Abstract
Nosocomial or late-onset sepsis is a common complication among premature infants, with a frequency inversely correlated with birth weight. Increased susceptibility to infection is due in part to an immature humoral (antibody-mediated) immune response. This study investigated the pharmacokinetics (PKs) and safety of a donor-selected specific intravenous immune globulin (IVIG) preparation, INH-A21 (Veronate), for prevention of sepsis in premature infants. Thirty-six infants weighing between 500 and 1,250 g during the first postnatal week were eligible to begin a series of up to four intravenous infusions of 500 or 750 mg/kg of body weight INH-A21. Blood samples were analyzed for antibodies against the Ser-Asp dipeptide repeat G (SdrG) and clumping factor A (ClfA) surface proteins of staphylococci. Sparse sampling and population PK analyses were performed to derive PK parameters. Following administration of the 500- and 750-mg/kg doses, the estimated average steady-state levels of anti-ClfA were 6.1 U/ml and 9.2 U/ml, respectively, and those of anti-SdrG were 5.2 U/ml and 7.7 U/ml, respectively. The elimination half-lives for anti-ClfA and anti-SdrG were 719 h and 701 h, respectively, and the clearances were 0.18 ml/h and 0.21 ml/h, respectively. In the final model, the values of the PK parameters were independent of gestational age. Both doses of INH-A21 were well tolerated, and the safety profile was similar to those of other IVIG preparations. These results suggest that a shorter dosing interval should be utilized between the first and second doses to achieve and maintain higher titers of anti-ClfA and anti-SdrG antibodies. Further studies examining INH-A21 for the prevention of late-onset sepsis in infants within the weight range studied are warranted.
Advances in medical care of very low-birth-weight (VLBW) infants have dramatically improved survival (13). Prolonged hospitalization of surviving premature infants increases the risk of complications, especially nosocomial (late-onset) infections. The NICHD Neonatal Network reported a late-onset sepsis rate of 16% among VLBW infants with birth weights between 501 and 1,500 g. The sepsis rate increased with decreasing birth weight, reaching 40% for the smallest infants (weights, 500 to 600 g) (6). Late-onset sepsis increases mortality, prolongs hospitalization, and contributes to adverse neurodevelopmental outcomes (3, 21, 22).
The predominant organisms responsible for late-onset sepsis in VLBW infants are coagulase-negative staphylococci (CoNS), primarily Staphylococcus epidermidis (2, 6, 21). Antimicrobial options for the treatment of CoNS infections are limited; and persistent infections, despite appropriate antibiotic therapy, have been reported (16). Staphylococcus aureus, especially strains resistant to multiple antibiotics, are also an increasing threat and in a recent study caused invasive disease in 7% of VLBW infants (8).
New approaches to the prevention of late-onset sepsis in premature infants are urgently needed. One strategy has been to enhance host defense by the administration of prophylactic intravenous immune globulin (IVIG). Systematic review of the clinical trials with nonselected donor IVIG preparations suggests that repeated administration of IVIG to premature infants is safe but is without consistent clinical benefit (9, 10, 12, 15). In contrast, IVIG preparations with high titers of specific antibodies do provide successful prophylaxis against selected pathogens (1, 20).
INH-A21 (Veronate) is an experimental donor-selected staphylococcal human IVIG under investigation as a prophylactic agent against staphylococcal infection in premature infants. It is a nanofiltered, solvent-detergent-treated, sucrose-free IVIG preparation containing elevated levels of antibodies against the staphylococcal fibrinogen binding proteins clumping factor A (ClfA) and Ser-Asp dipeptide repeat G (SdrG), which are found on virtually all S. aureus isolates and on approximately 60% of S. epidermidis strains, respectively. Neither of these antigens is expressed by other staphylococcal species. These antigens belong to a family of surface proteins called microbial surface components that recognize adhesive matrix molecules and play an important role in the adherence of bacteria to human tissues and serum-conditioned implanted biomedical materials (17, 18). INH-A21 is isotonic and contains 0.35 M NaCl in a 0.15 M glycine buffer.
The present study investigated the pharmacokinetics (PKs) and safety of two different doses of INH-A21 in a population of premature infants.
