Abstract
Pradofloxacin (PRA) is an 8-cyano-fluoroquinolone (FQ) being developed to treat bacterial infections in dogs and cats. Its mutant prevention concentrations (MPC) were determined for Escherichia coli ATCC 8739 at 0.225 μg/ml, and for Staphylococcus aureus ATCC 6538 at 0.55 μg/ml. At drug concentrations equal to or above the MPC, growth (implying selective clonal expansion) of first-step FQ-resistant variants, naturally present in large bacterial populations, was inhibited. MPC90 derived from 10 clinical isolates each of E. coli and Staphylococcus intermedius, the latter species being of greater clinical relevance than S. aureus in companion-animal medicine, amounted to 0.2 to 0.225 and 0.30 to 0.35 μg/ml, respectively. MPCs of other veterinary FQs were assessed to determine relative in vitro potencies. The MPCs of marbofloxacin, enrofloxacin, danofloxacin, sarafloxacin, orbifloxacin, and difloxacin were 1.2-, 1.4-, 2.3-, 2.4-, 5-, and 7-fold higher than the MPC of PRA for E. coli ATCC 8739, and 6-, 6-, 19-, 15-, 15-, and 31-fold higher than the MPC of PRA for S. aureus ATCC 6538, respectively. MPC curves revealed a pronounced heterogeneity in susceptibility within populations of ≥4 × 109 CFU employed, extending to 10-fold above the MICs. The duration of incubation and, for S. aureus, inoculum density profoundly affected the MPCs. With appropriate dosing, PRA may combine high therapeutic efficacy with a high potential for restricting the selection for FQ resistance under field conditions in the species analyzed.
Bacterial resistance to fluoroquinolones (FQs) emerges primarily through stepwise accumulation of specific point mutations in the genes encoding the target enzymes of FQs, DNA gyrase and topoisomerase IV, causing a variety of amino acid exchanges in their catalytic regions (2, 24). Such mutants may be amplified by consecutive steps of clonal enrichment under selective drug pressure during therapy (11, 14-16, 40, 42). The primary target is the enzyme in which a mutation conferring decreased drug susceptibility is detected first. Target selectivity has been shown to vary by the Gram type of a species, but also due to the chemical structure of the FQ (17). A new concept, based on the in vitro mutant prevention concentration (MPC), may offer a strategy to prevent selective enrichment of resistant variants in vivo by appropriate drug dosing (1, 6, 14-17, 22).
During in vitro MPC analysis, large bacterial populations are dissected according to the capability of distinct fractions of CFU to still form a visible colony, despite the presence of drug (5, 14, 15, 40). In practice, aliquots of a concentrated liquid culture containing 109 to 1010 CFU are streaked onto series of agar plates, each containing a specific drug concentration. Such inoculum size assures the presence of first-step FQ-resistant variants, which occur at a probability of about 10−7 to 10−8, and represents population sizes encountered at sites of infection (6, 15). If the numbers of colonies recovered during incubation are plotted over the drug concentrations, two distinct phases of decline of CFU are revealed (13-16). The bulk of the population is inhibited at drug concentrations close to the MIC, while a fraction of about 104 to 105 CFU are still capable of forming colonies (5, 26). However, these CFU are successively inhibited upon further increases of the drug concentration.
The MPC is defined as the drug concentration allowing no residual visible growth. Hence, at least static activity is exerted on even the most refractory variants present. Growth of such clones would require the concurrent presence of a second target mutation conferring FQ resistance, which may be contained in a much larger population of about 1014 CFU (14). As a consequence, clonal expansion of variants is inhibited at drug concentrations ≥MPC. Therefore, establishing MPC conditions in patients for an appropriate time may provide for treatment success while simultaneously restricting the emergence of resistance in clinical settings (1, 6, 11, 14-18, 22). Most notably, the selection of FQ-resistant clones of Streptococcus pneumoniae was effectively prevented in a mono-infection model in rabbits when MPC conditions had been established (9, 10, 18).
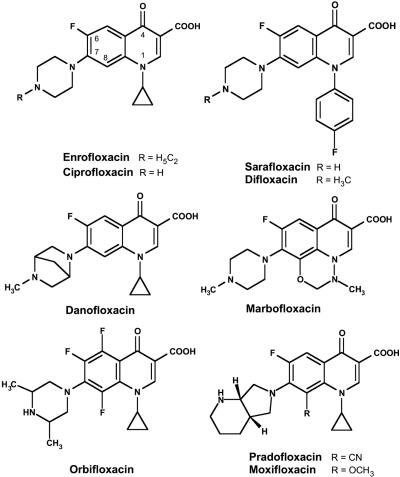
Pradofloxacin (PRA) is a new FQ being developed to treat bacterial infections in dogs and cats (4, 36). It is distinguished from enrofloxacin (ENR), the first veterinary FQ (21), by two structure elements: a bicyclic amine, pyrrolidino-piperidine, replacing the ethyl-piperazine moiety located at position C-7 of ENR, and a cyano group which is attached to the C atom at position 8 (Fig. 1). FQs substituted at position C-8 by a methoxy group were demonstrated to have greatly improved bactericidal activity, which was more pronounced for clones of Escherichia coli, Staphylococcus aureus, and other pathogens with reduced FQ susceptibility (6, 17, 27, 28, 41). The MPCs of such FQs were considerably lower than the MPCs of drug analogues carrying hydrogen at C-8 (13, 17, 40).
FIG. 1.
Chemical structures of the fluoroquinolones evaluated.
PRA has been shown in vitro to be highly active against clinical isolates of E. coli and S. intermedius from dogs and cats. Typical MIC90 values for 155 wild-type strains of E. coli were 0.03 μg/ml for PRA and 0.06 μg/ml for ENR. MIC90 values for 200 wild-type strains of S. intermedius were 0.06 μg/ml for PRA and 0.125 μg/ml for ENR (J. Abraham, K. Ewert, and A. de Jong, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-568, 2002). It was speculated that the low MICs might also translate into MPC values that would be clinically achievable based on conventional dosing of PRA.
In this study, MPCs of PRA for E. coli and S. aureus were determined and preliminary results have been previously reported (H.-G. Wetzstein, 103rd Annu. Meet. Am. Soc. Microbiol., abstract Z-010, 2003). MPCs obtained for control strains were then compared with those of 10 clinical isolates each of E. coli and S. intermedius; the latter species being of much greater significance than S. aureus in companion animal medicine (12, 20, 25). Furthermore, MPCs of other veterinary FQs, including ENR, danofloxacin (DAN), difloxacin (DIF), marbofloxacin (MAR), orbifloxacin (ORB), and sarafloxacin (SAR), were recorded, because relative in vitro potentials may translate into probabilities for clinical efficacy and restriction of FQ resistance (1, 6, 15, 17). As a standardized test method is not yet available, particular attention was paid to several methodological details that could potentially affect MPC results, such as the duration of incubation and the effect of inoculum density.
