Abstract
Indwelling prostheses and subcutaneous delivery devices are now routinely and indispensably employed in medical practice. However, these same devices often provide a highly suitable surface for bacterial adhesion and colonization, resulting in the formation of complex, differentiated, and structured communities known as biofilms. The University of Washington Engineered Biomaterials group has developed a novel drug delivery polymer matrix consisting of a poly(2-hydroxyethyl methacrylate) hydrogel coated with ordered methylene chains that form an ultrasound-responsive coating. This system was able to retain the drug ciprofloxacin inside the polymer in the absence of ultrasound but showed significant drug release when low-intensity ultrasound was applied. To assess the potential of this controlled drug delivery system for the targeting of infectious biofilms, we monitored the accumulation of Pseudomonas aeruginosa biofilms grown on hydrogels with and without ciprofloxacin and with and without exposure to ultrasound (a 43-kHz ultrasonic bath for 20 min daily) in an in vitro flow cell study. Biofilm accumulation from confocal images was quantified and statistically compared by using COMSTAT biofilm analysis software. Biofilm accumulation on ciprofloxacin-loaded hydrogels with ultrasound-induced drug delivery was significantly reduced compared to the accumulation of biofilms grown in control experiments. The results of these studies may ultimately facilitate the future development of medical devices sensitive to external ultrasonic impulses and capable of treating or preventing biofilm growth via “on-demand” drug release.
The high frequency of device-related biofilm infections has spurred a rapidly growing field of research directed at controlling or eliminating biofilm formation. Various device-related infections have been well documented on vascular catheters, prosthetic hips, knees, and other orthopedic implants (8, 23, 29). Increasingly, biofilm formation is recognized as a significant virulence factor in many of these infections (4, 5, 9, 26, 29, 30, 31). Among the more common pathogens are Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa. The capacity to grow on any surface and the high resistance to traditional systemic antibiotic treatments (3, 18, 19, 27) have posed serious challenges to the design of effective biofilm treatment or prevention strategies (13). Once a biofilm is established, it becomes increasingly difficult to eradicate as the infection matures, and often, patients must undergo revision surgery for the debridement of infected tissue or, in many cases, implant removal and replacement (21). This process increases the vulnerability of the patient to further nosocomial infections.
In recent years biomaterial scientists (8) have devoted significant efforts to creating biocompatible materials that prevent or minimize biofilm infection by inhibiting the formation and survival of biofilms. One strategy is to incorporate drug delivery into the device, which targets the site at which biofilm formation is likely to occur (1, 6, 7, 25). However, in many of these systems the release of antibiotics is through passive diffusion, which results in rapid dissipation to sublethal concentrations and which increases the risk of selection of antibiotic-resistant strains. To address this problem the University of Washington Engineered Biomaterials group has adapted an ultrasonic energy-responsive, biocompatible coating for controlled antibiotic release. Originally, this method was developed for controlled insulin release (15, 16, 17); however, it was apparent that such a delivery system might have high potential for the control of biofilms. The drug reservoir consists of a poly(2-hydroxyethyl methacrylate) (pHEMA) hydrogel film that can be “loaded” with antibiotic, either as a solid or in solution, during polymerization. The pHEMA is then coated with ordered methylene chains. The closely packed methylene chains create a self-assembled barrier membrane that minimizes passive release. The concept of using ultrasound to induce controlled drug delivery is attractive because at low power levels it is noninvasive and has been used effectively in other areas of medical treatment and diagnosis (20). Although the characteristics of release of certain drugs from the coated pHEMA hydrogels have been documented, it remains unclear whether these hydrogels would be effective against biofilm formation and growth. The purpose of our research was to investigate the effectiveness of ciprofloxacin-loaded, methylene chain-coated pHEMA hydrogels against the formation and growth of Pseudomonas aeruginosa biofilms.
MATERIALS AND METHODS
Polymers.
The pHEMA polymer hydrogels were manufactured as described previously (15, 16, 17). The hydrogels had a thickness of 0.38 mm and were cut to the dimensions of a standard microscope slide (24 by 60 mm) so that they could be accommodated in biofilm flow cells. For those hydrogels “loaded” with the antibiotic, ciprofloxacin was added to the HEMA solution in excess of saturation at a concentration of 11.1 mg/ml before polymerization. The solubility of ciprofloxacin in water at physiological pH (7.4) and 25°C is approximately 100 μg/ml (32).
Bacteria and nutrients.