MATERIALS AND METHODS
The study was conducted at seven neonatal tertiary care centers in the United States. The protocol and parental consent forms were reviewed and approved by the institutional review boards of each participating institution. Written informed consent was obtained from the parent(s) or legal guardian of each infant enrolled. An independent data and safety monitoring board reviewed the available data at specified intervals throughout the study. This study was conducted in accordance with the guidelines of Good Clinical Practice established by the International Conference on Harmonization (http://www.fda.gov/cder/guidance/959fnl.pdf). The protocol was open for enrollment in August 2002, with the last patient follow-up in January 2003. A single lot of INH-A21 was used throughout the study, and it contained 0.776 U of anti-ClfA and 0.675 U of anti-SdrG per mg immunoglobulin G (IgG).
Study population.
Infants aged 3 to 7 days (postnatal age, 48 to 168 h), with body weights of ≥500 g and ≤1,250 g, were eligible for the study. Infants were excluded from the study if any of the following criteria were met: they had received or were likely to receive IVIG, a white blood cell transfusion, fresh frozen plasma, or a cryoprecipitate infusion prior to the first study drug administration; they had received or had prior exposure to an investigational agent; they had a culture-proven infection at the time of the planned first infusion of study drug; they had congenital heart disease; they had some other condition at the time of infusion(s) that, in the opinion of the investigator, would not allow safe administration of the study drug; or they had a severe congenital anomaly, an inborn error of metabolism, or a prenatal diagnosis of congenital immunodeficiency. All infants who received at least a partial dose were included in the evaluation for safety. Pharmacokinetic analyses were performed for all infants who received two doses of INH-A21 and for whom samples were available for analysis.
Study design and procedures.
This was an open-label, dose-escalation study. The first 18 infants (cohort 1) received INH-A21 at 500 mg/kg of body weight (10 ml/kg). The schedule of infusions followed that used for IVIG by Baker et al. (2). This schedule was reported to achieve and maintain significant levels of total IgG over a period of time corresponding to that for the risk of late-onset sepsis. The first infusion was given between postnatal days 3 and 7. Subsequent doses were given as follows: dose 2, 7 days after dose 1; dose 3, 14 days after dose 2; and dose 4, 14 days after dose 3. INH-A21 was infused only if intravenous (i.v.) access was present for routine medical care. The initial infusion rate was 0.5 ml/kg/h and was increased to 1.5 ml/kg/h and to a maximum of 3 ml/kg/h at 15-min intervals while the infant's vital signs were monitored. Vital signs were also measured at the end of the infusion. Investigators were instructed to consider the fluid volume of the study drug when calculating the total daily fluid volume to be provided to the infant. The infusion of INH-A21 was interrupted for the following changes in vital signs: decrease in systolic blood pressure to more than 25% of the baseline value and heart rate less than 120 beats per minute or greater than 200 beats per minute. INH-A21 was to be permanently discontinued if a fluid bolus or pharmacologic support was given for a blood pressure change that occurred during the infusion, the heart rate was less than 100 beats per minute, or there was any change in vital signs that required intervention other than interruption of the study drug.
Seven days after the enrollment of cohort 1, safety data were reviewed by the data and safety monitoring board prior to enrollment into the second cohort, which received a 750-mg/kg dose of INH-A21 on the same schedule that it was given to cohort 1. Infants were monitored from study day 1 through study day 70, study end, or termination, whichever came first.
Pharmacokinetic analysis.
All serum samples were assayed at a central laboratory (Inhibitex, Inc.) for the concentrations of total IgG by radial immunodiffusion assay and for the concentrations of anti-ClfA and anti-SdrG by enzyme-linked immunoassay, as described previously (19). The results of the anti-ClfA and anti-SdrG assays are reported in units per ml. For these analyses, for the lot of INH-A21 used in this study, 1 unit of anti-ClfA activity is equivalent to 1.25 mg of INH-A21 and 1 unit of anti-SdrG activity is equivalent to 1.43 mg of INH-A21.
Samples for PK testing were obtained according to the following schedule: (i) before the first infusion, (ii) 1 h (±5 min) after dose 1 infusion, (iii) 24 to 48 h after dose 1 administration, (iv) within 48 h prior to dose 2 administration, (v) within 48 h prior to dose 3 administration, (vi) within 48 h prior to dose 4 administration, (vii) 24 to 48 h after dose 4 administration, and (viii) 14 days (±2 days) after dose 4 administration. If i.v. access was discontinued before the second, third, or fourth dose was administered and a sample could not be obtained at 24 to 48 h, a serum or plasma PK sample was obtained immediately before or right after the i.v. access had been removed. Whenever possible, PK samples were obtained from serum or plasma remaining from the samples drawn for laboratory tests during routine infant care. If samples were not available, blood samples were drawn.
