Abstract
Epigallocatechin gallate (EGCg), the main polyphenol component of green tea, has several antibacterial properties. Here we show that sub-MICs of EGCg appear to decrease slime production, therefore inhibiting biofilm formation by ocular staphylococcal isolates previously characterized for the presence of ica genes by the Congo red agar plate assay and for adhesion to microtiter plates.
Biofilm formation is a three-stage process (6) that significantly contributes to the pathogenesis of staphylococcal infections. The first stage, docking, is mainly due to hydrophobic interactions, whereas the next two stages, locking and maturation, are mediated by capsular polysaccharide adhesins, PIA (polysaccharide intercellular adhesin) and PNAG (poly-N-acetylglucosamine polysaccharide), both of which are synthesized by the gene products of the ica operon (icaADBC), with the main contribution coming from an N-acetylglucosaminyltransferase encoded by the icaA gene (5, 12). The expression of the icaA gene alone results in low enzymatic activity, but coexpression with icaD leads to a significant increase in activity (8).
Green tea polyphenols and, more specifically, epigallocatechin gallate (EGCg) are known to possess both direct bactericidal activity (18, 19) and the ability to potentiate the effects of certain antibiotics (15, 16, 17, 21). Moreover, they have already been shown to have at least an indirect influence on biofilm production, in that they can retard the formation of dental plaque (9, 11, 20).
In this study, we have investigated the effects of sub-MICs of EGCg (99% pure; Sigma) (Fig. 1A, inset) on biofilm formation by 20 different ocular staphylococcal isolates derived from patients with community-acquired ocular infections and belonging to our private collection. The different isolates included 8 Staphylococcus aureus isolates and 12 Staphylococcus epidermidis isolates. Moreover, two American Type Culture Collection strains (S. epidermidis ATCC 35984 and S. epidermidis ATCC 12228) were used as reference controls.
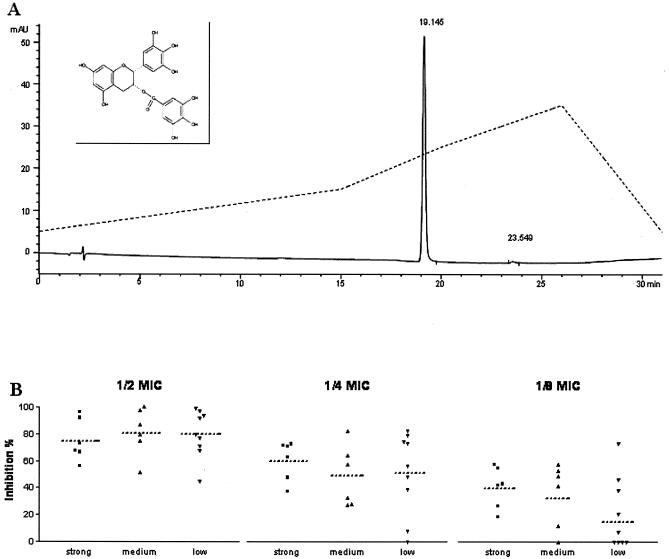
FIG. 1.
(A) Chromatogram (continuous line) of EGCg (Sigma) loaded on a reverse-phase column (Symmetry Shield RP18; 5 μm; 150 by 4.6 mm) eluted with a gradient (dotted line) of acetonitrile in phosphate buffer. (Inset) Structure of EGCg. (B) Inhibitory activity of EGCg. Biofilms produced in the presence of decreasing sub-MICs (one-half, one-quarter, and one-eighth) of EGCg are shown. The relative inhibition of biofilm production was quantitated by safranin staining and calculated, after subtraction of the blank, as 100 − [(OD570 treated/OD570 control) × 100] (16). The values were sorted for strong, medium, and low biofilm producers. Dotted lines indicate the average inhibition values for each group. The difference between groups at one-half and one-eighth the MIC was statistically significant (P < 0.05).