To aid with the visualization of live biofilms without staining, we used the constitutive green fluorescing protein (GFP)-producing P. aeruginosa PAO1 strain carrying plasmid pMF230 Car (22) for all experiments. We determined that the MIC of ciprofloxacin against P. aeruginosa (pMF230) was 0.125 μg/ml by using ciprofloxacin-loaded Etest strips (AB Biodisk). Overnight (20-h) batch cultures grown on full strength Luria-Bertani (LB) broth were used to inoculate the hydrogel containing flow cell biofilm reactors. The doubling time under biofilm growth conditions was approximately 2 h (data not shown). To avoid the potential complication of interactive effects between multiple antibiotics, we cultured pMF230 biofilms without the addition of the selective marker carbenicillin. We periodically compared confocal images taken in the transmitted mode with maximum projections made from image stacks in the GFP channel. The images were in good agreement, indicating that there was no noticeable plasmid loss over the culturing period (data not shown).
Planktonic experiments.
To assess whether enhanced killing may be due to a “bioacoustic effect” (i.e., an enhanced antibiotic killing of bacteria in the presence of ultrasound [20, 24]) rather than local concentrated antibiotic delivery, we exposed planktonic cells to the same treatments used for the flow cell biofilms. Stationary-phase, 48-h shake flask cultures grown at 37°C on full-strength LB broth were exposed to (i) ultrasound (20 min in a 43-kHz Branson 200 ultrasonic bath) with added ciprofloxacin (5 μg/ml), (ii) ciprofloxacin only, (iii) ultrasound only, and (iv) no ultrasound or ciprofloxacin (control). The flasks were incubated for a further 1 h 40 min (total time = 2 h) before serial dilutions were made and plated on solid agar.
Flow cell biofilm reactor.
Hydrogels (unloaded or loaded with ciprofloxacin) hydrated in purified water for 24 h were incorporated into flow cells (BST-FC81; BioSurface Technologies, Inc., Bozeman, Mont.), which consisted of a polycarbonate base with a lumen that was 5.08 cm long, 1.27 cm wide, and 0.203 cm deep (Fig. 1). The flow cell lumen was bounded on the top by a rectangular microscope coverslip (24 by 60 mm) and on the bottom by a sheet of hydrogel supported by a standard microscope slide. The flow cell was inoculated with 2 ml of an overnight batch culture (ca. 2 × 109 CFU/ml) by injection through a septated Y fitting port immediately upstream of the flow cell so that the flow cell was completely filled. The flow cell was allowed to incubate for 30 min before a continuous flow of 1/10 strength LB broth was pumped (Cole-Parmer 7553-80 peristaltic pump) at 1 ml/min through the flow cell into a waste carboy. The hydraulic residence time in the flow cell was 1.8 min, which was much less than the doubling time (∼120 min), so that planktonically growing populations would be continually washed out. A bubble trap-mixing chamber was positioned upstream to remove in-line bubbles and allow aeration with a sterile filtered airstream. The flow cell experiments were conducted at 25°C.
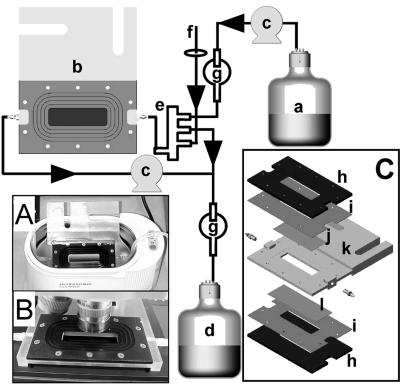
FIG. 1.
Standard flow cell configuration used for methylene chain-coated pHEMA hydrogel controlled release and pHEMA hydrogel biofilm control experiments. Nutrients (a) were pumped through the flow cell (b) by using peristaltic pumps (c) and placed into an effluent waste vessel (d). A mixing chamber (e) was used as a bubble trap, and aeration was supplied from a fish tank pump through a sterile 0.22-μm-syringe filter (f); fluid depth was maintained by a gravity feed overflow to waste. Backcontamination was prevented by using bubble traps (g). (A and B) The flow cell immersed in a water bath for ultrasound application and positioned on the confocal microscope respectively. (C) Exploded view of the flow cell; (h) aluminum plates; (i) silicone gasket; (j) microscope coverslip; (k) polycarbonate body; (l) hydrogel and microscope slide. The hydrogel was positioned on top of the microscope slide and is not shown separately. The flow cell was held together by screws between plates (h).
Ultrasound application.
Ultrasound was applied by immersing the complete flow cell assembly in a 43-kHz ultrasonic bath (Branson 200), consistent with the methods used in previous studies. To establish that the ultrasonic bath could stimulate the controlled release of ciprofloxacin in the flow cell, the effluent concentration of ciprofloxacin was monitored over a 4-h period, during which ultrasound was applied for 15 min at three distinct time points. The ciprofloxacin concentration from 5 ml of effluent collected by an adaptation of the method used by Kwok et al. (15) was measured with a spectrometer (A339) in duplicate experiments. The absorption was measured at 339 nm, which corresponds to the third maximal absorption peak at pH 7 (33). The ciprofloxacin concentration was found from a standard curve (by using the linear regression expression ciprofloxacin concentration (μg/ml) = 46.02 A339 − 0.0176 [r2 = 0.99; n = 6 over a range of 0.15 to 5 μg/ml]). During the biofilm experiments all ultrasound applications were delivered in a 20-min pulse.
pHEMA hydrogels: biofilm control.