The PK analysis was performed by using NONMEM (version V.1; Globomax, Hanover, MD). A two-compartment open model (Advan 3, Trans 4) was chosen based on prior PK studies of IVIG products in newborn infants, and the FOCE subroutine was used to fit the data (4). An endogenous IgG production model was employed that accounted for maternal transfer in utero and that allowed production by the newborn infant. Although the overall number of subjects was limited, attempts were made to elucidate potential associations of anti-SdrG and anti-ClfA clearances with dosing group, birth weight, gestational age at birth, APGAR score, and gender. Pharmacokinetic model covariates were assessed by a univariate approach and required an objective function change of 7.9 (P < 0.005) for inclusion. Empirical Bayesian estimates were made for the individual subjects.
Safety evaluations.
Infants were evaluated for safety over 70 days or until discharge to home, death, or transfer to another institution. Serious adverse events were defined according to the criteria of the International Conference on Harmonization (www.fda.gov/medwatch/report/iche2a.pdf). Adverse events were graded by the investigator as mild, moderate, or severe. Events known to occur in association with prematurity were predefined (morbidities associated with prematurity) and collected separately, and the frequencies were calculated.
Statistical analysis.
Statistical analyses were descriptive. All continuous variables were summarized by using the number of subjects (n), mean, standard deviation, median, minimum, and maximum. Categorical measures were summarized by using the frequency and the percentage of the observed levels.
RESULTS
Subjects.
Of 37 infants randomized in this study, 36 were enrolled, stratified by weight, and treated; 21 infants weighed 500 to 900 g (lower enrollment weight stratum), and 15 infants weighed 901 to 1,250 g (higher enrollment weight stratum). Twenty-nine (81%) infants completed the study through study day 70. Six infants were lost to follow-up after transfer to another institution, and one infant died. The mean gestational age for all infants was 27.2 weeks (range, 24 to 32 weeks; median, 27.0 weeks). The mean birth weight for all infants was 904 g (ranges, 564 to 1,035 g in the lower enrollment weight stratum and 940 to 1,213 g in the higher enrollment weight stratum). The median birth weight for all infants was 877 g. Fifty-six percent of the infants were female, and 78% were Caucasian.
Pharmacokinetics.
A total of 202 PK samples from 36 subjects were collected. However, 10 samples displayed inconsistent concentrations of anti-ClfA, anti-SdrG, and total IgG compared to their recorded dosing histories (for example, an apparent trough level 1 to 2 h after a reported dose or an elevated level compared to a prior level without an intervening dose). Some samples to be collected prior to the second or third infusions had collection times that fell during or shortly after the infusion. These were excluded from the analysis.
In defining the population PK model, baseline levels of anti-ClfA and anti-SdrG immunoglobulin were observed, indicating significant maternal transfer to the infants or fetal production. This pretherapy activity was included in the model. However, endogenous postnatal infant production of anti-ClfA and anti-SdrG appeared to be negligible and was removed from the model. Infant weight was the only statistically significant covariate with PK parameters found in this evaluation. However, the weight effect did not reach significance for all parameters, notably, clearance. Gestational age did not significantly influence anti-ClfA or anti-SdrG PK parameters, indicating that identical anti-SdrG and anti-ClfA immunoglobulin exposures were achieved across the developmental spectrum studied. Dose levels (500 mg/kg versus 750 mg/kg) had no impact on clearance (CL), the volume of distribution of the central compartment (V1), or the steady-state volume of distribution (Vss), indicating predictable concentrations at various dose levels. The low intersubject variability combined with a low residual error (the difference between the predicted and the observed concentrations not explained by intersubject variability) suggests that consistent levels were achieved across the study population.