Table 1 summarizes the characterization of bacterial isolates for biofilm production. The MIC of EGCg for each strain was determined in tryptic soy broth (TSB) by a broth dilution method according to the guidelines of the CLSI (formerly the NCCLS) (13). The quality of the lot of EGCg was controlled by reverse-phase high-pressure liquid chromatography (HPLC) and showed just one single, sharp peak, suggesting a very high degree of purity (Fig. 1A). The MICs obtained ranged from 125 to 500 mg/ml, similar to that which we reported recently (17); however, they were slightly higher than those already described by other authors (22, 24).
TABLE 1.
Characterization of microbial strainsa
| Strains | Presence of icaA/icaD | CRA test | EGCg MIC (μg/ml) | Strain classification based on biofilm production (absorbance at OD492) | OD492 (% biofilm inhibition) EGCg at:
|
||
|---|---|---|---|---|---|---|---|
| 1/2× MIC | 1/4× MIC | 1/8× MIC | |||||
| S. aureus 815 CT | +/+ | B | 500 | Strong (1.30) | 0.35 (72.8) | 0.37 (71.1) | 0.55 (57.7) |
| S. epidermidis ATCC 35984 | +/+ | B | 500 | Strong (1.07) | 0.03 (96.3) | 0.31 (70.3) | 0.62 (42.0) |
| S. aureus 810 CT | +/+ | B | 500 | Strong (1.00) | 0.44 (55.6) | 0.37 (63.3) | 0.57 (42.5) |
| S. epidermidis 813 | +/+ | B | 250 | Medium (0.76) | 0.37 (50.0) | 0.55 (27.0) | 0.60 (21.0) |
| S. epidermidis 14 ME | +/+ | AB | 250 | Medium (0.75) | 0.00 (100) | 0.27 (64.3) | 0.35 (53.4) |
| S. epidermidis 807CT | +/+ | AB | 250 | Medium (0.70) | 0.09 (86.9) | 0.51 (27.2) | 0.58 (17.2) |
| S. aureus 5 ME | +/+ | B | 500 | Medium (0.70) | 0.01 (97.6) | 0.30 (56.1) | 0.36 (47.7) |
| S. aureus CZ 11 | +/+ | B | 500 | Medium (0.68) | 0.14 (79.3) | 0.12 (82.3) | 0.32 (52.5) |
| S. epidermidis 23S | +/+ | B | 125 | Medium (0.68) | 0.17 (73.9) | 0.24 (65.7) | 0.36 (47.1) |
| S. aureus 74CCH | +/+ | B | 500 | Medium (0.68) | 0.20 (70.3) | 0.46 (32.0) | 0.41 (39.7) |
| S. epidermidis 10 NC | +/+ | AB | 500 | Low (0.55) | 0.04 (91.5) | 0.07 (87.3) | 0.28 (48.8) |
| S. epidermidis 809 | +/+ | B | 250 | Low (0.52) | 0.12 (75.8) | 0.32 (39.3) | 0.40 (23.1) |
| S. epidermidis 20 ME | +/+ | B | 500 | Low (0.52) | 0.00 (100) | 0.11 (77.5) | 0.64 (0) |
| S. epidermidis 15 NC | +/+ | AB | 500 | Low (0.45) | 0.13 (69.4) | 0.08 (81.4) | 0.15 (80.2) |
| S. epidermidis 21 Me | +/+ | B | 250 | Low (0.38) | 0.21 (43.0) | 0.35 (0) | 0.40 (0) |
| S. epidermidis 26 Me | +/+ | B | 250 | Low (0.32) | 0.01 (95.5) | 0.14 (56.2) | 0.35 (0) |
| S. aureus 6 ME | −/+ | Bx | 500 | Strong (1.55) | 0.52 (66.3) | 0.81 (47.5) | 0.70 (54.8) |
| S. aureus 808 CT | −/+ | Bx | 500 | Strong (1.12) | 0.09 (91.5) | 0.70 (37.7) | 0.82 (26.7) |
| S. epidermidis 9 NC | −/+ | Bx | 500 | Low (0.54) | 0.11 (79.7) | 0.14 (73.9) | 0.55 (0) |
| S. epidermidis ATCC 12228 | −/− | R | 250 | Low (0.55) | 0.00 (100) | 0.04 (92.7) | 0.4 (27.2) |
| S. epidermidis 7753 | −/− | R | 500 | Low (0.48) | 0.03 (92.9) | 0.25 (48.2) | 0.28 (41.2) |
| S. epidermidis 7777 | −/− | R | 500 | Low (0.43) | 0.14 (67.3) | 0.45 (0) | 0.43 (0) |
CRA, Congo red agar; B, black (slime producers); AB, almost black (slime producers); R, red (slime nonproducers); Bx, bordeaux (slime nonproducers); +, presence of the gene by PCR analysis; −, absence of the gene by PCR analysis.