P. aeruginosa PAO1 (pMF230) biofilms were grown on pHEMA-coated ultrasonically responsive hydrogels for 3 days. Four sets of triplicate independent experiments were conducted to determine if the methylene chain-coated polymers, in conjunction with low-intensity ultrasonic energy, had significant effects on the biofilm structure over a 72-h time period. These four sets of experiments were designed to separate the effects of the applied ultrasound and the release of ciprofloxacin into the bulk fluid. Biofilms were grown in flow cells containing either ciprofloxacin-loaded or unloaded hydrogels. In one set of experiments the biofilms were exposed to ultrasound for 20 min daily. In a control set of experiments, the flow cells were placed in the ultrasonic bath, but no ultrasound was applied (Table 1).
TABLE 1.
Matrix of combinations of biofilm exposure to ciprofloxacin and ultrasound and P values for the COMSTAT “biomass” parameter for biofilms grown on various configurations compared with the negative control
| Hydrogel configuration | Ciprofloxacin exposure | Ultrasound application |
P value
|
||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |||
| Experiment | + | + | 0.627 | 0.021a | <0.001a |
| Control for ultrasound only | − | + | 0.222 | 0.232 | 0.803 |
| Control for ciprofloxacin only | + | − | 0.219 | 0.332 | 0.602 |
| Negative controlb | − | − | |||
Significantly different result (P < 0.05).
Negative by ciprofloxacin exposure and ultrasound application.
Image acquisition and COMSTAT image analysis.
A Leica TCS-NT confocal scanning laser microscope was used to collect images of biofilms growing on the hydrogels in the flow cells in situ. A Leica ×40 long-working-distance microscope objective was used to collect all image stacks. For the hydrogel systems not exposed to ultrasound, 10 microscopic fields were imaged at 24, 48, and 72 h. For the hydrogel systems that were exposed to ultrasound, five fields were imaged before and after (five different fields) ultrasound application. The biofilm structure was quantified from the confocal stacks by using the image analysis software package COMSTAT (Technical University of Denmark, Lyngby) (11). The software interfaces with Matlab and utilizes Matlab's image analysis software toolbox. COMSTAT offers an array of functions and is capable of generating up to 10 different statistical parameters for the purpose of quantifying the three-dimensional biofilm structure. For the purposes of the present study, four COMSTAT parameters were utilized to determine the differences between biofilms grown under each of the four hydrogel system configurations. These parameters were maximum biofilm thickness, average biofilm thickness, roughness coefficient, and biomass. The maximum biofilm thickness was the maximum distance from the substratum that the biofilm colony reaches. The average thickness was the average biofilm height taken over the entire field of view. The roughness coefficient is a measure of variability in biofilm thickness and, consequently, is an indicator of biofilm heterogeneity. A roughness coefficient approaching zero would represent a flat slab. The biomass was calculated by normalizing the volume of the biofilm by the surface area of the field of view, which gives the biomass parameter as biofilm volume per unit surface area. The biomass parameter represents the volume of biofilm cells present in a given confocal image stack.
Selection of representative biofilm images.
Representative images of the various biofilms were selected nonsubjectively by calculating an “experimental error” (E) for each stack and then selecting the image with the lowest E for Fig. 5. E was found by summing the parameter errors (EP; [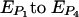 ] associated with each of the four individual parameters calculated by COMSTAT for a particular hydrogel configuration:
] associated with each of the four individual parameters calculated by COMSTAT for a particular hydrogel configuration:
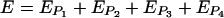 |
(1) |
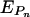 was found from the equation
was found from the equation
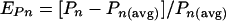 |
(2) |
where Pn was the parameter value for a particular stack and Pn(avg) is the average parameter value for all five stacks from each of the three independent replicate experiments for a particular hydrogel configuration.
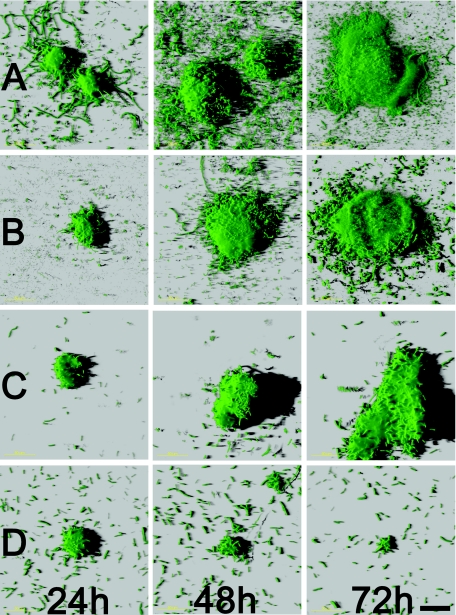
FIG. 5.