The PKs of INH-A21 activities, anti-ClfA and anti-SdrG, were well described by the population PK models used in this analysis, as illustrated in Fig. 1 to 4. Similar PK behaviors were observed overall for both anti-ClfA and anti-SdrG; however, there was no correlation between the terminal half-lives of the two antibodies. There were also no PK differences noted between the two dose groups and birth weight strata following i.v. administration of INH-A21. The total IgG concentrations were similar to those seen with multiple dosings on a similar schedule (Fig. 5). While limited data were collected after dose 3 and there was greater “noise” in the data with later samples, the final population PK models predicted later concentrations without becoming significantly biased.
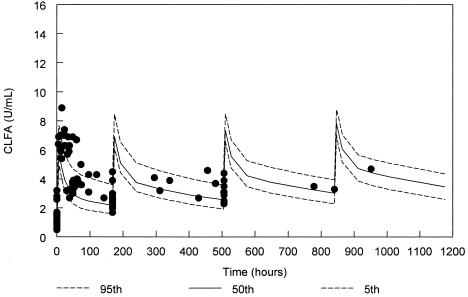
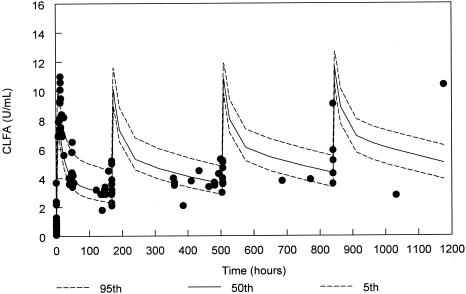
FIG. 1.
ClfA antibody levels for the 500-mg/kg cohort from the final population PK model with 90% confidence interval and actual datum points (solid circles). Due to variability in administration times for doses 2 to 4, samples are displayed based on times relative to the scheduled dose rather than the absolute clock times after the first dose.
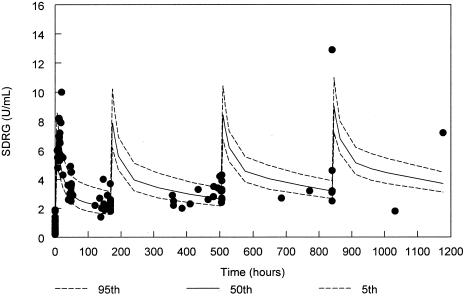
FIG. 4.
SdrG antibody levels for the 750-mg/kg cohort from the final population PK model with 90% confidence interval and actual datum points (solid circles). Due to variability in the administration times for doses 2 to 4, samples are displayed based on times relative to the scheduled dose rather than the absolute clock time after the first dose.
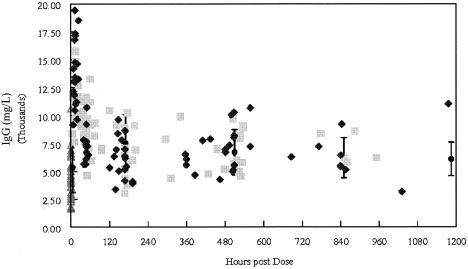
FIG. 5.
IgG concentrations from infants in the present study (gray squares, 500-mg/kg group; black diamonds, 750-mg/kg group) compared to the mean ± standard deviation reported by Baker et al. (2) (solid circles), adjusted for dose (to 750 mg/kg). Open triangles, predose concentrations.
Based on the final population PK models and population-estimated clearance, the average steady-state concentrations expected with 500 mg/kg and 750 mg/kg for anti-ClfA were 6.1 U/ml and 9.2 U/ml, respectively; and those for anti-SdrG were 5.2 U/ml and 7.7 U/ml, respectively. The estimated concentrations prior to dose 3 were approximately 20 to 25% higher than those prior to dose 2. For all infants, the mean (standard deviation) measured baseline (maternally transferred) plasma levels of anti-ClfA were 1.27 (±0.9) U/ml, and those of anti-SdrG were 0.8 (±0.6) U/ml. For anti-ClfA and anti-SdrG, the final PK model estimates for CL were 0.18 ml/h and 0.21 ml/h, respectively; those for V1 were 66 ml and 73 ml, respectively; those for Vss were 179 ml and 204 ml, respectively; and those for the elimination half-life were 719 h and 701 h, respectively. These are similar to the average of the Bayesian estimated PK parameters (Table 1). The overall intersubject variabilities were 14% for clearance and 16% for the volume of distribution, and the average residual errors expressed as the coefficient of variation for the population models were 13% for early samples (≤10 days) and 24% for late samples (>10 days).