MATERIALS AND METHODS
FQ drugs evaluated.
In pradofloxacin, 8-cyano-1-cyclopropyl-7-([1S,6S]-2,8-diazabicyclo-[4.3.0]-nonan-8-yl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid (4), the amine moiety is linked (in relation to the cyano group) to an α-β unsaturated system, a bond inherently susceptible to hydrolysis under alkaline conditions. Other veterinary FQs evaluated included DAN, DIF, ENR, MAR, ORB, and SAR (Fig. 1); SAR was withdrawn in the United States in 2001 (8). Two FQs used in human medicine, ciprofloxacin (CIP) and moxifloxacin (MXF), served as reference compounds to facilitate comparison of the present results with data from the literature. Chemical purity of FQs was ≥99.7%, except for DAN (98.7%) and ORB (97.9%). MAR was synthesized, while DAN, DIF, ORB, and SAR were purified from commercial formulations by W. Hallenbach, Bayer CropScience AG, Monheim am Rhein, Germany. Stock solutions in distilled water contained up to 0.5 mg FQ per ml and were stored at room temperature protected from light.
Organisms.
The following control strains were used: E. coli ATCC 8739, E. coli ATCC 25922, E. coli DSM 10650, S. aureus ATCC 6538, S. aureus DSM 11823, and S. intermedius ATCC 29663. E. coli WT, which had been employed for FQ target identification (2), was kindly provided by P. Heisig, University of Hamburg, Germany. Ten clinical strains of E. coli, isolated in Germany in 2004 from cases of urinary tract and wound infections in dogs and cats, were selected by site of isolation and MICs and kindly provided by S. Friederichs, Bayer HealthCare AG, Leverkusen, Germany. Similarly, 10 strains of S. intermedius from dogs in a pyoderma study conducted in Germany and France in 2001 and 2002 (36) were kindly provided by B. Stephan, Bayer HealthCare AG.
Analysis of E. coli isolates by the BD BBL Crystal Enteric/Nonfermenter ID kit (Becton, Dickinson & Co., Sparks, MD) provided confidence values for identification of ≥0.9986. For S. intermedius strains, analyzed with the BD BBL Crystal Gram-Positive ID kit, such values were ≥0.9774. Other characteristics used for identification included a negative Slidex Staph kit assay (bioMérieux SA, Marcy-I'Etoile, France), which specifically identified S. aureus (including its small colony variants by a delayed precipitation reaction), a pronounced β-hemolysis on Columbia Agar with 5% defibrinated sheep blood (Oxoid GmbH, Wesel, Germany), and a positive rabbit plasma coagulase test, Bactident coagulase (Merck KGaA, Darmstadt, Germany). Clonal independence could be confirmed by patterns of restriction fragments generated from 16S rRNA genes, the 16S-23S rRNA gene spacer region, and randomly amplified polymorphic DNA (H.-V. Tichy and H.-G. Wetzstein, unpublished data).
Preparation of media and inoculum.
Culture media were prepared according to the instructions given by the manufacturers. Stock cultures were kept at −80°C in Microbank vials (Pro-Lab Inc., Ontario, Canada). Working bacterial cultures were prepared on casein-peptone soymeal-peptone agar USP (Merck, Darmstadt, Germany), stored for ≤4 weeks at 4 to 8°C and subcultured for a maximum of 5 passages. Liquid cultures were grown in casein-peptone soymeal-peptone broth USP, pH 7.3 ± 0.2 (Merck), horizontally agitated in a water bath of 37°C at a frequency of 100 per min. To produce the concentrated cell suspension, 1-liter Erlenmeyer flasks containing 270 ml of medium were inoculated with 1% of an actively growing preculture, sealed with a cotton plug, and harvested by centrifugation after 17 h of incubation. Pellets, washed once in 0.9% saline, were resuspended to a density of about 5 × 109 CFU/ml, based on optical density at 576 nm read from a calibration curve correlating optical density with CFU/ml.
MPC testing.
BioAssay dishes for agar diffusion assays, providing a culture area of 530 cm2 (Nalge Nunc Internatl. Corp., Rochester, NY), were filled with 150 ml Difco Balanced Sensitivity Test medium, pH 7.4 ± 0.2 (Becton Dickinson & Co.), containing a FQ at a specific concentration. Following 20 h of drying, aliquots of 0.8 ml of a concentrated cell suspension containing 4 × 109± 2 × 109 CFU were streaked to the surface of the plates. Regrowth was monitored by counting colonies for up to 14 days, thus, assuring the inclusion of slow-growing small colony variants (SCVs). Plates were incubated in sealed plastic bags at 36°C ± 1°C in the dark to prevent photo degradation of the drugs. Water loss from the agar amounted to 0.7 ± 0.2 g per day. The apparent increase in drug concentration of about 3% per week was not corrected for.
To determine the MPC graphically, CFU (geometric means ± standard deviation) were plotted over the respective FQ concentrations. The lower detection limit was 1 colony per plate. If no colony was recovered, the corresponding symbol was placed directly below the detection limit indicated in the figures. Symbols placed at a lower position resulted from averaged CFU values either obtained for several plates or in several experiments. To determine CFU beyond the upper detection limit, set at 4,000 colonies per plate, 10-fold dilutions of the inoculum had to be prepared and plated onto series of plates, each containing the same FQ concentration. Experiments were performed on separate days, and in triplicate unless otherwise specified. A control plate without drug was inoculated from a 10−7 dilution of the inoculum to check its size and to monitor the course of regular growth. Additional media evaluated for utility in determining MPCs comprised casein-peptone soymeal-peptone agar USP, mentioned above, and Mueller-Hinton agar, pH 7.3 ± 0.1, according to standard M6-A (Oxoid GmbH, Wesel, Germany).
MIC determination.
MICs were determined by an agar dilution method on Difco Balanced Sensitivity Test medium (see above) following the method described in (32). For each strain, 104 CFU were applied per spot using a multipoint inoculator (Mast Laboratories Ltd., Liverpool, United Kingdom). Plates were incubated at 36°C ± 1°C and checked for visible growth after 20 and 44 h incubation in the dark under ambient atmosphere. The MIC was defined as the lowest drug concentration inhibiting visible growth. E. coli ATCC 25922 served as the control strain.