Biofilm images from methylene chain-coated pHEMA hydrogel experiments. Images are shown for each observation time period and each system configuration. Biofilms grown on hydrogels configurations: no ultrasound application or ciprofloxacin (row A); ultrasound application, no ciprofloxacin (row B); no ultrasound application, ciprofloxacin treatment (row C); and ultrasound application and ciprofloxacin (row D). Scale bar, 50 μm.
Statistical analysis.
Means and standard errors were based on the average of each parameter over three experiments calculated by COMSTAT from 10 confocal image stacks per experiment.
For experiments that included ultrasound, the confocal image stacks acquired before and immediately after ultrasound application were grouped to increase statistical power, due to the following rationale. Sixteen of 18 tests generated P values greater than 0.05, indicating that there were no statistically significant differences between the confocal image stacks acquired before and after ultrasound application (data not shown). When the three experiments were grouped and analysis of variance (ANOVA) tests were performed on a daily basis, there were no statistically significant differences by any of the tests (P > 0.232). Based on these results, the populations of before ultrasound application and after ultrasound application were grouped into one population for further ANOVA studies on the statistical differences in biomass between each of the hydrogel system configurations.
ANOVA was used to determine the statistical significance of the results generated by COMSTAT. The commercially available software package MINITAB 13 was used to perform all ANOVA comparisons. All ANOVA operations were performed on the biomass parameter. The P values generated by ANOVA comparisons were considered significantly different when P was <0.05.
Effluent collection.
To assess the effect of ultrasound exposure on detachment and the viability of detached cells, the effluent from the two hydrogel systems that were exposed to ultrasound application were collected over the application time period of 20 min. The optical density at 660 nm (OD660; Spectronic Genesys 5 spectrophotometer) was used to assess the total biomass effluent concentration. Enumeration and the viability of detached cells were assessed by culturing samples from serial dilutions (Ringer's buffer) and plating (LB agar).
RESULTS
Planktonic control.
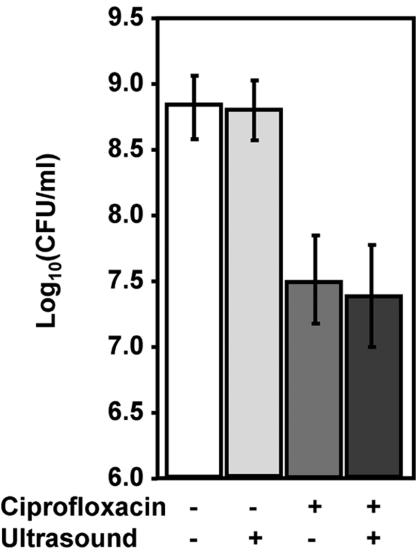
Ultrasound alone had no significant effect (P = 0.896) on the viability of planktonic cells in comparison to the control (no ultrasound application or ciprofloxacin treatment) (Fig. 2). However, ciprofloxacin alone (5 μg/ml) caused a significant (P < 0.001) 1.5-log reduction. There was no significant difference in cell viability between samples exposed to ciprofloxacin alone and those exposed to both ciprofloxacin and ultrasound (P = 0.604).
FIG. 2.
Effects of various combinations of ultrasound (20 min, 43 kHz) and ciprofloxacin (5 μg/ml) on viability of stationary-phase flask cultures. Data represent the geometric mean and 1 standard deviation (n = 5 independent experiments).
Ultrasonic controlled release of ciprofloxacin in the flow cell.
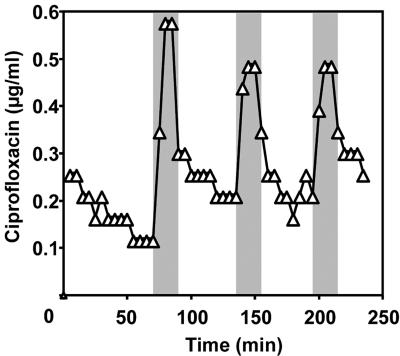
Before the application of ultrasound there was a residual release of ciprofloxacin into the effluent so that the concentration was approximately 0.1 and 0.3 μg/ml for the duplicate experiments (Fig. 3). When the ultrasound was applied there was a corresponding sharp spike in effluent concentration. The peak concentrations were 0.52 ± 0.04 and 1.8 ± 0.40 μg/ml for the duplicate experiments (n = 3 peaks), which reflected four- and sevenfold increases over the background concentration, respectively. When ultrasound was turned off there was an exponential decay over the 45 min back to the background level.
FIG. 3.