TABLE 1.
Bayesian estimated pharmacokinetic parameters derived from the population PK model
| Cohort and parameter | Wt (g) | Gestational age (wk) | Anti-SdrG
|
Anti-ClfA
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 (ml/kg) | Vss (ml/kg) | CL (ml/h/kg) | t1/2βa (h) | t1/2αb (h) | V1 (ml/kg) | Vss (ml/kg) | CL (ml/h/kg) | t1/2β (h) | t1/2α (h) | |||
| 500 mg/kg | ||||||||||||
| Avg | 858 | 27.7 | 79 | 218 | 0.25 | 644 | 14.1 | 72 | 193 | 0.22 | 641 | 12.7 |
| SD | 160 | 2.2 | 10 | 28 | 0.05 | 134 | 1.8 | 20 | 53 | 0.04 | 119 | 0.1 |
| 750 mg/kg | ||||||||||||
| Avg | 837 | 26.6 | 79 | 162 | 0.26 | 633 | 14.1 | 75 | 201 | 0.22 | 651 | 12.7 |
| SD | 198 | 1.2 | 9 | 79 | 0.07 | 168 | 1.6 | 28 | 75 | 0.05 | 143 | 0.1 |
t1/2β, elimination half-life.
t1/2α, distribution half-life.
Safety.
As expected for this population of infants, all infants had at least one adverse event. Two infants had adverse events considered by the investigator to be drug related: one infant in the lower-enrollment-weight, 500-mg/kg group experienced hypertension; and one infant in the higher-enrollment-weight, 750-mg/kg group experienced hypoglycemia. No infants discontinued the study medication due to an adverse event. Three severe adverse events were reported. One infant (birth weight, 624 g; gestational age, 24 weeks) had received one infusion of INH-A21, 500 mg/kg, and died at postnatal age 8 days (3 days after the dose of INH-A21) following severe respiratory failure. Another infant developed severe sepsis due to Acinetobacter at postnatal age 25 days after receiving two doses of INH-A21, 500 mg/kg, with the second dose having been given 2 weeks prior to the onset. In the lower-enrollment-weight, 750-mg/kg group, one infant suffered a severe vascular accident in the right arm related to a brachial arterial puncture performed 4 days after the second dose of INH-A21. None of these severe events were considered to be related to INH-A21. There were no significant differences in the frequencies of morbidities commonly associated with prematurity across the dose groups (Table 2). Six infants had definitive CoNS infections (defined by positive cultures for blood samples taken from two separate sites); of these, four were in the lower-enrollment-weight stratum. One infant, also in the lower-enrollment-weight stratum, had S. aureus sepsis. This infant had received three doses of INH-A21, 750 mg/kg, but developed sepsis 30 days after the third infusion. The plasma anti-ClfA level was measured and was found to be 2.8 U/ml on the day before the onset of sepsis.
TABLE 2.
Summary of frequencies of selected morbidities commonly associated with prematuritya
| Morbidity | No. (%) of patients by wt stratum and dose
|
All patients (n = 36) | |||
|---|---|---|---|---|---|
| 500 g to 900 g
|
901 g to 1,250 g
|
||||
| 500 mg/kg (n = 11) | 750 mg/kg (n = 10) | 500 mg/kg (n = 7) | 750 mg/kg (n = 8) | ||
| Anemiab | 8 (73) | 9 (90) | 3 (43) | 8 (100) | 28 (78) |
| Apnea with or without bradycardia | 3 (27) | 7 (70) | 3 (43) | 2 (25) | 15 (42) |
| Bradycardia not temporally associated with infusion of study drug | 4 (36) | 4 (40) | 1 (14) | 2 (25) | 11 (31) |
| Bronchopulmonary dysplasia | 8 (73) | 8 (80) | 4 (57) | 5 (63) | 25 (69) |
| Necrotizing enterocolitis | 0 | 1 (10) | 2 (29) | 0 | 3 (8) |
| Patent ductus arteriosus | 2 (18) | 1 (10) | 0 | 0 | 3 (8) |
| Periventricular or intraventricular hemorrhage | 0 | 2 (20) | 0 | 1 (13) | 3 (8) |
| Retinopathy of prematurity | 3 (27) | 3 (30) | 0 | 2 (25) | 8 (22) |
Only morbidities diagnosed after enrollment and not morbidities for all infants with a diagnosis at any time are included. Data for patients who received any INH-A21 are included in this table, and data are for patients experiencing at least one morbidity in the designated category.
