Abstract
A study explored the pharmacokinetics of tenofovir (300 mg administered once daily) and nelfinavir (1,250 mg twice daily) when coadministered in 29 healthy volunteers. Tenofovir, nelfinavir, and M8 pharmacokinetics was unaltered when tenofovir and nelfinavir were coadministered, and tenofovir administration did not affect the M8/nelfinavir area under the concentration-versus-time curve over the dosing interval (AUCtau) ratio. No interaction between tenofovir and nelfinavir was observed.
Sixteen antiretroviral agents are now licensed for use in Europe and the United States. Although these drugs can be combined into numerous different regimens, clinically relevant drug interactions may occur, limiting the number of effective combinations available to any one patient (1).
Nelfinavir (NFV) mesylate is a well-established human immunodeficiency virus type 1 (HIV type 1) protease inhibitor approved for administration twice daily (1,250 mg). NFV is naturally boosted by food and is metabolized by the cytochrome P450 isoforms 3A4 (which includes the majority of the protease inhibitors), 2C19, and 2D6 (12). Formation of its active metabolite, M8, is mediated primarily by 2C19, whereas M8 metabolism occurs through 3A4 (9).
The nucleotide reverse transcriptase inhibitor tenofovir disoproxil fumarate (TDF) is a prodrug, converted by plasma and tissue esterases to tenofovir and subsequently phosphorylated intracellularly to form tenofovir diphosphate (6), and when administered at 300 mg once daily has shown efficacy in both antiretroviral-naïve and -experienced patients (5, 13).
TDF is not metabolized by cytochrome P450 isoenzymes (2); it is not an inducer or inhibitor of these enzymes and is excreted in urine (6). Therefore, the potential for widespread drug interactions with drugs metabolized hepatically should be low. However, despite not sharing a common elimination pathway, clinically relevant drug interactions between TDF and protease inhibitors have been reported; the most notable of these is the reduction in atazanavir concentrations (4). Therefore, an atazanavir dose of 300 mg with 100 mg of ritonavir is recommended when atazanavir is used in combination with TDF. Interestingly, an increase (32%) in tenofovir plasma exposure following coadministration with lopinavir-ritonavir (8, 11) has also been observed; however, no dose adjustments are recommended for this combination. This highlights the complexity and unpredictability of some pharmacokinetic drug interactions for which the mechanisms remain unclear.
A recent study conducted with HIV-infected patients showed that the addition of TDF to an NFV-containing regimen had no effect on the pharmacokinetics of NFV (10).
The purpose of this study was to assess the pharmacokinetics of tenofovir, NFV, and M8 after administration of TDF and NFV mesylate separately and in combination in male and female HIV-seronegative healthy volunteers.
(Some of the data shown in this article have previously been submitted as a poster presentation at the 7th International Congress on Drug Therapy in HIV Infection, Glasgow, United Kingdom, 14 to 18 November 2004.)
Approval for the study was obtained from the local ethics committee (Riverside Research Ethics Committee), and all subjects gave written informed consent to participate in the study.
Subjects were randomized to either of the two treatment sequence groups (group I or II). Study drugs were administered within 5 min of consuming a standardized meal. On study days 7 (all subjects on TDF), 21 (group I on TDF and NFV, group II on NFV), and 35 (group I on NFV, group II on TDF and NFV) serial venous blood samples were collected to determine steady-state pharmacokinetics over 24 h after TDF dosing and over 12 h after NFV dosing.
The safety and tolerability of the study medications were evaluated on the basis of clinical adverse events, laboratory tests, vital signs, and physical examinations. Concentrations of tenofovir in plasma were determined by a validated high-performance liquid chromatographic method using mass spectrometric detection at Gilead Sciences, Inc. (Durham, NC). The standard curve of the assay spans concentration ranges of 10.0 to 1,000 ng/ml, with coefficients of determination for each analyte of 0.99 or greater. Between-batch precision (percent relative standard deviation [SD]) and accuracy (percent bias) for the lower limit of quantification samples (10.0 ng/ml) were within 7.9% and 5.2%, respectively. Within- and between-batch precision and accuracy measurements for quality control samples were within 8.3% and 8.1%, respectively. Nelfinavir and M8 concentrations were determined in serum by a validated high-performance liquid chromatographic method using mass spectrometric detection at Covance Bioanalytical Services, LLC (Indianapolis, IN). The standard curves of the assay span nelfinavir and M8 concentration ranges of 10.0 to 10,000 ng/ml, with within- and between-batch precision and accuracy for lower limits of quantification samples (10.0 ng/ml) meeting standard acceptance criteria for analytical standards and quality control samples.