Ultrasonic release characteristics of ciprofloxacin (effluent concentration) from the pHEMA hydrogel incorporated into the flow cell from one of the duplicate experiments. The shaded areas indicate the 15-min periods during which ultrasound was applied.
Biofilm development and COMSTAT analysis.
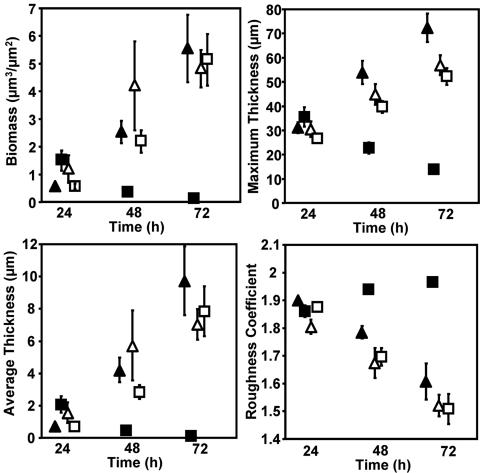
After 24 h biofilms formed on both ciprofloxacin-loaded and unloaded hydrogel surfaces. The biofilms were similar in structural morphology and consisted of distinct microcolonies separated by interstitial voids and channels. However, P. aeruginosa biofilms showed significant structural differences when the ultrasound regime was initiated (Fig. 4). The biomass, maximum biofilm thickness, and average biofilm thickness all increased over time for biofilms grown on methylene-coated hydrogels without ciprofloxacin loading both with and without ultrasound application. The same was true for biofilms grown on hydrogels with ciprofloxacin loading when ultrasound was not applied. The biomass, maximum biofilm thickness, and average biofilm thickness all decreased over time for biofilms grown on ciprofloxacin-loaded hydrogels exposed to ultrasound. The roughness coefficients displayed opposite trends for all hydrogel system configurations. The biofilms that were grown on hydrogels loaded with ciprofloxacin and to which ultrasound was applied demonstrated decreasing microcolony sizes over the 3-day observation period compared with those of biofilms grown on the control hydrogel system configurations, which all showed increasing sizes (Fig. 5).
FIG. 4.
Biofilm development over a 3-day time period. Each datum point represents the average of the respective parameter over three experiments calculated by COMSTAT from 10 confocal image stacks per experiment. Hydrogel configurations: no ciprofloxacin or ultrasound application (Δ); ciprofloxacin treatment, no ultrasound application (▴); no ciprofloxacin treatment, ultrasound application (□); ciprofloxacin treatment and ultrasound application (▪). Bars indicate 1 standard error. The datum points are staggered on the x axis for clarity.
Statistical significance by ANOVA.
Biofilms grown on methylene chain-coated hydrogels without ciprofloxacin and without ultrasound application were compared to the other hydrogel system configurations on each observation day. Table 1 shows the P values for the results for each hydrogel configuration compared to the results for hydrogels without ciprofloxacin and without ultrasound application. There were no significant differences in biomass populations after 1 day. On day 2 and day 3, however, the biomass populations for the hydrogels with ciprofloxacin and with ultrasound application were significantly different, with all P values being <0.05. There were no significant differences in biofilm biomass between hydrogels without ciprofloxacin and ultrasound application and hydrogels with ciprofloxacin and ultrasound application on days 2 and 3 (P > 0.05).
Effluent biomass.
The viable counts from effluent collected during ultrasound application with and without ciprofloxacin are reported in Table 2. There were no significant differences (P = 0.32) between the two hydrogels in the cell concentrations measured by determination of the absorbance (OD660) on day 1. However, the OD660 increased to 0.07 by day 3 for hydrogels with no ciprofloxacin but remained steady for hydrogels with ciprofloxacin. Similarly, for the hydrogel with no ciprofloxacin, the viable counts increased from 1.5 × 104 CFU/ml (from the geometric mean) on day 1 to 1.1 × 107 CFU by day 3. The effluents of the hydrogels with ciprofloxacin did not yield any detectable CFU for any of the triplicate experiments on any of the days (detection limit = 17 CFU/ml).
TABLE 2.
Viable cell concentration and OD660 in the bulk fluid effluent collected during ultrasound application from flow cells
| Hydrogel configurationa | Day | Viable cell concn (log10 CFU/ml)b | OD660 |
|---|---|---|---|
| US+, cipro− | 1 | 4.18 ± 0.18 | 0.046 ± 0.003 |
| 2 | 6.96 ± 1.14 | 0.067 ± 0.010 | |
| 3 | 7.06 ± 1.12 | 0.070 ± 0.010 | |
| US+, cipro+ | 1 | NDc | 0.044 ± 0.001 |
| 2 | ND | 0.043 ± 0.001 | |
| 3 | ND | 0.041 ± 0.001 |
US+, cipro−, ultrasound application without ciprofloxacin; US+, cipro+, ultrasound application with ciprofloxacin.