Each ocular isolate was then characterized for biofilm-related properties. Biofilm-forming ability was tested by determination of adhesion to microtiter plates (5) and was quantitated by safranin staining and reading of the absorbance at 492 nm. The biofilm production of the different strains was then arbitrarily classified as strong (optical density [OD], >0.9 OD), medium (0.6 < OD < 0.9), or low (OD, <0.6). The isolates were evaluated for the presence of the icaA and the icaD genes by PCR analysis (1, 2); and slime production was detected by the Congo red agar assay (1, 7), in which slime-producing strains formed almost-black to black colonies, whereas nonproducing strains develop bordeaux to red colonies. We found (Table 1) that most of the ocular isolates were slime producing (16 of 20 isolates) and possessed both the icaA and the icaD genes (15 of 20 isolates). Three strains were found to be icaA negative and icaD positive and showed a reduced slime formation ability. In the absence of ica genes, the bacteria were negative for slime production and produced only a thin biofilm. These data are consistent with previous observations indicating that in the absence of the ica operon, staphylococci are still able to adhere to the substrate surface, the genetically distinct first step in biofilm formation, but are not able to build a multilayered biofilm due to a defect in cell-cell adhesion (5, 10), which is mediated by PIA. However, the slime-forming ability, as evaluated by the Congo red agar assay, did not correlate with the biofilm-forming ability on microtiter plates; and purple colonies could even form more biofilm than black colonies, among which the whole range of biofilm-forming ability, from very low to strong, could be found. This suggests that the docking phase during biofilm formation can be very critical and that a poor docking ability impairs biofilm production, despite the full activity of the ica operon.
The effects of EGCg on biofilm production are illustrated in Fig. 1B. For this purpose, the biofilm assay was carried out in the presence of sub-MICs of EGCg (one-half, one-quarter, and one-eighth the MIC) diluted in TSB growth medium. The relative inhibition of biofilm production was quantitated by safranin staining and is reported as the percent inhibition with respect to that for the untreated controls (14). Despite a high variability of the inhibitory response among the different strains, a general inhibitory effect on biofilm production was clear at all concentrations tested. The inhibitory response was similar for all three groups (strong, medium, and low biofilm producers) at each EGCg dilution, although it was significantly higher for strains tested at one-half the MIC than for strains tested at one-eighth the MIC. Therefore, the response seems to be independent of the amount of biofilm produced; however, it is proportional to the dose of EGCg.