The maximum observed plasma concentration (Cmax), the time taken to achieve the maximum concentration (Tmax), the last observed quantifiable plasma concentration of the drug (Clast), and the area under the concentration-versus-time curve over the dosing interval (AUCtau) for tenofovir, NFV, and M8 were calculated using a nonlinear curve fitting software package (WinNonlin, professional edition, version 4.1; Pharsight Corporation, Mountain View, CA).
All statistical calculations were performed using SAS, version 8.2 (SAS Institute; Cary, NC). The ratio of the geometric means and associated 90% confidence intervals (CIs) were constructed for the pharmacokinetic parameters Cmax, Clast, and AUCtau by use of the parameters for tenofovir or NFV administered alone as a reference. The study was designed to detect a 30% difference in the pharmacokinetic parameters with coadministration, and no change in the pharmacokinetics was to be concluded when the 90% CIs for the ratio of geometric least-squares means were within 70% to 143% for Cmax, Clast, and AUCtau.
Among the 32 (10 females; age and weight, 29 ± 7 years and 73.9 ± 9.9 kg, respectively) (means ± SD) subjects enrolled, 29 completed all phases of the study (2 were discontinued because of protocol violations and 1 because of personal reasons).
The adverse events observed were of mild or moderate severity, with no grade 3 or 4 or serious adverse event reported. A total of 13 of 32 (40.6%) subjects experienced an adverse event while on TDF alone, 20 of 31 (64.5%) while on NFV alone, and 18 of 30 (60.0%) while on both TDF and NFV. The most frequently reported treatment-related adverse events were diarrhea, headache, fatigue, nausea, and loose stools.
The calculated pharmacokinetic parameters of tenofovir and NFV (and M8) when administered alone and when coadministered are summarized in Table 1.
TABLE 1.
Pharmacokinetic parameters expressed as means and coefficient of variation percentages and statistical comparison of steady-state PK parameters of tenofovir, nelfinavir, and M8
| Parameter | Mean (% coefficient of variation) | Geometric least-squares mean ratio (%) | 90% CI | |
|---|---|---|---|---|
| Tenofovir PK | TDR (300 mg) (n = 29) | TDF (300 mg) + NFV (1,250 mg) (n = 29) | ||
| Cmax (ng/ml) | 310 (26.2) | 306 (30.1) | 97.7 | 91.0-105 |
| Clast (ng/ml) | 52.1 (19.9) | 57.7 (27.0) | 109 | 102-117 |
| AUCtau (ng · h/ml) | 2,620 (17.8) | 2,680 (26.1) | 101 | 95.1-107 |
| Nelfinavir PK | NFV (1,250 mg) (n = 29) | TDF (300 mg) + NFV (1,250 mg) (n = 29) | ||
| Cmax (ng/ml) | 4,310 (29.8) | 4,040 (37.9) | 91.7 | 85.1-98.7 |
| Clast (ng/ml) | 752 (61.0) | 771 (78.7) | 101 | 84.6-119 |
| AUCtau (ng · h/ml) | 27,400 (35.3) | 26,100 (41.1) | 93.1 | 85.1-102 |
| Metabolite M8 | NFV (1,250 mg) (n = 29) | TDF (300 mg) + NFV (1,250 mg) (n = 29) | ||
| Cmax (ng/ml) | 2,070 (42.7) | 1,920 (44.0) | 91.5 | 84.0-99.7 |
| Clast (ng/ml) | 263 (78.7) | 268 (93.1) | 97.9 | 83.7-115 |