RESULTS
MPCs of PRA for control strains of E. coli.
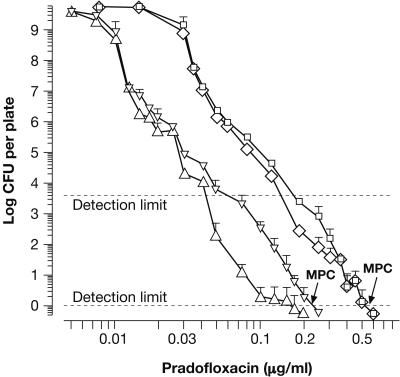
Residual CFU, obtained for large populations of E. coli ATCC 8739 after plating onto agar containing PRA at 0.005 to 0.25 μg/ml, were used to generate two curves revealing an essentially biphasic decline of CFU over increasing PRA concentrations (Fig. 2; compare with Fig. 2 in reference 13). The first represents colonies exhibiting regular growth, i.e., increase in size and morphology similar to that of unexposed controls. Mostly, those colonies were outnumbered by SCVs, which were included in the second curve representing total CFU. SCVs predominantly appeared after ≥ 3 days of incubation and were especially prominent at PRA concentrations between 0.04 and 0.2 μg/ml. However, a few colonies, apparently exhibiting regular growth, were detected even close to the MPC. The MPC of PRA for E. coli ATCC 8739 fell between 0.2 and 0.25 μg/ml (Fig. 2; Table 1). Hence, the average MPC of 0.225 μg/ml, also determined graphically, was employed in subsequent calculations.
FIG. 2.
Phenotypic heterogeneity in large populations of E. coli ATCC 8739 and S. aureus ATCC 6538 as revealed upon successive inhibition of colony formation by increasing concentrations of PRA. Graphs representing residual CFU of E. coli after 5 days (▵, regular colony morphology; ▿, total CFU including SCVs) and of S. aureus after 7 days of incubation (⋄, regular morphology; □, total CFU including SCVs) were compiled from 7 and 11 experiments, respectively. At the MPC, no visible growth was detectable. Subsequent increases in CFU, observed until day 14, did not affect the MPCs. Susceptibility of individual clones varied between 1 and 10 times the MIC (see Table 1). The upper detection limit indicates the maximum number of CFU resolved on one agar plate.
TABLE 1.
Potencies of veterinary fluoroquinolones expressed in terms of MICs and MPCsa
| Compound |
E. coli ATCC 8739
|
S. aureus ATCC 6538
|
||||
|---|---|---|---|---|---|---|
| MIC (μg/ml) | MPC (μg/ml) | MPC/MIC | MIC (μg/ml) | MPC (μg/ml) | MPC/MIC | |
| Pradofloxacin | 0.015-0.03++ | 0.2-0.25 | 9.4 | 0.03-0.06++ | 0.5-0.6** | 12 |
| Danofloxacin | 0.06 | 0.5-0.55 | 8.8 | 0.125-0.25 | 10-11* | 56 |
| Difloxacin | 0.125-0.25 | 1.5-1.6 | 8.3 | 0.125 | 16-18* | 136 |
| Enrofloxacin | 0.03-0.06+ | 0.3-0.35 | 7.8 | 0.06-0.125++ | 3-3.5* | 35 |
| Marbofloxacin | 0.03 | 0.25-0.3 | 9.2 | 0.25-0.5 | 3-3.5 | 9 |
| Orbifloxacin | 0.125 | 1-1.25 | 9.0 | 0.5 | 8-9 | 17 |
| Sarafloxacin | 0.03-0.06 | 0.5-0.6 | 12.2 | 0.125-0.25 | 8-9 | 45 |
| Ciprofloxacin | 0.015-0.03 | 0.1-0.15 | 5.6 | 0.25-0.5+ | 6 | 16 |
| Moxifloxacin | 0.06-0.125 | 0.5-0.6 | 6.0 | 0.03-0.06 | 0.8-1 | 20 |
MICs have been compiled from three, six, or seven (+) and 10 to 14 (++) independent experiments; the more frequent result is printed in bold. MPCs were determined in three, five (*), or nine (**) experiments. In calculations, mean values were employed.
MPCs of PRA for three supplementary control strains of E. coli were lower than those obtained for E. coli ATCC 8739, and also approximately two- to threefold lower than the MPCs of selected comparator FQs such as ENR, MAR and CIP (Table 2), indicating a difference in potency to be described in more detail below for E. coli ATCC 8739. SCVs were much more prominent (than shown in Fig. 2) for E. coli DSM 10650 and E. coli WT, increasing the MPCs by 30 to 50%, but were much less prevalent in E. coli ATCC 25922 with no effect on the MPC. MICs of PRA tended to be the lowest, but still were in the same order of magnitude as the MICs of comparator FQs (Table 2).
TABLE 2.
Susceptibility of supplementary control strainsa
| Strain | Pradofloxacin
|
Enrofloxacin
|
Marbofloxacin
|
Ciprofloxacin
|
||||
|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | MPC (μg/ml) | MIC (μg/ml) | MPC (μg/ml) | MIC (μg/ml) | MPC (μg/ml) | MIC (μg/ml) | MPC (μg/ml) | |
| E. coli | ||||||||
| ATCC 25922 | 0.008-0.015 | 0.075-0.1 | 0.015-0.03 | 0.15-0.175 | 0.015-0.03 | 0.2-0.25 | 0.008-0.015 | 0.1-0.15 |
| DSM 10650 | ≤0.008 | 0.075-0.1 | ≤0.008 | 0.125-0.15 | 0.008-0.015 | 0.175-0.2 | ≤0.008 | ND |
| WT | 0.015-0.03 | 0.125-0.15 | 0.03-0.06 | 0.4-0.5 | 0.03 | 0.5 | 0.015-0.03 | 0.3 |
| S. aureus DSM 11823 | 0.06 | 0.2-0.25 | 0.125-0.25 | 1 | 0.5 | ND | 0.25-0.5 | ND |
| S. intermedius ATCC 29663 | 0.03 | 0.15 | 0.06-0.125 | 2-2.5 | 0.25 | ND | 0.125 | ND |
MICs were determined in three to five experiments; the more frequent result is printed in bold. ND, not determined.
MPC90 of PRA for clinical isolates of E. coli.