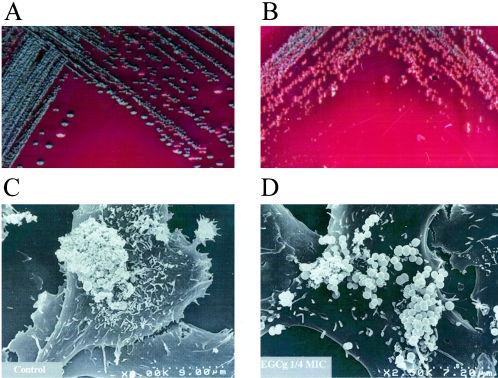
EGCg inhibition of biofilm formation might be ascribed to an inhibitory effect on bacterial growth or slime production, or both. Hence, we verified that the effects on bacterial growth were indeed negligible at these sub-MIC dilutions (data not shown) and checked the effects of EGCg on slime production by the Congo red agar plate assay. EGCg at doses corresponding to one-sixth the MIC calculated for the slime-producing strain under testing were thus spread and air-dried on Congo red agar plates to see whether the developing colonies would turn from black to red. Figure 2A and B shows that, indeed, a slime-producing strain that formed black colonies on Congo red agar plates formed pink or pale gray colonies in the presence of this sub-MIC dilution of EGCg, thus indicating a loss of slime-producing ability. These results are further supported by direct observation by scanning electron microscopy (SEM) of bacterial colonies adhering onto a monolayer of rabbit corneal epithelial cells (SIRC) in vitro. To this end, a suspension of 5 × 105 bacterial cells of a strong biofilm producer strain was added overnight at 37°C to SIRC grown at confluence on 15-mm-diameter glass coverslips to allow the adhesion of the bacteria to the underlying monolayer. Glass coverslips were then prepared for SEM as described previously (4) and observed with an S-400 scanning electron microscope (Hitachi). Figure 2C and D shows representative fields of single bacterial cells of a black slime-producing strain attached to corneal epithelial cells: it is evident that in the absence of EGCg (Fig. 2C) the bacterial cells formed a tight colony covered by a polysaccharide secretion. In the presence of EGCg at concentrations one-quarter the MIC (Fig. 2D), the bacterial cells grew as looser colonies, and the amount of secreted polysaccharides was less.
FIG. 2.
(A and B) Bacterial colonies of S. epidermidis ATCC 35984 grown on Congo red agar plates to show decreasing levels of slime production (from black to gray, bordeaux, and red) in the presence of EGCg. (A) Black colonies of the untreated control; (B) EGCg at one-sixth the MIC (83.35 μg/ml). (C and D) Scanning electron photomicrographs of S. epidermidis ATCC 35984 grown on a monolayer of rabbit corneal epithelial cells (SIRC) in the absence of EGCg (C) or in the presence of EGCg at one-quarter the MIC (D). Dotted bars, 9 μm (C) and 7.2 μm (D).
Taken together, these observations suggest that EGCg interferes with the polysaccharides that form the glycocalyx, disrupting their interactions either reciprocally or with the cell wall and thus reducing the amount of slime that accumulates. However, EGCg is also known to bind to the peptidoglycan, breaking the integrity of the bacterial cell wall (22-24), and could therefore also interfere with the initial docking phase of biofilm formation, which requires hydrophobic interactions between the bacterial cell wall and the surface to be colonized (3).
Acknowledgments
We thank Valeria Moschetti, head of analytical chemistry at Sifi Research and Development, for HPLC analysis of EGCg and Pino Mondio of the Biomedical Science Department, Section of General and Cellular Biology and Molecular Genetics of the University of Catania, for expert collaboration in the realization of the SEM artwork. Vittorio Alonzo of the Pharmaco-Biological Department, Section of Microbiology, Faculty of Pharmacy, University of Messina, is gratefully acknowledged for critical reading of the manuscript; and Eileen Collazo, Sifi Medical Division, is acknowledged for proofreading of the English language.