Data represent the geometric mean and 1 standard deviation.
ND, nondetectable (detection limit = 17 CFU/ml; log10 = 1.2).
DISCUSSION
By incorporating methylene chain-coated pHEMA hydrogels into a flow cell system and by utilizing GFP-expressing P. aeruginosa and confocal microscopy, we were able to assess the efficacy of an ultrasonically controlled antibiotic delivery system against live biofilms by direct observation without requiring culturing. By positioning a flow cell in a standard laboratory ultrasonic bath, we were able to stimulate pulsed releases of ciprofloxacin from the pHEMA hydrogels in a similar manner to that previously achieved with “free-floating” pieces of hydrogel (15). Successful adaptation to the flow cell configuration allowed the coated hydrogel to be held in place for direct observation of the attachment of bacterial cells and subsequent quantification of biofilm development directly on the surface.
The ultrasonic release of ciprofloxacin administered in a 20-min pulse daily significantly reduced the accumulation of an established 24-h P. aeruginosa biofilm over a 3-day period by a factor of approximately 50 from approximately 5 μm3/μm2 in the controls (ultrasound alone, ciprofloxacin alone, or neither ciprofloxacin nor ultrasound) to 0.1 μm3/μm2. In terms of cell numbers, if a biofilm cell concentration of 1010 cells/ml of biofilm (not including channels) is assumed (D. deBeer and P. Stoodley unpublished results) this converts to a reduction from 5 × 106 to 1 × 105 cells/cm2. A similar reduction (a factor of ca. 60) was reflected in average biofilm thickness, which decreased from approximately 8 μm in the controls to 0.14 μm in the treatment experiment. Since we started treatment after a biofilm had been allowed to develop for 24 h and we saw a reduction in biofilm from the initial condition as well relative to the condition for the controls, we believe that this technology holds promise not only for retarding biofilm accumulation but also for eradicating established biofilms. The ability to retard biofilm accumulation may have been even more pronounced if controlled delivery had been initiated sooner rather than at the 24 h over which the biofilm was allowed to become established. The results of the planktonic control experiment suggest that biofilm suppression was due to growth inhibition and killing from the localized release of high concentrations of ciprofloxacin in the immediate vicinity of the biofilm and not a bioacoustic effect. Although we did not directly measure the local concentration of ciprofloxacin at the surface of the hydrogel, it was likely to approach saturation (ca. 100 μg/ml) since the internal hydrogel ciprofloxacin concentration was greatly in excess at 11.1 mg/ml. Thus, the biofilm cells may have been exposed to concentrations approximately 800 times the planktonic cell MIC (0.125 μg/ml). For severe or complicated bone and joint infections, an oral dose of 750 mg (adult, usual), which achieves peak serum levels ranging from 1.97 to 5.39 μg/ml (12), every 12 h for 4 to 6 weeks is recommended (19a). The ratio of the ciprofloxacin concentration in synovial fluid to that in plasma has been measured to be 0.9 (2). The aqueous solubility of ciprofloxacin under physiological conditions (pH 7.4, 37°C) is approximately 200 μg/ml (32). Thus, in the context of an orthopedic implant equipped with an ultrasound-activated hydrogel delivery system, if ciprofloxacin is saturated at the implant surface, the local concentration may be 2 orders of magnitude greater than that which can be achieved by systemic delivery. In terms of the lives of these hydrogels in our experiments, the hydrogel had an area of 14.4 cm2 and contained approximately 6 μg of ciprofloxacin. With a background leach rate of 0.2 μg ml−1 min−1, the ciprofloxacin would be lost after 21 days (if a constant leach rate is assumed). With three 15-min pulses per day at an ultrasound-induced release rate of 5.4 μg/pulse (measured from curve areas in Fig. 3), the hydrogel would last 20 days. However, these time estimates are specific to our system and will vary according to the hydrogel dimensions, the antibiotic concentration, and the external mass transfer conditions.
Interestingly, we measured biofilm accumulation in the ciprofloxacin-treated, ultrasound application-negative control, even though in our release experiment the background leach rate resulted in an effluent ciprofloxacin concentration in the range of 0.1 to 0.3 μg/ml. The concentration at the surface would have been higher and would certainly have been above the measured MIC (0.125 μg/ml). This indicates that the nascent biofilm cells have a higher MIC than planktonic cells, even when they are freshly attached and the biofilm is thin. Another possible explanation is that we inoculated the flow cells with 2 ml of an overnight culture containing approximately 2 × 109 CFU/ml. This is much higher than that used to obtain a conventional MIC (2 × 104 to 105 CFU/ml), and it is known that MIC is sensitive to the inoculum concentration. Regardless, ideally, we aim to achieve a background leach rate of zero (i) to minimize sublethal exposure, with the possibility for the selection of resistant strains, and (ii) to lengthen the useful life of the hydrogel.