There was a statistically significant difference among treatment groups (P = 0.041 for the proportion of infants that developed anemia). The difference was not considered clinically relevant.
DISCUSSION
This was a phase I study for assessment of the PK parameters and initial safety and tolerance of INH-A21. The PKs for anti-ClfA and anti-SdrG in this study could be characterized by a two-compartment model by use of a population approach. The peak and trough concentrations for total IgG at the 500-mg/kg dose were similar to those reported by Baker et al. (2). In our population PK model, the PK parameters appeared to be independent of gestational age, with increased predose trough levels of anti-ClfA and anti-SdrG with each dose.
The dose level (500 mg/kg versus 750 mg/kg) had no impact on clearance, the volume of distribution of the central compartment, or the steady-state volume of distribution, indicating predictable concentrations at various dose levels. Low intersubject variability combined with a low residual error suggests that consistent levels were achieved across the study population. The PK models, with their long terminal half-lives, suggest that activity will be maintained for an extended period after the final dose is administered. However, the long half-life of IgG and the observed increase in trough (predose) levels over the course of the dosing regimen used in this study imply that shorter intervals between doses could provide overall higher levels of anti-ClfA and anti-SdrG during the period of peak risk of infection without increasing the total volume of INH-A21 infused.
The performance of PK studies with VLBW infants is challenging due to considerations of blood volume. We were able to scavenge samples for PK analysis and obtained, on average, 5.3 usable samples per subject. The baseline anti-ClfA and anti-SdrG titers acquired in utero and the complexity of the pharmacokinetics prevented a standard PK analysis for each subject. Although the model fit the data well, the limited quantity of PK data beyond the first 2 weeks of therapy limited the precision for terminal half-life estimation and its variability. The sparse data also prevented a robust assessment of developmental changes in antibody elimination or distribution. However, the quantity of PK data collected during the first two doses allowed good characterization of PK disposition early in therapy, a time interval that corresponds to the period of highest risk for infection. It also predicted the concentrations observed after the third dose without significant bias, suggesting good estimates for the typical model parameters.
The optimal levels of anti-ClfA and anti-SdrG required for the prevention of staphylococcal infection in infants are unknown. In rabbit models of S. aureus and S. epidermidis infections, single INH-A21 doses as low as 200 mg/kg to 300 mg/kg reduced bacterial colony counts (Inhibitex, Inc., submitted for publication). This is in spite of the very rapid elimination of INH-A21 in this species. Optimal dosing, defined by maximum possible activity, has not been identified, nor have higher doses been tested in these models. At the 750-mg/kg dose used in this study, the model-predicted steady-state average of anti-ClfA and anti-SdrG activities exceeded the peak concentrations seen in rabbits at 300 mg/kg (5.1 U/ml for anti-ClfA and 4.75 U/ml for anti-SdrG). This dose level is a reasonable upper limit based on the volume infused relative to the daily fluid requirement for premature infants. Additional dose-ranging studies examining preliminary efficacy will be reported elsewhere.
Overall, INH-A21 was well tolerated in this population of VLBW infants. The safety profile of INH-A21 was similar to those of other IVIG preparations (2, 7, 14, 15). Interruptions in administration of IVIG have been reported to occur in less than 1% of infusions and occurred in only 1 of 94 infusions of INH-A21 in this study. Changes in vital signs, including blood pressure, are known to occur with IVIG infusions and are easily managed by reducing the rate of infusion. While VLBW infants are at risk for hypoglycemia, INH-A21 does not contain glucose; thus, a source of glucose needs to be maintained during infusion. Among the morbidities generally considered to be associated with prematurity, only apnea and anemia were more common at the higher dose (750 mg/kg), but this was not consistent across the weight strata. The clinical significance of this finding, therefore, is uncertain. The higher frequency of anemia observed at the higher dose may be the consequence of chance and multiple comparisons. Hemolytic anemia has been reported in patients receiving high-dose IVIG therapy for autoimmune diseases (23). On the other hand, the difference noted in this study could have been the result of hemodilution, as has been noted by others (11). Assessment of the frequencies of anemia in premature infants is complex because these patients are constantly receiving red blood cell transfusions to compensate for the sampling for laboratory tests performed in the course of routine medical care. There was no significant difference in the mean total volume of packed red blood cells transfused to each dose group during the study (data not shown). Necrotizing enterocolitis (NEC) occurred in 3 (8%) infants, but it did not appear to be correlated with the IVIG dose or the weight stratum. While this study was not placebo controlled or large enough to identify a correlation, this rate of NEC is similar to that expected for this birth weight range, so no association between NEC and INH-A21 could be identified (5).