MPCs of PRA for ten clinical isolates of E. coli, determined after 5 days of incubation, fell into three categories (i) ≤0.15 μg/ml, three strains; (ii) 0.15 to 0.175 μg/ml, five strains; and (iii) 0.175 to 0.2 μg/ml, two strains. Therefore, MPC90 amounted to 0.175 to 0.2 μg/ml. This value increased to 0.2 to 0.225 μg/ml after 14 days of incubation. In four strains, SCVs already had increased the MPCs by about 10 to 20%, compared to MPCs based on colonies exhibiting regular growth.
MPCs of PRA for control strains of S. aureus and S. intermedius.
Residual colony formation of S. aureus ATCC 6538 in the presence of 0.075 to 0.6 μg PRA/ml is depicted in Fig. 2. Both curves, comprising either regular colonies or total CFU including SCVs, indicated a biphasic drop of CFU with increasing drug concentrations. Mostly, SCVs outnumbered colonies showing regular growth, especially at concentrations between 0.15 to 0.3 μg/ml. SCVs detected after 5 to 7 days of incubation had lost pigmentation and mostly had an irregular colony edge. However, SCVs did not contribute to the MPC of PRA, which fell between 0.5 and 0.6 μg/ml. The mean value of 0.55 μg/ml, determined graphically, was used in subsequent calculations.
MPCs of PRA for two supplementary control strains of S. aureus and S. intermedius were in the order of 0.15 to 0.25 μg/ml, indicating a higher susceptibility (Table 2). SCVs did not contribute to these MPC values. The MPCs of PRA were 4- and 15-fold lower than those of ENR, pointing to a potency difference described below for S. aureus ATCC 6538. Quite notably, the MPCs of PRA were similar to or even lower than the MICs of MAR and CIP (Table 2); consequently, the MPCs of the latter FQs were not evaluated.
MPC90 of PRA for clinical isolates of S. intermedius.
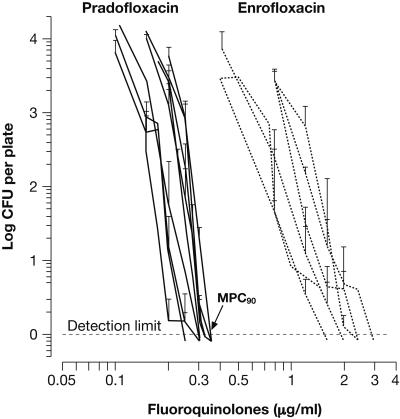
A first MPC90 of PRA was determined for 10 clinical isolates of S. intermedius. MPCs read after 7 days fell into three categories: (i) ≤0.25 μg/ml, seven strains; (ii) 0.25 to 0.3 μg/ml, two strains; and (iii) 0.35 to 0.375 μg/ml, one strain, thus providing an MPC90 of 0.25 to 0.3 μg/ml. For two strains, MPCs had already been determined by SCVs; the respective increases amounted to about 30%. Due to the continued appearance of SCVs in five strains, at 14 days incubation, the MPC90 was increased to 0.3 to 0.35 μg/ml. The curves, shown in Fig. 3, represent total CFU for the strains of categories i and ii. MPCs of ENR were about six- to eightfold higher, confirming the difference in potency already noted for two control strains (Table 2).
FIG. 3.
Determination of MPC90 of PRA for clinical isolates of S. intermedius. Each graph represents total CFU (geometric mean ± standard deviation from triplicate experiments) recovered after 14 days of incubation; the curve of the least sensitive strain was omitted (see text). Representing the other veterinary FQs, MPC curves obtained with ENR (depicted for six representative strains) confirmed the higher potential of PRA also observed with control strains. The inoculum size was in the order of 5 × 109± 2 × 109 CFU per plate. Such bundles of curves may facilitate determination of a mean MPC plus a concentration range, which may be more characteristic for a species than MPC90.
Comparative MPCs of veterinary FQs.
The least susceptible control strains, E. coli ATCC 8739 and S. aureus ATCC 6538, were used to assess relative potencies of six veterinary FQs (Fig. 4). Numerical MPC values and MICs are listed in Table 1, along with data on the reference compounds CIP and MXF.
FIG. 4.
Comparative MPCs of veterinary FQs for E. coli ATCC 8739 and S. aureus ATCC 6538. Graphs represent total CFU (geometric means ± standard deviation) and comprise as references the lower parts of the respective graphs shown for PRA in Fig. 2. FQs analyzed included PRA (•), ENR (▪), MAR (▵), DAN (⋄), SAR (▿), ORB (⧫), and DIF (○). Inocula were in the order of 4 × 109 CFU per plate, and incubation times are given in Fig. 2.
E. coli ATCC 8739. Compared to the MPC of PRA (0.225 μg/ml), MPCs of MAR, ENR, DAN, SAR, ORB, and DIF were 1.2-, 1.4-, 2.3-, 2.4-, 5-, and 7-fold higher, respectively (Table 1). MPCs of five FQs were in the range of 0.4 ± 0.2 μg FQ/ml, while those of ORB and DIF were >1 μg/ml. All MPC curves had similar shapes (Fig. 4). The MPC of CIP was 0.1 to 0.15 μg/ml and found to be the lowest of all compounds tested. SCVs had a pronounced effect on the MPCs of DAN, ENR, CIP, and MXF, causing MPCs about twice as high as would have been obtained on the basis of regular colonies. MICs were in the order of 0.03 to 0.06 μg/ml, except for ORB and DIF. The quotient MPC/MIC amounted to between 8 to 12 for veterinary FQs, and to 6 for the reference FQs (Table 1).
S. aureus ATCC 6538. MPCs of ENR, MAR, ORB, SAR, DAN, and DIF were 6-, 6-, 15-, 15-, 19-, and 31-fold higher, respectively, compared with the PRA MPC of 0.55 μg/ml (Table 1). MPC curves obtained for SAR and DIF extended over an exceptionally wide concentration range (Fig. 4). Hence, the quotients MPC/MIC were highly variable, ranging from about 10 for PRA and MAR up to 136 for DIF (Table 1). The MPC of MXF was comparable with that of PRA (Table 1). SCVs had a significant influence on the MPC of DIF only, increasing it by approximately 30%. In contrast to the MPCs, MICs indicated only moderate differences in potency with the MICs of PRA and MXF being the lowest (Table 1).
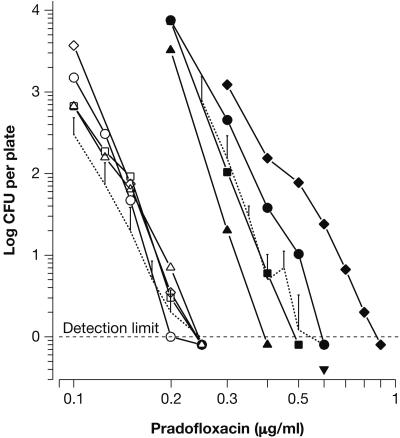
Dependency of the MPC on inoculum density.
