Abstract
The susceptibility of typical class D β-lactamases to inhibition by acyl phosph(on)ates has been determined. To a large degree, these class D enzymes behaved very similarly to the class A TEM β-lactamase towards these reagents. Dibenzoyl phosphate stood out in both cases as a lead compound towards a new class of effective inhibitors.
The resistance of bacteria to β-lactam antibiotics is to a large extent due to β-lactamases (14). There is a wide range of β-lactamases, although from a structural standpoint, they can be divided into four classes, A, B, C, and D (11). Each class contains many variants of widely differing substrate specificities and thus clinical importance. Of the canonical four classes, it is perhaps class D that at present is least well studied at the molecular level.
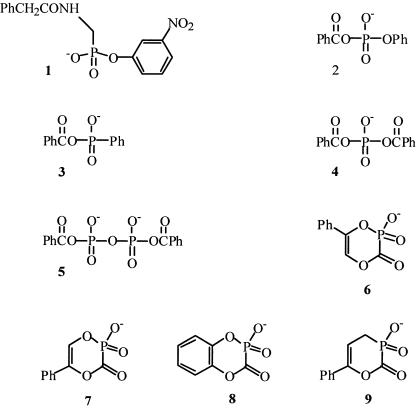
The class D β-lactamases, also generally known as oxacillinases because of their general specificity for oxacillin and its derivatives, represent a diverse class of enzymes (1) that hydrolyze a broad spectrum of substrates (2). Like class A and C β-lactamases, the class D enzymes are serine hydrolases, catalyzing substrate hydrolysis by way of a covalent acyl-enzyme (acyl-serine) intermediate and thus by a double-displacement mechanism. Structural studies indicate that the class D β-lactamases are more closely related to class A than to class C, although there are distinct differences in active-site structures and thus, presumably, in chemical mechanisms (20). Although class D β-lactamases are clinically significant, there are no specific inhibitors known for them other than certain inhibitory β-lactams (8, 12) and a series of anthraquinone dyes (15). The classical mechanism-based inhibitors of class A β-lactamases are not generally effective against class D (16). A variety of phosph(on)ates have been found to be covalent inhibitors of class A and class C β-lactamases (4, 5, 9, 17-19). This paper describes the screening of a panel of these compounds, 1 to 9, against the class D OXA-1 β-lactamase. This enzyme is representative of one subclass of the D enzymes (1) and is clinically important in its own right; a crystal structure is also available (20). The more effective compounds of 1 to 9 (Fig. 1) were also tested against the OXA-10 enzyme, a representative of another major class D subgroup (1).
FIG. 1.
Structures of phosph(on)ates 1 to 9.
The OXA-1 and OXA-10 β-lactamases were prepared and purified as described previously (8, 20). The various phosph(on)ates were available from previous studies (4, 5, 9, 17-19). The phosphates 2 and 4 to 8 and the phosphonates 1, 3, and 9 were all irreversible or slowly reversible inhibitors of the OXA β-lactamases, and the inactivation step could be described simply by scheme 1 as follows:
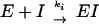 |
where E is the free enzyme and I is the inhibitor. Second-order rate constants of inactivation were obtained from measurements of the loss of enzyme activity as a function of time (18, 19). All kinetics studies were performed at 25°C in a buffer at pH 7.5 containing 20 mM MOPS (3-morpholinopropanesulfonic acid) and 50 mM sodium bicarbonate (3). The enzyme concentration in reaction mixtures was 1.4 μM, and the inhibitor concentrations were 0 to 0.5 mM, depending on reactivity (Table 1). Aliquots of reaction mixtures were diluted 15-fold into 0.5 mM benzylpenicillin solution for assay. The values of ki, obtained as described above, are reported in Table 1.
TABLE 1.
Inhibition of class D β-lactamases by phosph(on)ates
| Phosph(on)atea |
ki (s−1 M−1)c
|
|
|---|---|---|
| OXA-1 | OXA-10 | |
| 1 | 0.90 ± 0.25 | 2.9 ± 0.6 |
| 2 | 45 ± 10 | ND |
| 3 | 10 ± 3 | ND |
| 4 | (5.2 ± 0.2) × 104b | (1.7 ± 0.4) × 105b |
| 5 | ≤0.04 | ND |
| 6 | 0.6 ± 0.1 | ND |
| 7 | (2.8 ± 0.2) × 102 | (1.0 ± 0.3) × 103 |
| 8 | 27 ± 2 | ND |
| 9 | (3.3 ± 0.6) × 102b | ND |
The concentrations of 1 to 9 employed ranged up to 0.5 mM, 60 μM, 60 μM, 1.0 μM, 1.0 mM, 0.3 mM, 8.0 μM, 40 μM, and 12.6 μM, respectively.
