Recently, it was reported that sera from healthy volunteers given atovaquone-proguanil (Malarone) inhibited parasite transmission and asexual blood stage development for up to 6 weeks after treatment (1). The lengthy persistence of drug activity was quite unexpected because earlier studies had shown that proguanil and atovaquone had elimination half-lives of about 14 to 20 h (2-4, 10, 11) and 2 to 3 days, (5, 9-11), respectively. This drug combination acts synergistically against malaria parasites and avoids the rapid selection of atovaquone-resistant parasites whenever parasites are exposed to the action of atovaquone alone (7).
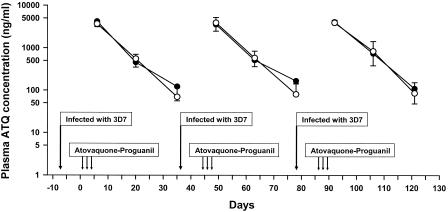
The present study was designed to quantitate the persistence of atovaquone in three Caucasian volunteers who had participated in a study to assess the effects of repeated subclinical infections on the development of immunity to Plasmodium falciparum (8). The three volunteers (mean weight, 84.8 ± 21.9 kg) had been inoculated intravenously with about 30 parasitized erythrocytes of P. falciparum (3D7 strain) on three occasions at 35-day intervals, and on each occasion, treatment with atovaquone-proguanil (1,000 mg atovaquone-400 mg proguanil daily for 3 days) was started 8 days later. Blood samples were collected at 6, 20, and 35 days after the onset of treatment (Fig. 1). None of the volunteers developed symptoms of malaria.
FIG. 1. .
Mean plasma atovaquone (ATQ) concentrations versus time after repeated atovaquone-proguanil treatment for uncomplicated Plasmodium falciparum malaria in three volunteers infected on three occasions. Plasma ATQ concentrations were measured by HPLC (•) and bioassay (○).
Mean plasma atovaquone concentrations-versus-time curves measured by high-pressure liquid chromatography (HPLC) (9) were similar after each treatment with atovaquone-proguanil (Fig. 1). Comparable values were obtained by bioassay of samples using the 3D7 atovaquone-sensitive strain (6). The average elimination half-life of atovaquone in the three volunteers was 5.9 days by HPLC and 4.9 days by bioassay. The prolonged persistence of atovaquone was further illustrated by the complete suppression of schizont formation when plasma collected up to 35 days after treatment was incubated with parasites of the 3D7 strain (50% inhibitory concentration [IC50], ∼2 ng/ml). In contrast, plasma collected 6 days after treatment failed to inhibit the maturation of parasites of three atovaquone-resistant strains (TM90-C2b, TM91-C32b, TM93-C1088), with mean IC50 values exceeding 4,000 ng/ml (7). These findings suggest that residual atovaquone may have contributed to the suppression of malaria in the volunteers challenged with the 3D7 strain 35 days after treatment (8).
The lengthy 5- to 6-day elimination half-life of atovaquone in these three Caucasian volunteers is twice as long as observed previously in African and Asian patients treated with atovaquone (5, 10, 11). Although proguanil concentrations were not measured in this study, numerous previous studies have shown that proguanil and its cycloguanil metabolite are eliminated much more quickly than atovaquone (2-4, 10, 11), and none would be present within a week after atovaquone-proguanil treatment to potentiate the antimalarial activity of atovaquone. The prolonged presence of atovaquone is of little concern as long as this expensive drug continues to be used mainly by travelers to malarious areas. However, if atovaquone-proguanil were used more widely by expatriate residents living in areas of endemicity, long-lasting low atovaquone concentrations in the absence of any residual proguanil may facilitate the rapid selection of atovaquone-resistant parasites.
Acknowledgments
We thank Hamish Barbour for HPLC analysis and Anthony Kotecki and Kerryn Rowcliffe for bioassay analysis.
The opinions expressed are those of the authors and do not necessarily reflect those of the Australian Defence Health Service or any extant Australian Defence Force policy.
REFERENCES
- 1.Butcher, G. A., and R. E. Sinden. 2003. Persistence of atovaquone in human sera following treatment: inhibition of Plasmodium falciparum development in vivo and in vitro. Am. J. Trop. Med. Hyg. 68:111-114. [PubMed] [Google Scholar]
- 2.Edstein, M. D., S. Looareesuwan, C. Viravan, and D. E. Kyle. 1996. Pharmacokinetics of proguanil in malaria patients treated with proguanil plus atovaquone. Southeast Asian J. Trop. Med. Public Health 27:216-220. [PubMed] [Google Scholar]
- 3.Helsby, N. A., S. A. Ward, G. Edwards, R. E. Howells, and A. M. Breckenridge. 1990. The pharmacokinetics and activation of proguanil in man: consequences of variability in drug metabolism. Br. J. Clin. Pharmacol. 30:593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussein, Z., J. Eaves, D. B. Hutchinson, and C. J. Canfield. 1996. Population pharmacokinetics of proguanil in patients with acute P. falciparum malaria after combined therapy with atovaquone. Br. J. Clin. Pharmacol. 42:589-597. [DOI] [PubMed] [Google Scholar]
- 5.Hussein, Z., J. Eaves, D. B. Hutchinson, and C. J. Canfield. 1997. Population pharmacokinetics of atovaquone in patients with acute malaria caused by Plasmodium falciparum. Clin. Pharmacol. Ther. 61:518-530. [DOI] [PubMed] [Google Scholar]
- 6.Kotecka, B. M., and K. H. Rieckmann. 1993. Chloroquine bioassay using malaria microcultures. Am. J. Trop. Med. Hyg. 49:460-464. [DOI] [PubMed] [Google Scholar]
- 7.Looareesuwan, S., C. Viravan, H. K. Webster, D. E. Kyle, D. B. Hutchinson, and C. J. Canfield. 1996. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am. J. Trop. Med. Hyg. 54:62-66. [DOI] [PubMed] [Google Scholar]
- 8.Pombo, D. J., G. Lawrence, C. Hirunpetcharat, C. Rzepczyk, M. Bryden, N. Cloonan, K. Anderson, Y. Mahakunkijcharoen, L. B Martin, D. Wilson, S. Elliott, S. Elliott, D. P. Eisen, J. B. Weinberg, A. Saul, and M. F. Good. 2002. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360:610-617. [DOI] [PubMed] [Google Scholar]
- 9.Rolan, P. E., A. J. Mercer, B. C. Weatherley, T. Holdich, H. Meire, R. W. Peck, G. Ridout, and J. Posner. 1994. Examination of some factors responsible for a food-induced increase in absorption of atovaquone. Br. J. Clin. Pharmacol. 37:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabchareon, A., P. Attanath, P. Phanuaksook, P. Chanthavanich, Y. Poonpanich, D. Mookmanee, T. Chongsuphajaisiddhi, B. M. Sadler, Z. Hussein, C. J. Canfield, and D. B. Hutchinson. 1998. Efficacy and pharmacokinetics of atovaquone and proguanil in children with multidrug-resistant Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 92:201-206. [DOI] [PubMed] [Google Scholar]
- 11.van Vugt, M., M. D. Edstein, S. Proux, K. Lay, M. Ooh, S. Looareesuwan, N. J. White, and F. Nosten. 1999. Absence of interaction between artesunate and atovaquone-proguanil. Eur. J. Clin. Pharmacol. 55:469-474. [DOI] [PubMed] [Google Scholar]