E. coli ATCC 8739 and S. aureus ATCC 6538 were used to determine the extent by which inoculum density affected the MPCs of PRA. Results are presented in Fig. 5. Inocula ranged from 4 × 108, spread on 10 plates for each drug concentration in order to evaluate 4 × 109 CFU, to 1010 CFU per plate. For E. coli, all MPC curves targeted the concentration range between 0.2 and 0.25 μg/ml. Thus, inoculum density had no significant effect on the MPC. In contrast, a pronounced effect was observed for S. aureus. MPCs of PRA varied from around 0.4 to 0.9 μg/ml. The increase was especially pronounced, if density was raised from 4 × 109 to 1010 CFU per plate. In one experiment, each of 10 plates containing 0.6 μg PRA/ml had been seeded with an inoculum of 3.4 × 109 CFU. The resulting average CFU was 0.4 per plate. Its inclusion in Fig. 5 fully confirmed the MPC of 0.55 μg/ml graphically determined. However, even at the inoculum density used for routine testing, the MPC may already have been slightly overestimated (Fig. 5).
FIG. 5.
Effect of inoculum density on the MPCs of PRA for E. coli ATCC 8739 (open symbols) and S. aureus ATCC 6538 (solid symbols). The graphs, mostly comprising means of total CFU, were generated in four and five separate experiments, respectively. Agar plates containing PRA at the indicated concentrations were seeded with either: 4 × 108 CFU (Δ▴, 10 plates); 1 × 109 CFU (□▪, four plates); 4 × 109 CFU (○•, one plate); 3.4 × 109 CFU (▾, 10 plates; performed with S. aureus only); or 1010 CFU (⋄⧫, one plate). Serving as references, the dotted lines represent the lower part of the curves (total CFU) shown in Fig. 2. The duration of incubation was as indicated in Fig. 2.
Effect of culture medium.
To estimate the effect of the culture medium on MPCs, E. coli ATCC 8739 and S. aureus ATCC 6539 were used in duplicate experiments to inoculate three media in parallel. The MPCs determined amounted to 0.2 to 0.25 μg/ml for E. coli and 0.5 μg/ml for S. aureus.
DISCUSSION
MPC curves illustrate dual targeting.
The MPC curves determined for E. coli ATCC 8739 and S. aureus ATCC 6538 revealed sharp initial drops in CFU of about 4 log10, occurring at PRA concentrations close to the respective MICs (Fig. 2; Table 1). Regrowth of the bulk population, comprising 4 × 109 CFU, was prevented, obviously due to inhibition of a first FQ target. The primary targets of CIP and other FQs in E. coli and S. aureus have been identified as DNA gyrase and topoisomerase IV, respectively (2, 17, 24). Nevertheless, subpopulations comprising 104 to 105 CFU were still capable of forming colonies, thus possessing phenotypically an FQ-resistant first target. However, upon further increase of the PRA concentration, the number of colonies declined moderately until a second pronounced drop, just before the MPCs were attained at 0.225 μg/ml for E. coli and 0.55 μg/ml for S. aureus. Regrowth of first-step FQ-resistant variants was prevented through inhibition of a second FQ target, possibly topoisomerase IV in E. coli and DNA gyrase in S. aureus (2, 24).
It should be noted that neither the targets nor their order have been confirmed, as of yet, for any of the veterinary FQs. Additional mechanisms causing phenotypic FQ resistance such as active efflux may be involved and interfere with such target assignments (17). Regardless, growth in the presence of PRA at concentrations ≥MPC would clearly have required the concurrent presence of two mutations conferring reduced susceptibility or resistance to both targets. Such double mutants, theoretically, should occur at a frequency of 10−12 to 10−14 and hence ought to be absent in a population of the size used as the inoculum (6, 14-17). Consequently, for large wild-type populations, the MPC apparently defines the sensitivity of the secondary FQ target, while the sensitivity of the first target is approximated by the MIC. Therefore, selective amplification of first-step FQ-resistant subpopulations can only be prevented by appropriate inhibition of the secondary (unchanged) drug target.
All FQs studied may cover both target enzymes, provided that the respective drug concentrations could be achieved, particularly at the site of infection. For E. coli ATCC 8739, MPCs of MAR, ENR, DAN, and SAR were about 1.2- to 2.4-fold, those of ORB and DIF five- to sevenfold higher than the MPC of PRA. The relatively low MPCs in the first group may explain the good clinical efficacy of these agents against E. coli, especially, when combined with a key pharmacokinetic parameter, the area under the drug concentration curve (AUC) in serum or tissues, as AUCs ranging from 10 to 30 are attainable by appropriate dosing of PRA (19), ENR, DIF and MAR (39). The MPC of CIP was only slightly below that of PRA. MPCs published recently for other Enterobacteriaceae, namely Salmonella enteritidis and Salmonella enterica serovar Typhimurium, were 0.25 and 0.5 μg/ml for CIP and ENR, respectively (34). Similar MICs of all FQs (except DIF and ORB) for E. coli indicated a comparable affinity for their primary target. The relative difference in sensitivity between the first and second target, expressed by the quotient MPC/MIC, was almost constant.
For S. aureus ATCC 6538, MICs in the same order of magnitude suggested comparable antibacterial potential; only the MICs of MAR and ORB were slightly increased (Table 1). However, MPCs varied from 0.55 μg/ml for PRA up to 17 μg/ml for DIF. Such variation in affinity for the secondary target, reflected by MPC/MIC quotients ranging from 10 to 135, may be predictive for resistance selection during treatment; the quotient of 10 obtained for MAR was due to its relatively high MIC. The MPC of MXF was only slightly above that of PRA (Table 1). In contrast, the MPC of CIP, determined for S. aureus MT5 at 0.9 μg/ml after 2 days incubation (13), was well below the value reported in Table 1 and, most likely, attributable to the shorter incubation period. The MPC90 of MXF was previously reported to be 0.25 μg/ml for 122 methicillin-susceptible clinical isolates of S. aureus (30), and this value was similar to the MPC of PRA determined with S. aureus DSM 11823 (Table 2). In animals, drug levels required to achieve MPC conditions should readily be attained with PRA (19). For the other FQs, 6- (ENR, MAR), 15- (ORB and SAR), 19- (DAN), and 31-fold (DIF) higher MPCs suggest much higher drug doses will need to be administered to achieve and maintain MPC drug levels. Maximum drug concentrations attained during routine dosing in various animal species have been reviewed recently (39).