In addition to the killing of bacteria on an implant surface, it is also important to consider the viability of cells that may detach from the biofilm during a treatment. This may result not only in systemic infection (10) but also in the dissemination of the biofilm infection, as suggested by the findings of Kurihara et al. (14). One concern of introducing ultrasonic pressure waves at locations where the biofilm is already established is that detachment and dispersion might be augmented (28), with detrimental effects to the health of the patient. However, our studies show that although the total number of detached cells was similar on day 1 (as assessed by determination of the OD660), culture demonstrated that the presence of ciprofloxacin had reduced the viable cell concentration by over 3 log units, from 1.5 × 104 CFU/ml to less than 1.7 × 101 CFU/ml. This CFU reduction may be due to the killing of cells in the biofilm prior to detachment, as well as the killing of any surviving detached biofilm cells by exposure to antibiotics while they are in the bulk fluid. The detached cell effluent concentration increased, as determined by measurement of the viable count and the OD660 measurement for the hydrogel with no ciprofloxacin. This is expected, since it is reasonable to assume that the detachment rate will be directly related to the biomass of the attached biofilm, which also increased over the 3 days. In the effluent from the hydrogel with ciprofloxacin, the OD660 decreased slightly from 0.044 to 0.041 in a trend consistent with that of the biofilm biomass (Fig. 4). Importantly, no viable cells were cultured from the effluent, suggesting that the ciprofloxacin released during ultrasound application acted not only against biofilm bacteria growing on the surface but also against detached cells that were washed downstream of the biofilm.
Finally, our in vitro system represents the first step in assessing the potential of ultrasound-induced release of antibiotics from hydrogels to control biofilms. The system may be used for future optimization studies in which the pulse times, the type of antibiotic, and the challenge organism may be varied over more extensive time periods. For example, the immediate application of ultrasound after inoculation may determine if biofilm formation can be eliminated altogether with an immediate dose of ciprofloxacin. It would be beneficial to approach the problem in this manner since bacterial cells coming to the surface in a planktonic state are generally more susceptible to antibiotics. Another advantage of our controlled-release technology is that it has the potential to be tailored for efficacy against nonvegetative, resistant persister populations or even spore formers in a manner analogous to Tyndalization. Sequential releases may allow the outgrowth of nonvegetative populations, which could then be killed while they are growing between antibiotic applications.
Ultimately, one might envision medical devices implanted with an antibiotic reservoir. If the device ever became infected with a biofilm, using externally applied high-intensity focused ultrasound, the physician might attack the biofilm by triggering antibiotic release from the reservoir without the need for the use of massive systemic antibiotics or surgical intervention.
Acknowledgments
Funding for this project was received from NSF grant EEC-0121881 (to B.D.R. and J.W.C.), NSF grant EEC-9529161 (to B.D.R.), grant RO1 GM60052 (to P.S.), and grant DC04173 (to Garth Ehrlich, Center for Genomic Sciences).
From Montana State University we thank Mike Franklin for providing strain P. aeruginosa PAO1 (pMF230) and Betsey Pitts for confocal assistance. From the University of Washington we thank Pierre Mourad for discussions on ultrasound application.
REFERENCES
- 1.Ackart, W. B., R. L. Camp, and W. L. Wheelwright. 1975. Antimicrobial polymers. Biomed. Mater. Res. 9:55-68. [DOI] [PubMed] [Google Scholar]
- 2.Bosseray, A., P. Leclercq, G. Manquat, J. P. Stahl, and M. Micoud. 1992. Letter. Penetration of ciprofloxacin into synovial fuid after oral dosing. J. Antimicrob Chermother. 30:874-875. [DOI] [PubMed] [Google Scholar]
- 3.Brown, M. R. W., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-783. [DOI] [PubMed] [Google Scholar]
- 4.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 5.Donlan, R. M. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francolini, I., P. Norris, A. Piozzi, G. Donelli, and P. Stoodley. 2004. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob. Agents Chemother. 48:4360-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golomb, G., and A. Shpigelman. 1991. Prevention of bacterial colonization on polyurethane in vitro by incorporated antbacterial agent. J. Biomed. Mater. Res. 25:937-952. [DOI] [PubMed] [Google Scholar]