Reversible (see the text).
ND, experiment not done.
We see from these data that the “classical” substrate-like phosphonate 1 had little activity against the OXA-1 enzyme. Of the acyclic acyl phosphates 2 to 5, the diacyl phosphate 4 shows significant activity. Of the cyclic acyl phosph(on)ates 6 to 9, the 4-phenyl phosphate 7 and its phosphonate analogue 9 are most effective. It is interesting to note that this pattern of activity closely mimics that against the class A TEM β-lactamase (4, 9, 17, 18).
The reactivity of dibenzoyl phosphate, 4, with the OXA-1 β-lactamase is quite striking, and even without structural optimization, it is comparable to that of clavulanic acid (ki = 2.2 × 105 s−1 M−1 under the same conditions). The inactivation by 4 was found to be slowly reversible (5, 7), corresponding to a directly measured turnover number (kcat) of (4.9 ± 0.9) ×10−3 s−1 (the half-life of the EI complex was thus 2.4 min; cf. that of clavulanic acid [0.5 s]). The effective Km of 4 as a substrate, or its Ki as an inhibitor, is given by kcat/ki, with a value of 94 nM (cf. that of clavulanic acid [6.4 μM]). Inactivation of the enzyme by the cyclic phosphonate 9 was also reversible, with a rate constant of 1.5 × 10−2 s−1. In contrast, reactivation from treatment with 7 did not occur at a measurable rate, the difference perhaps indicating phosphorylation of the enzyme by 7 and 9 (6).
The data of Table 1 also suggest that the pattern of reactivity established for the OXA-1 enzyme also applies to OXA-10. Dibenzoyl phosphate was again the most reactive. In this case, the reactivation rate constant (kcat) was (4.2 ± 0.2) ×10−2 s−1 and thus the effective Ki was (0.24 ± 0.02) μM.
The slow reversibility of the reaction of these class D enzymes with 4 is suggestive of the formation of a covalent benzoyl-enzyme species, as is well established with a class C β-lactamase (5, 9) and is likely also with the class A TEM enzyme (9). It is notable, however, that no reversal of reaction with 2 and 3 was observed, despite the fact that these compounds should generate the same benzoyl-enzyme as would 4. This difference may reflect nonspecific modification by 2 and 3 at the high concentrations of these species (cf. that of 4) required for inhibition. In the case of the class C β-lactamase of Enterobacter cloacae P99, a direct relationship, based on transition state analogy, was demonstrated between the reactivity of the enzyme with acyl phosphates and its inhibition by aryl boronates (5). In the present instance, we found that the OXA-1 β-lactamase was not strongly inhibited by benzeneboronic acid (Ki = 3.5 mM); the class A TEM β-lactamase is also not strongly inhibited by simple aryl boronic acids (10). The relatively high reactivity of 4 with both class A and D enzymes must therefore reflect the strong interaction of the PhCOOPO2− leaving group with the active sites of these enzymes.
In conclusion, this paper reports that typical phosph(on)ates inhibit typical class D β-lactamases to much the same degree that they do the class A TEM β-lactamase. It seems likely that this result reflects the overall similarity of these active sites despite the fact that details of catalysis must be different (13, 20). The reactivity of dibenzoyl phosphate 4, now evident with all classes of serine β-lactamases, suggests new avenues of inhibitor design for these enzymes. Further optimization of 4 should include an increase in the on rate constant (ki) and some decrease in the off rate constant.
Acknowledgments
This research was supported by U.S National Institutes of Health grant AI-17986 (R.F.P.) and by FRFC grants 2.4.508.01 and 2.4.524.03 (FNRS, Brussels, Belgium) and PAI P5/33 (Politique Scientifique Fedérale, Belgium).