Besides the bioavailable drug concentration (7), the duration of time such drug concentration is to be maintained above the MPC during therapy will be of primary importance in pharmacodynamic estimations. For once daily dosing, at least 6 to 12 h is deemed to be necessary (6). To adequately understand coverage of the dosing interval, bactericidal and bacteriostatic activities of FQs, such as postantibiotic effects, need to be addressed (on top, the immune defenses) in addition to MPCs, since pathogen elimination is considered to be of primary importance for outcome of therapy and prevention of resistance selection (3).
MPCs of PRA, determined with control strains of E. coli and S. aureus were in the same order of magnitude as MPC90 for recent clinical isolates of E. coli and S. intermedius. Comparison of MPC90 of PRA for S. intermedius with MPCs determined for ENR (Fig. 3), the next potent of the veterinary FQs, confirmed the principal difference in drug potency that was also observed with control strains. It remains to be analyzed, however, how the vast differences in MPCs (especially for staphylococci) translate into treatment success and prevention of FQ resistance. It has been stated that the ideal FQ should have similar affinities for both targets, DNA gyrase and topoisomerase IV (6, 17, 33). However, as both enzymes differ in mode of expression as well as the molecular mechanisms (23), this may be feasible for specific FQs only, e.g., clinafloxacin (33). Nonetheless, in animals, PRA is expected to cover both targets in E. coli and S. intermedius due to the low MPC drug concentrations suggested in this study.
Mutant selection window.
At drug concentrations below the MPC, first-step FQ-resistant clones are likely to continue growth, replacing the wild-type population. Several cycles of acquisition of an appropriate mutation, followed by clonal expansion under drug selective pressure, are thought to precede the manifestation of clones with clinically relevant degrees of FQ resistance (1, 6, 17, 24, 26). MPC curves obtained with ORB and DIF for S. aureus (Fig. 4) spanned an unusually wide range of drug concentrations, resulting in a broad mutant selection window (16). While its upper limit is defined by the MPC, the lower limit may be approximated by either MIC (42), an MIC99 (5), or even the initial drops in CFU shown in Fig. 2.
Clinical observations made in human medicine (11), comprehensively discussed by others (6, 22), and in a rabbit infection model with Streptococcus pneumoniae (9, 10, 18) represent first evidence in support of the validity of the MPC concept in vivo. Ideally, MPC-based dosing should be applied from initial drug treatment onward. Otherwise, due to the natural presence of first-step FQ-resistant variants in large wild-type populations, clonal selection will gradually erode the potential of even the most active FQ. Consequently, it has already been suggested to use the most potent drugs within a class (3, 37, 38). Ultimately, it may be necessary to reevaluate less potent drugs within a class to determine if higher dosages or more frequent dosing can satisfy MPC-based strategies (22).
Diagnostic potential of MICs.
The MPC curves shown in Fig. 2 revealed a pronounced heterogeneity in susceptibility present in large bacterial populations. The most resistant variants were capable of colony formation even in the presence of PRA at about 10 times the MIC. Such heterogeneity remains undetected during MIC testing, for which an inoculum of 104 CFU is employed (32). Such inocula comprise just the most sensitive fraction of either a liquid preculture or a resuspended colony derived from one clinical isolate, which itself represented another large population. The comparably small extent of variation in susceptibility among CFU used as inoculum in MIC testing can also be inferred from the curves shown in Fig. 2. If inocula of 105 to 106 CFU were employed, more refractory CFU would be included, most likely increasing the MIC by one dilution step. Although MICs may refer to the sensitivity of the first target only, pharmacodynamics of FQs is currently based on serum and organ drug concentrations and MICs (6). If inhibition of the second FQ target was of any therapeutic relevance, MPC-based pharmacodynamics would provide the framework for estimating therapeutic efficacy.
Methodological aspects in MPC testing.
The type of culture dish used in our experiments provided about 10-times the surface area of a regular agar plate and thus, a broader range for enumerating residual CFU. Three media tested in parallel provided similar MPCs for the primary control strains, thereby indicating comparable nutritive capacities and, possibly, similar extents of interference due to unspecific drug binding or complex formation between PRA and divalent cations. Spreading bacteria on a larger surface should minimize protective effects for surviving cells due to multilayered cellular debris. At the same time, additional selective pressure may be reduced, possibly exerted by lytic enzymes released from dead cells. Binding of PRA to cells has been investigated: about 5% of 14C-labeled PRA, applied at MPC levels, was bound by 3 × 1010 CFU of S. aureus or 2 × 1011 CFU of E. coli, resuspended in 10 ml of growth medium (H.-G. Wetzstein, unpublished results). Hence, unspecific drug binding may result in overestimated MPCs.
Because of the large agar volume (150 ml), a larger amount of drug was present, likely to better ensure the intended exposure concentration. In experimental protocols, drug concentrations may be controlled on the basis of drug purity, standard solutions, and careful adjustment of the volumes of drug and agar applied. Effective protection of plates from daylight to prevent photodegradation of FQs may be more demanding for large-scale experiments. Initial drying of the agar plates was required to prevent nonspecific spread of the inoculum on the wet surface. The latter would have increased CFU, causing overestimated MPCs. As well, initial drying and long-term incubation resulted in water loss due to evaporation, and under the present experimental conditions, this was in the order of 3% per week, even if plates were stored in sealed polyamide bags. Therefore, the MPC values reported may have been slightly underestimated.
Incubation time has a pronounced effect on the MPC. Two to fourfold increased MPCs, observed between 24 and 48 h of incubation, have been reported recently (34). Bacterial populations recovered in the present study comprised colonies exhibiting a regular increase in size as well as slow-growing SCVs, the abundance of which varied by the strain studied. Comprehensive detection of SCVs required at least 5 (E. coli) and 7 days (S. aureus). For clinical isolates, up to 14 days may be required; however, the clinical significance of SCVs has yet to be determined. Incubation time had a pronounced effect on both the shape and location of MPC curves (Fig. 2), being shifted towards higher drug concentrations as well as higher CFU over time. SCVs often outnumbered colonies exhibiting regular growth. For some strain/FQ combinations, SCVs even doubled the MPC. SCVs were carefully recorded because of their undefined relevance in the selection of FQ resistance (33, 35). Generally, SCVs are thought to exhibit a resistance phenotype caused by impaired heme or quinone biosynthesis (29, 31). The resulting ATP deficiency, however, may also be characteristic of SCVs appearing under drug selective pressure (33).