| AUCtau (ng · h/ml) | 11,600 (51.4) | 11,000 (51.1) | 93.3 | 83.3-105 |
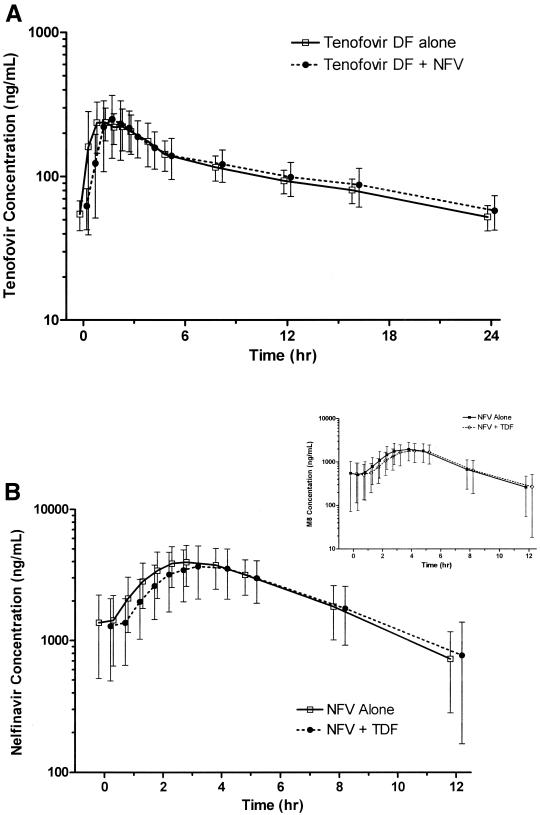
Figure 1A shows the plasma concentration-versus-time profiles for tenofovir and NFV when administered alone or in combination.
FIG. 1.
(A) Steady-state tenofovir (TFV) concentration-versus-time profiles in the absence and in the presence of nelfinavir. (B) Steady-state nelfinavir (and M8 [inset]) concentration-versus-time profiles in the absence and in the presence of TDF. Values are expressed as means ± SD (error bars).
Overall, the 90% CIs for the ratios of the geometric means after coadministration of tenofovir and NFV compared with the drugs administered alone for Cmax, Clast, and AUCtau were contained within the equivalence bounds of 70% to 143% (Table 1).
The steady-state ratios of M8 (active metabolite) to NFV (parent compound) for AUCtau were similar when NFV was administered alone (arithmetic mean, 0.44) or with TDF (0.45).
Here, we report equivalent steady-state pharmacokinetics of tenofovir, NFV, and M8 when TDF and NFV were dosed either alone or together, demonstrating that TDF and NFV do not have a pharmacokinetic drug interaction.
Furthermore, the ratios of the M8 (metabolite) AUC to the NFV (parent compound) AUC were similar in the absence and in the presence of TDF, indicating that tenofovir does not alter the extent of NFV metabolism.
The lack of an interaction between TDF and NFV was not unexpected, as the drugs do not share a common elimination pathway. These pharmacokinetic results agree with data obtained from HIV-infected subjects, which demonstrated the lack of an effect of TDF on NFV pharmacokinetics (10). Furthermore, no clinically meaningful interactions have been observed with other protease inhibitors, such as saquinavir-ritonavir administered at standard doses of 1,000/100 mg twice daily to both healthy volunteers (14) and HIV patients (3) and indinavir administered at 800 mg three times daily (7), which are also cleared predominantly by cytochrome P450 enzymes (mainly 3A4). However, there are a few noteworthy exceptions, such as the decreased atazanavir concentrations during coadministration with tenofovir (4) and the increased tenofovir plasma concentrations in the presence of atazanavir or lopinavir-ritonavir (4, 8, 11). Dose adjustments are unnecessary, but being extra vigilant for a potential increase in tenofovir-related adverse events may be warranted.
In this study, coadministration of TDF and NFV mesylate was generally well tolerated, with mild to moderate adverse events developing in 90.6% of the subjects overall.
In conclusion, this study provides reassuring pharmacokinetic data showing the lack of a pharmacokinetic interaction between TDF (300 mg once daily) and NFV mesylate (administered 1,250 mg twice daily) under steady-state conditions.
Acknowledgments
The source for financial support was Gilead Sciences, Inc.
