM415, a new peptide deformylase inhibitor, compared with those of other agents. Antimicrob. Agents Chemother. 48:4033-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallo, S. F., and J. B. Baseman. 2000. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb. Pathog. 29:301-309. [DOI] [PubMed] [Google Scholar]
- 10.Duffy, L. B., D. M. Crabb, X. Bing, and K. B. Waites. 2003. Bactericidal activity of levofloxacin against Mycoplasma pneumoniae. J. Antimicrob. Chemother. 52:527-528. [DOI] [PubMed] [Google Scholar]
- 11.Ednie, L. M., G. Pankuch, and P. C. Appelbaum. 2004. Antipneumococcal activity of LBM415, a new peptide deformylase inhibitor, compared with those of other agents. Antimicrob. Agents Chemother. 48:4027-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farber, J. M. 1990. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc. Natl. Acad. Sci. USA 87:5238-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foy, H. M., J. T. Grayston, G. E. Kenny, E. R. Alexander, and R. McMahan. 1966. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA 197:859-866. [PubMed] [Google Scholar]
- 14.Fritsche, T. R., G. J. Moet, and R. N. Jones. 2004. Commercial broth microdilution panel validation and reproducibility trials for NVP PDF-713 (LBM 415), a novel inhibitor of bacterial peptide deformylase. Clin. Microbiol. Infect. 10:857-860. [DOI] [PubMed] [Google Scholar]
- 15.Gendrel, D., J. Raymond, F. Moulin, J. L. Iniguez, S. Ravilly, F. Habib, P. Lebon, and G. Kalifa. 1997. Etiology and response to antibiotic therapy of community-acquired pneumonia in French children. Eur. J. Clin. Microbiol. Infect. Dis. 16:388-391. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalo, J. A., C. M. Lloyd, D. Wen, J. P. Albar, T. N. Wells, A. Proudfoot, A. C. Martinez, M. Dorf, T. Bjerke, A. J. Coyle, and J. C. Gutierrez-Ramos. 1998. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188:157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156:766-775. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, W. W., B. Lu, W. Gao, V. Csizmadia, K. Faia, J. A. King, S. T. Smiley, M. Ling, N. P. Gerard, and C. Gerard. 2000. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J. Exp. Med. 192:1515-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy, R. D., H. S. Jafri, K. Olsen, J. Hatfield, J. Iglehart, B. B. Rogers, P. Patel, G. Cassell, G. H. McCracken, and O. Ramilo. 2002. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect. Immun. 70:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy, R. D., H. S. Jafri, K. Olsen, M. Wordemann, J. Hatfield, B. B. Rogers, P. Patel, L. Duffy, G. Cassell, G. H. McCracken, and O. Ramilo. 2001. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect. Immun. 69:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy, R. D., A. M. Rios, S. Chavez-Bueno, H. S. Jafri, J. Hatfield, B. B. Rogers, G. H. McCracken, and O. Ramilo. 2003. Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae-induced pneumonia. Antimicrob. Agents Chemother. 47:1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, X., K. T. Nguyen, V. C. Jiang, D. Lofland, H. E. Moser, and D. Pei. 2004. Macrocyclic inhibitors for peptide deformylase: a structure-activity relationship study of the ring size. J. Med. Chem. 47:4941-4949. [DOI] [PubMed] [Google Scholar]
- 23.Ito, S., Y. Abe, K. Kinomoto, T. Saitoh, T. Kato, Y. Kohli, M. Kuriyama, T. Sakai, and T. Ishizaki. 1995. Fulminant Mycoplasma pneumoniae pneumonia with marked elevation of serum soluble interleukin-2 receptor. Intern. Med. 34:430-435. [DOI] [PubMed] [Google Scholar]
- 24.Jones, R. N., T. R. Fritsche, and H. S. Sader. 2004. Antimicrobial spectrum and activity of NVP PDF-713, a novel peptide deformylase inhibitor, tested against 1,837 recent gram-positive clinical isolates. Diagn. Microbiol. Infect. Dis. 49:63-65. [DOI] [PubMed] [Google Scholar]
- 25.Jones, R. N., G. J. Moet, H. S. Sader, and T. R. Fritsche. 2004. Potential utility of a peptide deformylase inhibitor (NVP PDF-713) against oxazolidinone-resistant or streptogramin-resistant gram-positive organism isolates. J. Antimicrob Chemother. 53:804-807. [DOI] [PubMed] [Google Scholar]