The current study developed a two-compartment population PK model that characterized the PK disposition of anti-ClfA and anti-SdrG in VLBW infants after INH-A21 administration. Due to their long elimination half-lives, the anti-ClfA and anti-SdrG titers persisted at concentrations that exceeded the levels associated with efficacy in an animal model of staphylococcal prophylaxis. Since the protective level in infants is unknown, an additional dose-ranging study should be performed to better define the pharmacodynamic relationship between the of INH-A21, plasma level, and any measurable reduction in the incidence of staphylococcal sepsis.
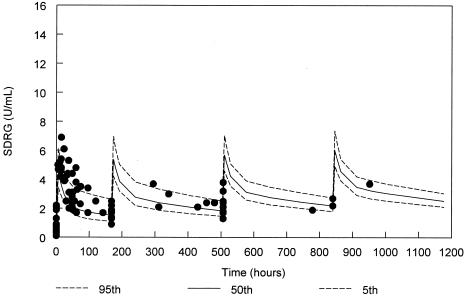
FIG. 2.
SdrG antibody levels for the 500-mg/kg cohort from the final population PK model with 90% confidence interval and actual datum points (solid circles). Due to variability in administration times for doses 2 to 4, samples are displayed based on times relative to the scheduled dose rather than absolute clock times after the first dose.
FIG. 3.
ClfA antibody levels for the 750-mg/kg cohort from the final population PK model with 90% confidence interval and actual datum points (solid circles). Due to variability in administration times for doses 2 to 4, samples are displayed based on times relative to the scheduled dose rather than the absolute clock times after the first dose.
Acknowledgments
We thank Maxine Okazaki and Amy Burdan for help with preparation of the manuscript and John Vernachio for assistance with the antibody assays.
This study was supported by Inhibitex, Inc., Alpharetta, Ga.
REFERENCES
- 1.Anonymous. 1997. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 99:93-99. [DOI] [PubMed] [Google Scholar]
- 2.Baker, C. J., M. E. Melish, R. T. Hall, D. T. Casto, U. Vasan, L. B. Givner, et al. 1992. Intravenous immune globulin for the prevention of nosocomial infection in low-birth-weight neonates. N. Engl. J. Med. 327:213-219. [DOI] [PubMed] [Google Scholar]
- 3.Barton, L., J. E. Hodgman, and Z. Pavlova. 1999. Causes of death in the extremely low birth weight infant. Pediatrics 103:446-451. [DOI] [PubMed] [Google Scholar]
- 4.Capparelli, E. V., and J. D. Connor. 2002. Population pharmacokinetics of IVIG in premature infants using an endogenous IgG production model. Clin. Pharmacol. Ther. 71:P4. [Google Scholar]
- 5.Fanaroff, A. A., M. Hack, and M. C. Walsh. 2003. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin. Perinatol. 27:281-287. [DOI] [PubMed] [Google Scholar]
- 6.Fanaroff, A. A., S. B. Korones, L. L. Wright, J. Verter, R. L. Poland, C. R. Bauer, J. E. Tyson, J. B. Philips III, W. Edwards, J. F. Lucey, C. S. Catz, S. Shankaran, and W. Oh. 1998. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. Pediatr. Infect. Dis. J. 17:593-598. [DOI] [PubMed] [Google Scholar]
- 7.Fanaroff, A. A., S. B. Korones, L. L. Wright, E. C. Wright, R. L. Poland, C. B. Bauer, J. E. Tyson, J. B. Philips III, W. Edwards, and J. F. Lucey. 1994. A controlled trial of intravenous immune globulin to reduce nosocomial infections in very-low-birth-weight infants. N. Engl. J. Med. 330:1107-1113. [DOI] [PubMed] [Google Scholar]
- 8.Healy, C. M., D. L. Palazzi, M. S. Edwards, J. R. Campbell, and C. J. Baker. 2004. Features of invasive staphylococcal disease in neonates. Pediatrics 114:953-961. [DOI] [PubMed] [Google Scholar]