REFERENCES
- 1.Back, D., G. Gatti, C. Fletcher, R. Garaffo, R. Haubrich, R. Hoetelmans, M. Kurowski, A. Luber, C. Merry, and C. F. Perno. 2002. Therapeutic drug monitoring in HIV infection: current status and future directions. AIDS 16(Suppl. 1):S5-S37. [DOI] [PubMed] [Google Scholar]
- 2.Birkus, G., M. Hájek, P. Kramata, I. Votruba, A. Holy, and B. Otová. 2002. Tenofovir diphosphate is a poor substrate and a weak inhibitor of rat DNA polymerases α, δ, and ɛ. Antimicrob. Agents Chemother. 46:1610-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boffito, M., D. Back, M. Stainsby-Tron, A. Hill, G. Di Perri, G. Moyle, M. Nelson, J. Tomkins, B. Gazzard, and A. Pozniak. 2005. Pharmacokinetics of saquinavir hard gel/ritonavir (1000/100 mg twice daily) when administered with tenofovir disoproxil fumarate in HIV-1-infected subjects. Br. J. Clin. Pharmacol. 59:38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bristol Meyer Squibb Company. 2004. Reyataz [atazanavir]: summary of product characteristics. Bristol Meyer Squibb Company, Princeton, N.J.
- 5.Gallant, J. E., S. Staszewski, A. L. Pozniak, E. DeJesus, J. M. Suleiman, M. D. Miller, D. F. Coakley, B. Lu, J. J. Toole, A. K. Cheng, and the 903 Study Group. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 292:191-201. [DOI] [PubMed]
- 6.Gilead Sciences. 2004. Viread [tenofovir diproxil fumarate]: summary of product characteristics. Gilead Sciences, Foster City, Calif.
- 7.Kearney, B., J. Flaherty, J. Wolf, J. Sayre, S. Gill, and D. Coakley. 2001. Lack of clinically relevant drug-drug interactions between tenofovir DF and efavirenz, indinavir, lamivudine, and lopinavir/ritonavir in healthy subjects, abstr. 171. Abstr. 8th Eur. Conf. Clin. Aspects Treatment HIV-Infect., Athens, Greece.
- 8.Kearney, B. P., A. Mittan, J. Sayre, F. Flaherty, I. Zhong, J. J. Toole, and A. K. Cheng. 2003. Pharmacokinetic drug interaction and long term safety profile of tenofovir DF and lopinavir/ritonavir, abstr. A-1617. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 9.Kerr, B., C. Lee, and G. Yuen. 1997. Overview of in vitro and in vivo drug interaction studies of nelfinavir mesylate (NFV), a new protease inhibitor, abstr. 373. Abstr. 4th Conf. Retrovir. Opportunistic Infect., Washington, D.C.
- 10.Kruse, G., S. Esser, H. Stocker, A. Breske, A. Koerber, M. Kopperman, H. Wiehler, B. Ross, C. Mocklinghoff, A. Hill, M. Becker, and M. Kurowski. 2005. The steady-state pharmacokinetics of nelfinavir in combination with tenofovir in HIV-infected patients. Antivir. Ther. 10:349-355. [PubMed] [Google Scholar]
- 11.Poirier, J., J. Meynard, J. Guiard-Schmid, P. Girard, W. Rozenbaum, and P. Jaillon. 2002. Lack of alteration of lopinavir and ritonavir through plasma concentrations in HIV-experienced patients treated with kaletra and tenofovir DF, abstr. H-1715. Abstr. 42nd Int. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 12.Roche. 2004. Viracept [nelfinavir]: summary of product characteristics. Roche, Welwyn Garden City, Hertfordshire, United Kingdom.
- 13.Squires, K., A. L. Pozniak, G. Pierone, C. R. Steinhart, Jr., D. Berger, N. C. Bellos, S. L. Becker, M. Wulfsohn, M. D. Miller, J. J. Toole, D. F. Coakley, A. Cheng, and the Study 907 Team. 2003. Tenofovir disoproxil fumarate in nucleoside-resistant HIV-1 infection: a randomized trial. Ann. Intern. Med. 139:313-320. [DOI] [PubMed] [Google Scholar]
- 14.Zong, J., G. Chttick, M. R. Blum, D. Hill, J. Begley, N. Adda, J. Shah, and B. P. Kearney. 2004. Pharmacokinetic assessment of tenofovir DF (TDF) and ritonavir (RTV)-boosted saquinavir (SQV/r) in healthy subjects, abstr. A-444. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.