- 26.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2000. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 27.Luster, A. D., and J. V. Ravetch. 1987. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J. Exp. Med. 166:1084-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marston, B. J., J. F. Plouffe, T. M. File, Jr., B. A. Hackman, S. J. Salstrom, H. B. Lipman, M. S. Kolczak, R. F. Breiman, et al. 1997. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. Arch. Intern. Med. 157:1709-1718. [PubMed] [Google Scholar]
- 29.Matsuoka, M., M. Narita, N. Okazaki, H. Ohya, T. Yamazaki, K. Ouchi, I. Suzuki, T. Andoh, T. Kenri, Y. Sasaki, A. Horino, M. Shintani, Y. Arakawa, and T. Sasaki. 2004. Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob. Agents Chemother. 48:4624-4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCracken, G. H., Jr. 1986. Current status of antibiotic treatment for Mycoplasma pneumoniae infections. Pediatr. Infect. Dis. 5:167-171. [DOI] [PubMed] [Google Scholar]
- 31.Meseguer, M. A., A. Alvarez, M. T. Rejas, C. Sanchez, J. C. Perez-Diaz, and F. Baquero. 2003. Mycoplasma pneumoniae: a reduced-genome intracellular bacterial pathogen. Infect. Genet. Evol. 3:47-55. [DOI] [PubMed] [Google Scholar]
- 32.Moser, B., and P. Loetscher. 2001. Lymphocyte traffic control by chemokines. Nat. Immunol. 2:123-128. [DOI] [PubMed] [Google Scholar]
- 33.Principi, N., S. Esposito, F. Blasi, and L. Allegra. 2001. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin. Infect. Dis. 32:1281-1289. [DOI] [PubMed] [Google Scholar]
- 34.Radisic, M., A. Torn, P. Gutierrez, H. A. Defranchi, and P. Pardo. 2000. Severe acute lung injury caused by Mycoplasma pneumoniae: potential role for steroid pulses in treatment. Clin. Infect. Dis. 31:1507-1511. [DOI] [PubMed] [Google Scholar]
- 35.Rios, A. M., A. Mejias, S. Chavez-Bueno, M. Fonseca-Aten, K. Katz, J. Hatfield, A. M. Gomez, H. S. Jafri, G. H. McCracken, Jr., O. Ramilo, and R. D. Hardy. 2004. Impact of cethromycin (ABT-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of Mycoplasma pneumoniae lower respiratory infection. Antimicrob. Agents Chemother. 48:2897-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabato, A. R., A. J. Martin, B. P. Marmion, T. W. Kok, and D. M. Cooper. 1984. Mycoplasma pneumoniae: acute illness, antibiotics, and subsequent pulmonary function. Arch. Dis. Child. 59:1034-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarze, J., E. Hamelmann, K. L. Bradley, K. Takeda, and E. W. Gelfand. 1997. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J. Clin. Investig. 100:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, C. B., W. T. Friedewald, and R. M. Chanock. 1967. Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy. N. Engl. J. Med. 276:1172-1175. [DOI] [PubMed] [Google Scholar]
- 39.Takahata, M., M. Shimakura, R. Hori, K. Kizawa, Y. Todo, S. Minami, Y. Watanabe, and H. Narita. 2001. In vitro and in vivo efficacies of T-3811ME (BMS-284756) against Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 45:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka, H., M. Narita, S. Teramoto, T. Saikai, K. Oashi, T. Igarashi, and S. Abe. 2002. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest 121:1493-1497. [DOI] [PubMed] [Google Scholar]
- 41.van Schaik, S. M., G. Enhorning, I. Vargas, and R. C. Welliver. 1998. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J. Infect. Dis. 177:269-276. [DOI] [PubMed] [Google Scholar]
- 42.Waites, K. B., D. M. Crabb, X. Bing, and L. B. Duffy. 2003. In vitro susceptibilities to and bactericidal activities of garenoxacin (BMS-284756) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob. Agents Chemother. 47:161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waites, K. B., D. M. Crabb, and L. B. Duffy. 2003. In vitro activities of ABT-773 and other antimicrobials against human mycoplasmas. Antimicrob. Agents Chemother. 47:39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waites, K. B., D. M. Crabb, and L. B. Duffy. 2003. Inhibitory and bactericidal activities of gemifloxacin and other antimicrobials against Mycoplasma pneumoniae. Int. J. Antimicrob. Agents 21:574-577. [DOI] [PubMed] [Google Scholar]
- 45.Waites, K. B., and D. F. Talkington. 2004. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 17:697-728. [DOI] [PMC free article] [PubMed] [Google Scholar]