- 9.Hill, H. R. 1993. Intravenous immunoglobulin use in the neonate: role in prophylaxis and therapy of infection. Pediatr. Infect. Dis. J. 12:549-558. [PubMed] [Google Scholar]
- 10.Jenson, H. B., and B. H. Pollock. 1997. Meta-analyses of the effectiveness of intravenous immune globulin for prevention and treatment of neonatal sepsis. Pediatrics 99:E2. [DOI] [PubMed] [Google Scholar]
- 11.Koffman, B. M., and M. C. Dalakas. 1997. Effect of high-dose intravenous immunoglobulin on serum chemistry, hematology, and lymphocyte subpopulations: assessments based on controlled treatment trials in patients with neurological diseases. Muscle Nerve 20:1102-1107. [DOI] [PubMed] [Google Scholar]
- 12.Lassiter, H. A. 1992. Intravenous immunoglobulin in the prevention and treatment of neonatal bacterial sepsis. Adv. Pediatr. 39:71-99. [PubMed] [Google Scholar]
- 13.Lemons, J. A., C. R. Bauer, W. Oh, S. B. Korones, L. A. Papile, B. J. Stoll, J. Verter, M. Temprosa, L. L. Wright, R. A. Ehrenkranz, A. A. Fanaroff, A. Stark, W. Carlo, J. E. Tyson, E. F. Donovan, S. Shankaran, and D. K. Stevenson. 2001. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics 107:E1. [DOI] [PubMed] [Google Scholar]
- 14.Ohlsson, A., and J. B. Lacy. 2001. Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants. Cochrane Database Syst. Rev., CD000361. [DOI] [PubMed]
- 15.Ohlsson, A., and J. B. Lacy. 2004. Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants. Cochrane Database Syst. Rev., CD000361. (Update.) [DOI] [PubMed]
- 16.Patrick, C. C., S. L. Kaplan, C. J. Baker, J. T. Parisi, and E. O. Mason, Jr. 1989. Persistent bacteremia due to coagulase-negative staphylococci in low birth weight neonates. Pediatrics 84:977-985. [PubMed] [Google Scholar]
- 17.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 18.Patti, J. M., and M. Hook. 1994. Microbial adhesins recognizing extracellular matrix macromolecules. Curr. Opin. Cell Biol. 6:752-758. [DOI] [PubMed] [Google Scholar]
- 19.Reilley, S., E. Wenzel, L. Reynolds, B. Bennett, J. M. Patti, and S. Hetherington. 2005. Open-label, dose escalation study of the safety and pharmacokinetic profile of tefibazumab in healthy volunteers. Antimicrob. Agents Chemother. 49:959-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shurin, P. A., J. M. Rehmus, C. E. Johnson, C. D. Marchant, S. A. Carlin, D. M. Super, G. F. Vanhare, P. K. Jones, D. M. Ambrosino, and G. R. Siber. 1993. Bacterial polysaccharide immune globulin for prophylaxis of acute otitis media in high-risk children. J. Pediatr. 123:801-810. [DOI] [PubMed] [Google Scholar]
- 21.Stoll, B. J., N. Hansen, A. A. Fanaroff, L. L. Wright, W. A. Carlo, R. A. Ehrenkranz, J. A. Lemons, E. F. Donovan, A. R. Stark, J. E. Tyson, W. Oh, C. R. Bauer, S. B. Korones, S. Shankaran, A. R. Laptook, D. K. Stevenson, L. A. Papile, and W. K. Poole. 2002. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110:285-291. [DOI] [PubMed] [Google Scholar]
- 22.Stoll, B. J., N. I. Hansen, I. Adams-Chapman, A. A. Fanaroff, S. R. Hintz, B. Vohr, and R. D. Higgins. 2004. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 292:2357-2365. [DOI] [PubMed] [Google Scholar]
- 23.Wilson, J. R., H. Bhoopalam, and M. Fisher. 1997. Hemolytic anemia associated with intravenous immunoglobulin. Muscle Nerve 20:1142-1145. [DOI] [PubMed] [Google Scholar]