REFERENCES
- 1.Arciola, C. R., L. Baldassarri, and L. Montanaro. 2001. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J. Clin. Microbiol. 39:2151-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arciola, C. R., S. Collamati, E. Donati, and L. Montanaro. 2001. A rapid PCR method for the detection of slime-producing strains of Staphylococcus epidermidis and S. aureus in periprosthesis infections. Diagn. Mol. Pathol. 10:130-137. [DOI] [PubMed] [Google Scholar]
- 3.Carpentier, B., and O. Cerf. 1993. Biofilms and their consequences, with particular reference to hygiene in the food industry. J. Appl. Bacteriol. 75:499-511. [DOI] [PubMed] [Google Scholar]
- 4.Civiale, C., G. Paladino, C. Marino, F. Trombetta, T. Pulvirenti, and V. Enea. 2003. Multilayer primary epithelial cell culture from bovine conjunctiva as a model for in vitro toxicity tests. Ophthalmol. Res. 35:126-136. [DOI] [PubMed] [Google Scholar]
- 5.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunne, W. M., Jr. 2002. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 15:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman, D. J., F. R. Falkiner, and C. T. Keane. 1989. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 42:872-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerke, C., A. Kraft, R. Sussmuth, O. Schweitzer, and F. Gotz. 1998. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J. Biol. Chem. 273:18586-18593. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton-Miller, J. M. 2001. Anti-cariogenic properties of tea (Camellia sinensis). J. Med. Microbiol. 50:299-302. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Gotz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, T., and Y. Chi. 2000. Experimental study on polyphenol anti-plaque effect in human. Zhonghua Kou Qiang Yi Xue Za Zhi 35:383-384. (In Chinese.) [PubMed] [Google Scholar]
- 12.Maira-Litran, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, vol. 17, p. 10-13. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Polonio, R. E., L. A. Mermel, G. E. Paquette, and J. F. Sperry. 2001. Eradication of biofilm-forming Staphylococcus epidermidis (RP62A) by a combination of sodium salicylate and vancomycin. Antimicrob. Agents Chemother. 45:3262-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiota, S., M. Shimizu, T. Mizushima, H. Ito, T. Hatano, T. Yoshida, and T. Tsuchiya. 1999. Marked reduction in the minimum inhibitory concentration (MIC) of beta-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis). Biol. Pharm. Bull. 22:1388-1390. [DOI] [PubMed] [Google Scholar]
- 16.Stapleton, P. D., S. Shah, J. C. Anderson, Y. Hara, J. M. Hamilton-Miller, and P. W. Taylor. 2004. Modulation of beta-lactam resistance in Staphylococcus aureus by catechins and gallates. Int. J. Antimicrob. Agents 23:462-467. [DOI] [PubMed] [Google Scholar]
- 17.Sudano Roccaro, A., A. R. Blanco, F. Giuliano, D. Rusciano, and V. Enea. 2004. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 48:1968-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toda, M., S. Okubo, H. Ikigai, T. Suzuki, Y. Suzuki, and T. Shimamura. 1991. The protective activity of tea against infection by Vibrio cholerae O1. J. Appl. Bacteriol. 70:109-112. [DOI] [PubMed] [Google Scholar]
- 19.Toda, M., S. Okubo, R. Ohnishi, and T. Shimamura. 1989. Antibacterial and bactericidal activities of Japanese green tea. Nippon Saikingaku Zasshi 44:669-672. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 20.Wolinsky, L. E., J. Cuomo, K. Quesada, T. Bato, and P. M. Camargo. 2000. A comparative pilot study of the effects of a dentifrice containing green tea bioflavonoids, sanguinarine or triclosan on oral bacterial biofilm formation. J. Clin. Dent. 11:53-59. [PubMed] [Google Scholar]
- 21.Yanagawa, Y., Y. Yamamoto, Y. Hara, and T. Shimamura. 2003. A combination effect of epigallocatechin gallate, a major compound of green tea catechins, with antibiotics on Helicobacter pylori growth in vitro. Curr. Microbiol. 47:244-249. [DOI] [PubMed] [Google Scholar]
- 22.Yoda, Y., Z. Q. Hu, W. H. Zhao, and T. Shimamura. 2004. Different susceptibilities of Staphylococcus and gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 10:55-58. [DOI] [PubMed] [Google Scholar]
- 23.Zhao, W. H., Z. Q. Hu, Y. Hara, and T. Shimamura. 2002. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2266-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao, W. H., Z. Q. Hu, S. Okubo, Y. Hara, and T. Shimamura. 2001. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]