Inoculum density had essentially no effect on the MPC of PRA for E. coli. In contrast, for S. aureus, density was found to be a significant factor, as MPCs were almost doubled over the drug concentration range tested. Even at the density of cells routinely used, the MPC of PRA may already have been slightly overestimated. A final aspect should be mentioned: E. coli and S. aureus were applied at twice and fourfold their maximum density naturally attained in liquid cultures. A fraction of cells may rapidly have attained the more refractory state which has been observed for cell aggregates or in biofilms.
MPC curves also characterize the inoculum used in other in vitro potency assays with regard to the variability in susceptibility introduced. The likelihood for the presence of persisters (first-step variants) and regrowth from populations of 106 to 107 CFU/ml, routinely used to determine kill kinetics or postantibiotic effects, will depend on the culture volume, i.e., the total population present. Moreover, the probability of isolating FQ-resistant clones depends on whether a drug concentration applied for selection was located above or below the MPC, i.e., above or within the mutant selection window. This is illustrated by an example taken from Fig. 2: at a concentration of 0.6 μg PRA/ml, such probability for S. aureus would have been <2.5 × 10−10 (the reciprocal of the inoculum), while at 0.3 μg/ml, it would have been in the order of 5 × 10−8. SCVs may also have to be included in such probability values.
Although the experimental procedure used to determine MPCs still needs further refinement and standardization, the MPC concept is expected to have great utility for evaluating relative in vitro potency of related drugs and for designing appropriate dosing regimens. In terms of MPC measurements for E. coli, S. aureus, and S. intermedius, PRA had the highest potential of all veterinary FQs evaluated. The relative potency of PRA even increases if first and second-step FQ-resistant isogenic variants of E. coli and S. aureus are considered (H.-G. Wetzstein and W. Hallenbach, Abstr. 104th Annu. Meet. Am. Soc. Microbiol., abstract Z-026, 2004). Although FQ resistance in household pets is still rare, concerning E. coli (12) and S. intermedius (20), it appears to be timely to evaluate MPC-based therapeutic concepts. With appropriate dosing, PRA may combine high therapeutic efficacy with an unprecedented potential in restricting the emergence of FQ resistance during therapy.
Acknowledgments
The technical assistance of, formerly, S. Ochtrop, S. Waldeyer, and I. Fietz, and, currently, N. Schröter and A. Seitz is gratefully acknowledged.
Footnotes
This work is dedicated to G. Gottschalk, University of Göttingen, on the occasion of his 70th birthday.
REFERENCES
- 1.Allen, G. P. 2003. The mutant prevention concentration (MPC): a review. J. Infect. Dis. Pharmacother. 6:27-47. [Google Scholar]
- 2.Bagel, S., V. Hüllen, B. Wiedemann, and P. Heisig. 1999. Impact of gyrA and parC mutations on quinolone resistance, doubling time, and supercoiling degree of Escherichia coli. Antimicrob. Agents Chemother. 43:868-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball, P., F. Baquero, O. Cars, T. File, J. Garau, K. Klugman, D. E. Low, E. Rubinstein, and R. Wise. 2002. Antibiotic therapy of community respiratory tract infections: strategies for optimal outcomes and minimized resistance emergence. J. Antimicrob. Chemother. 49:31-40. [DOI] [PubMed] [Google Scholar]
- 4.Bartel, S., T. Jaetsch, T. Himmler, H.-G. Rast, W. Hallenbach, E. Heinen, F. Pirro, M. Scheer, M. Stegemann, H.-P. Stupp, and H.-G. Wetzstein. November. 2001. Possibly substituted 8-cyano-1-cyclopropyl-7-(2,8-diazabicyclo-[4.3.0]-nonan-8-yl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolin carboxylic acids and their derivatives. U.S. patent 6,323,213 B1.
- 5.Blondeau, J. M., X. Zhao, G. Hansen, and K. Drlica. 2001. Mutant prevention concentration of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blondeau, J. M., G. Hansen, K. Metzler, and P. Hedlin. 2004. The role of PK/PD parameters to avoid selection and increase of resistance: mutant prevention concentration. J. Chemother. 16(Suppl. 3):1-19. [DOI] [PubMed] [Google Scholar]
- 7.Bregante, M. A., A. de Jong, A. Calvo, E. Hernandez, R. Rey, and M. A. Garcia. 2003. Protein binding of pradofloxacin, a novel 8-cyanofluoroquinolone, in dog and cat plasma. J. vet. Pharmacol. Therap. 26(Suppl. 1):87-88. [Google Scholar]
- 8.Cimons, M. 2001. FDA Planning to halt Ag use of two antibiotics. ASM News 67:9-10. [Google Scholar]
- 9.Croisier, D., M. Etienne, E. Bergoin, P.-E. Charles, C. Lequeu, L. Piroth, H. Portier, and P. Chavanet. 2004. Mutant selection window in levofloxacin and moxifloxacin treatments of experimental pneumococcal pneumonia in a rabbit model of human therapy. Antimicrob. Agents Chemother. 48:1699-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croisier, D., M. Etienne, L. Piroth, E. Bergoin, C. Lequeu, H. Portier, and P. Chavanet. 2004. In vivo pharmacodynamic efficacy of gatifloxacin against Streptococcus pneumoniae in an experimental model of pneumonia: impact of the low levels of fluoroquinolone resistance on the enrichment of resistant mutants. J. Antimicrob. Chemother. 54:640-647. [DOI] [PubMed] [Google Scholar]
- 11.Davidson, R., R. Cavalcanti, J. L. Brunton, D. J. Bast, J. C. de Azavedo, P. Kibsey, C. Fleming, and D. E. Low. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747-750. [DOI] [PubMed] [Google Scholar]
- 12.De Graef, E. M., A. Decostere, L. A. Devriese, and F. Haesebrouck. 2004. Antibiotic resistance among fecal indicator bacteria from healthy individually owned and kennel dogs. Microb. Drug Res. 10:65-69. [DOI] [PubMed] [Google Scholar]
- 13.Dong, Y., X. Zhao, J. Domagala, and K. Drlica. 1999. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1756-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drlica, K. 1999. Refining the fluoroquinolones. ASM News 65:410-415. [Google Scholar]
- 15.Drlica, K. 2001. A strategy for fighting antibiotic resistance. ASM News 67:27-33. [Google Scholar]
- 16.Drlica, K. 2003. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 52:11-17. [DOI] [PubMed] [Google Scholar]
- 17.Drlica, K., and M. Malik. 2003. Fluoroquinolones: action and resistance. Curr. Top. Med. Chem. 3:249-282. [DOI] [PubMed] [Google Scholar]
- 18.Etienne, M., D. Croisier, P.-E. Charles, C. Lequeu, L. Piroth, H. Portier, K. Drlica, and P. Chavanet. 2004. Effect of low-level resistance on subsequent enrichment of fluoroquinolone-resistant Streptococcus pneumoniae in rabbits. J. Infect. Dis. 190:1472-1475. [DOI] [PubMed] [Google Scholar]
- 19.Fraatz, K., R. Krebber, M. Edingloh, and E. Heinen. 2003. Oral bioavailability of pradofloxacin tablets and renal drug excretion in dogs. J. vet. Pharmacol. Therap. 26(Suppl. 1):88-89. [Google Scholar]
- 20.Ganiere, J.-P., C. Medaille, and C. Mangion. 2005. Antimicrobial drug susceptibility of Staphylococcus intermedius clinical isolates from canine pyoderma. J. Vet. Med. B 52:25-31. [DOI] [PubMed] [Google Scholar]
- 21.Greene, C. E., and S. C. Budsberg. 1993. Veterinary use of quinolones, p. 473-488. In D. C. Hooper and J. S. Wolfson (ed.), Quinolone antimicrobial agents, 2nd ed. American Society for Microbiology Press, Washington, D.C.