REFERENCES
- 1.Barlow, M., and B. G. Hall. 2002. Phylogenetic analysis shows that the OXA beta-lactamase genes have been on plasmids for millions of years. J. Mol. Evol. 55:314-327. [DOI] [PubMed] [Google Scholar]
- 2.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golemi, D., L. Maveyraud, S. Vakulenko, J.-P. Samama, and S. Mobashery. 2001. Critical involvement of a carbamylated lysine in catalytic function of class D β-lactamases. Proc. Natl. Acad. Sci. USA 98:14280-14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur, K., S. A. Adediran, M. J. K. Lan, and R. F. Pratt. 2003. Inhibition of β-lactamases by monocyclic acyl phosph(on)ates. Biochemistry 42:1529-1536. [DOI] [PubMed] [Google Scholar]
- 5.Kaur, K., and R. F. Pratt. 2001. Mechanism of reaction of acyl phosph(on)ates with the β-lactamase of Enterobacter cloacae P99. Biochemistry 40:4610-4621. [DOI] [PubMed] [Google Scholar]
- 6.Kaur, K., M. J. K. Lan, and R. F. Pratt. 2001. Mechanism of inhibition of the class C β-lactamase of Enterobacter cloacae P99 by cyclic acyl phosph(on)ates: rescue by return. J. Am. Chem. Soc. 123:10436-10443. [DOI] [PubMed] [Google Scholar]
- 7.Kuzmic, P. 1996. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal. Biochem. 237:260-273. [DOI] [PubMed] [Google Scholar]
- 8.Ledent, P., X. Raquet, B. Joris, J. Van Beeumen, and J.-M. Frère. 1993. A comparative study of class-D β-lactamases. Biochem. J. 292:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, N., and R. F. Pratt. 1998. Inhibition of serine β-lactamases by acyl phosph(on)ates: a new source of inert acyl [and phosph(on)yl] enzymes. J. Am. Chem. Soc. 120:4264-4268. [Google Scholar]
- 10.Martin, R., M. Gold, and J. B. Jones. 1994. Inhibition of the RTEM β-lactamase by boronic acids. Bioorg. Med. Chem. Lett. 4:1229-1234. [Google Scholar]
- 11.Matagne, A., A. Dubus, M. Galleni, and J.-M. Frère. 1999. The β-lactamase cycle: a tale of selective pressure and bacterial ingenuity. Nat. Prod. Rep. 16:1-19. [DOI] [PubMed] [Google Scholar]
- 12.Maveyraud, L., D. Golemi-Kotra, A. Ishiwata, O. Meroueh, S. Mobashery, and J.-P. Samama. 2002. High-resolution X-ray structure of an acyl-enzyme species for the class D OXA-10 β-lactamase. J. Am. Chem. Soc. 124:2461-2465. [DOI] [PubMed] [Google Scholar]
- 13.Maveyraud, L., D. Golemi, L. P. Kotra, S. Tranier, S. Vakulenko, S. Mobashery, and J.-P. Samama. 2000. Insights into class D β-lactamases are revealed by the crystal structure of the OXA-10 enzyme from Pseudomonas aeruginosa. Structure 8:1289-1298. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros, A. A. 1997. Evolution and dissemination of beta-lactamases accelerated by generations of beta-lactam antibiotics. Clin. Infect. Dis. 24(Suppl. 1):S19-S45. [DOI] [PubMed] [Google Scholar]
- 15.Monaghan, C., S. Holland, and J. W. Dale. 1982. The interaction of anthraquinone dyes with the plasmid-mediated OXA-2 beta-lactamase. Biochem. J. 205:413-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page, M. G. P. 2000. β-Lactamase inhibitors. Drug Resist. Updates 3:109-125. [DOI] [PubMed] [Google Scholar]
- 17.Pratt, R. F., and N. J. Hammar. 1998. Salicyloyl cyclic phosphate, a “penicillin-like” inhibitor of β-lactamases. J. Am. Chem. Soc. 120:3004-3006. [Google Scholar]
- 18.Rahil, J., and R. F. Pratt. 1991. Phosphonate monoester inhibitors of class A β-lactamases. Biochem. J. 275:793-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahil, J., and R. F. Pratt. 1992. Mechanism of inhibition of the class C β-lactamase of Enterobacter cloacae P99 by phosphonate monoesters. Biochemistry 31:5869-5878. [DOI] [PubMed] [Google Scholar]
- 20.Sun, T., M. Nukaga, K. Mayama, E. H. Braswell, and J. R. Knox. 2003. Comparison of β-lactamases of classes A and D: 1.5-Å crystallographic structure of the class D OXA-1 oxacillinase. Protein Sci. 12:82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]