- 8.Gristina, A. G., G. Giridhar, B. L. Gabriel, P. T. Naylor, and Q. N. Myrvik. 1993. Cell biology and molecular mechanisms in artificial device infections. Int. J. Artific. Organs 16:755-764. [PubMed] [Google Scholar]
- 9.Habash, M., and G. Reid. 1999. Microbial biofilms: their development and significance for medical device-related infections. J. Clin. Pharmacol. 39:887-898. [DOI] [PubMed] [Google Scholar]
- 10.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the environment to infectious disease. Nat. Rev. Microbiol. 2:95-108. [DOI] [PubMed] [Google Scholar]
- 11.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 12.Kays, M. B., B. R. Overholser, B. A. Mueller, S. M. Moe, and K. M. Sowinski. 2003. Effects of sevelamer hydrochloride and calcium acetate on the oral bioavailability of ciprofloxacin. Am. J. Kidney Dis. 42:1253-1259. [DOI] [PubMed] [Google Scholar]
- 13.Khoury, A. E., K. Lam, B. Ellis, and J. W. Costerton. 1992. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 38:174-178. [DOI] [PubMed] [Google Scholar]
- 14.Kurihara, N., Y. Inoue, T. Iwai, M. Umeda, Y. Huang, and I. Ishikawa. 2004. Detection and localization of periodontopathic bacteria in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 28:553-558. [DOI] [PubMed] [Google Scholar]
- 15.Kwok, C., P. D. Mourad, L. A. Crum, and B. D. Ratner. 2001. Self-assembled molecular structures as ultrasonically-responsive barrier membranes for pulsatile drug delivery. J. Biomed. Mater. Res. 57:151-164. [DOI] [PubMed] [Google Scholar]
- 16.Kwok, C. S., P. D. Mourad, L. A. Crum, and B. D. Ratner. 2000. Surface modification of polymers with self-assembled molecular structures: multitechnique surface characterization. Biomacromolecules 1:139-148. [DOI] [PubMed] [Google Scholar]
- 17.Kwok, C. S., W. Changxiu, S. Hendricks, J. D. Bryers, T. A. Horbett, and B. D. Ratner. 1999. Design of infection-resistant antibiotic-releasing polymers: I. Fabrication and formulation. J. Controlled Release 62:289-299. [DOI] [PubMed] [Google Scholar]
- 18.Mah, T., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 19.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 19a.MICROMEDEX Healthcare Series. 2005. Thomson Healthcare. [Online.] Thomson Micromedex, Greenwood Village, Colo. www.thomson.com.
- 20.Mourad, P. D. 1999. Biological effects of ultrasound. In J. G. Webster (ed.), Wiley encyclopedia of electrical and electronics engineering. John Wiley & Sons, Inc., New York, N.Y.
- 21.Neut, D., J. R. van Horn, T. G. van Kooten, H. C. van der Mei, and H. J. Busscher. 2003. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin. Orthopaed. Related Res. 413:261-268. [DOI] [PubMed] [Google Scholar]
- 22.Nivens, D. E., D. E. Ohman, J. Williams, and M. J. Franklin. 2001. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J. Bacteriol. 183:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Passerini, L., K. Lam, J. W. Costerton, and E. G. King. 1992. Biofilms on indwelling vascular catheters. Crit. Care Med. 20:665-673. [DOI] [PubMed] [Google Scholar]
- 24.Quian, Z., R. D. Sagers, and W. G. Pitt. 1997. The effect of ultrasonic frequency upon the enhanced killing of P. aeruginosa biofilms. Ann. Biomed. Res. 25:69-76. [DOI] [PubMed] [Google Scholar]
- 25.Robinson, J. R., and V. H. L. Lee. 1987. Controlled drug delivery: fundamentals and applications, 2nd ed. Marcell Decker, Inc., New York, N.Y.
- 26.Schierholz, J. M., and J. Beuth. 2001. Implant infections: a haven for opportunistic bacteria. J. Hosp. Infect. 49:87-93. [DOI] [PubMed] [Google Scholar]
- 27.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 28.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 29.Strachan, C. J. L. 1995. The prevention of orthopaedic implant and vascular graft infections. J. Hosp. Infect. 30:54-63. [DOI] [PubMed] [Google Scholar]
- 30.Vogley, H. C., A. Fleer, W. J. A. Dhert, and A. J. Verbout. 2000. Infection of an orthopaedic implant: treatment and prevention. Rev. Med. Microbiol. 11:223-231. [Google Scholar]
- 31.Widmer, A. F. 2001. New developments in diagnosis and treatment of infection in orthopedic implants. Clin. Infect. Dis. 33:94-106. [DOI] [PubMed] [Google Scholar]
- 32.Yu, X., G. L. Zipp, and G. W. Davidson III. 1994. The effect of temperature and pH on the solubility of quinolone compounds: estimation of heat of fusion. Pharm. Res. 11:522-527. [DOI] [PubMed] [Google Scholar]
- 33.Zupancic, M., I. Arcon, P. Bukovec, and A. Kodre. 2002. Physico-chemical study of the interaction of cobalt(II) ion with ciprofloxacin. Croat. Chem. Acta 75:1-12. [Google Scholar]