- 22.Hansen, G. T., and J. M. Blondeau. 2005. Mutant prevention concentration as a strategy to minimize antimicrobial resistance: a timely concept but will its acceptance be too late? Therapy 2:61-66. [Google Scholar]
- 23.Hiasa, H., M. E. Shea, C. M. Richardson, and M. N. Gwynn. 2003. Staphylococcus aureus gyrase-quinolone-DNA ternary complexes fail to arrest replication fork progression in vitro. J. Biol. Chem. 278:8861-8868. [DOI] [PubMed] [Google Scholar]
- 24.Hooper, D. C. 2003. Mechanisms of fluoroquinolone resistance, p. 41-67. In D. C. Hooper and E. Rubinstein (ed.), Quinolone antimicrobial agents, 3rd ed. American Society for Microbiology Press, Washington, D.C.
- 25.Ihrke, P. J., M. G. Papich, and T. C. Demanuelle. 1999. The use of fluoroquinolones in veterinary dermatology. Vet. Dermatol. 10:193-204. [DOI] [PubMed] [Google Scholar]
- 26.Li, X., N. Mariano, J. J. Rahal, C. M. Urban, and K. Drlica. 2004. Quinolone-resistant Haemophilus influenzae: determination of mutant selection window for ciprofloxacin, garenoxacin, levofloxacin, and moxifloxacin. Antimicrob. Agents Chemother. 48:4460-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, T., X. Zhao, and K. Drlica. 1999. Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activities by the C-8-methoxy group. Antimicrob. Agents Chemother. 43:2969-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu, T., X. Zhao, X. Li, A. Drlica-Wagner, J.-Y. Wang, J. Domagala, and K. Drlica. 2001. Enhancement of fluoroquinolone activity by C-8 halogen and methoxy moieties: action against a gyrase resistance mutant of Mycobacterium smegmatis and a gyrase-topoisomerase IV double mutant of Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNamara, P. J., and R. Proctor. 2000. Staphylococcus aureus small colony variants, electron transport and persistent infections. Int. J. Antimicrob. Agents 14:117-122. [DOI] [PubMed] [Google Scholar]
- 30.Metzler, K., G. M. Hansen, P. Hedlin, E. Harding, K. Drlica, and J. M. Blondeau. 2004. Comparison of minimal inhibitory and mutant prevention drug concentrations of 4 fluoroquinolones against clinical isolates of methicillin-susceptible and -resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 24:161-167. [DOI] [PubMed] [Google Scholar]
- 31.Mitsuyama, J., H. Yamada, J. Maehana, Y. Fukuda, S. Kurose, S. Minami, Y. Todo, Y. Watanabe, and H. Narita. 1997. Characteristics of quinolone-induced small colony variants in Staphylococcus aureus. J. Antimicrob. Chemother. 39:697-705. [DOI] [PubMed] [Google Scholar]
- 32.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals. Approved Standard M31-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 33.Pan, X.-S., P. J. Hamlyn, R. Talens-Visconti, F. L. Alovero, R. H. Manzo, and L. M. Fisher. 2002. Small-colony mutants of Staphylococcus aureus allow selection of gyrase-mediated resistance to dual-target fluoroquinolones. Antimicrob. Agents Chemother. 46:2498-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randall, L. P., S. W. Cooles, L. J. V. Piddock, and M. J. Woodward. 2004. Mutant prevention concentrations of ciprofloxacin and enrofloxacin for Salmonella enterica. J. Antimicrob. Chemother. 54:688-691. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz, F.-J., A. C. Fluit, A. Beeck, M. Perdikouli, and C. von Eiff. 2001. Development of chromosomally encoded resistance mutations in small-colony variants of Staphylococcus aureus. J. Antimicrob. Chemother. 47:113-115. [DOI] [PubMed] [Google Scholar]
- 36.Stephan, B., K. Hellmann, P. Liège, S. Granier, T. N. Knoppe, E. Heinen, and H. A. Greife. 2003. Clinical efficacy and safety of pradofloxacin in the treatment of canine pyoderma and wound infections under field conditions. J. Vet. Pharmcol. Ther. 26(Suppl. 1):217-218. [Google Scholar]
- 37.Thomson, K. S. 2000. Minimizing quinolone resistance: are the new agents more or less likely to cause resistance? J. Antimicrob. Chemother. 45:719-723. [DOI] [PubMed] [Google Scholar]
- 38.Tillotson, G. S., X. Zhao, and K. Drlica. 2001. Fluoroquinolones as pneumococcal therapy: closing the barn door before the horse escapes. Lancet Infect. Dis. 1:145-146. [DOI] [PubMed] [Google Scholar]
- 39.Walker, R. D. 2000. Fluoroquinolones, p. 315-338. In J. F. Prescott, J. D. Baggot, and R. D. Walker (ed.), Antimicrobial therapy in veterinary medicine, 3rd ed. Iowa State Press, Ames, Iowa.
- 40.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. USA 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, X., J.-Y. Wang, C. Xu, Y. Dong, J. Zhou, J. Domagala, and K. Drlica. 1998. Killing of Staphylococcus aureus by C-8-methoxy fluoroquinolones. Antimicrob. Agents Chemother. 42:956-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao, X., and K. Drlica. 2001. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 33(Suppl.):S147-S156. [DOI] [PubMed] [Google Scholar]