Abstract
Antifungal drugs can affect the cellular morphology of Cryptococcus neoformans in culture, which alters its interactions with phagocytes. We examined the effects of amphotericin B on C. neoformans during murine infection. The antifungal reduced capsule size and serum polysaccharide, which suggests an additional mechanism for amphotericin B's efficacy in cryptococcosis.
Cryptococcus neoformans is an encapsulated yeast-like fungus of worldwide distribution that is a relatively frequent cause of serious disease in immunocompromised patients. Amphotericin B (AMB) is recommended in combination with flucytosine for the treatment of cryptococcosis (16). AMB is purported to mediate its antifungal effects by binding to cell membrane sterols and damaging the cell membrane (1). Additionally, AMB may function as an immunomodulator because it can promote nitric oxide release (14, 19), enhance superoxide production (21, 22), affect cytokine secretion (23), and interact with Toll-like receptors (17). We have previously shown that subinhibitory concentrations of AMB significantly impact the morphology of C. neoformans grown in culture (15). In vitro, the polysaccharide capsule and cell size are reduced, the magnitude of cell charge is lowered, and there is increased capsule release. Also, alterations to the fungal cell by exposure to AMB result in significantly greater phagocytosis of the fungus, suggesting that exposure to this drug alters capsular structure to inhibit its antiphagocytic properties. In this study, we examine the effect of AMB on C. neoformans morphology and polysaccharide release during murine infection.
C. neoformans var. grubii (serotype A; ATCC, Manassas, VA) strain H99 was used for this study because it represents the most prevalent clinical serotype. The fungus was grown overnight in Sabouraud medium (30°C, at 150 rpm) and then collected by centrifugation, washed and suspended in phosphate-buffered saline (PBS), and counted in a hemacytometer. A suspension of 2 × 107 cells/ml was prepared, and 50 μl was injected intratracheally in female C57BL/6J mice (6 to 8 weeks old; Jackson Laboratories, Maine). AMB was administered intraperitoneally every other day at 1 mg/kg of body weight (high dose) or 0.25 mg/kg (low dose) beginning 1 h prior to infection. Control mice received PBS. Mice were sacrificed at either day 1 or day 7 after infection, and the lungs were removed and homogenized in 10 ml of PBS. Collagenase A was added at 1 mg/ml (Roche, IN), the extract was incubated for 90 min at 37°C with vortexing, and the cells were washed with distilled H2O to lyse mammalian cells. Aliquots of the cell preparations were plated onto Sabouraud agar (one colony = 1 CFU). India ink preparations were viewed with an Olympus AX70 (Melville, NY) microscope, and images were obtained (QImaging Retiga 1300 digital camera [Burnaby, British Columbia, Canada]) with QCapture Suite V2.46 software (QImaging). Capsule thickness was determined by subtracting the diameter of the cell body from that of the whole cell (capsule plus cell body). Three mice were sacrificed per sample, and at least 200 cells were counted per mouse. Significance was determined by t test or Kruskal-Wallis test.
Serum samples from mice were collected at day 7 after infection. The sera were treated with proteinase K (1 mg/ml; Roche, IN) and then used in a capture enzyme-linked immunosorbent assay (ELISA) to determine the quantity of circulating glucuronoxylomannan (GXM), the major component of C. neoformans polysaccharide (4). GXM levels were calculated relative to H99 GXM standards (2). The Kruskal-Wallis test was used to analyze differences in GXM levels.
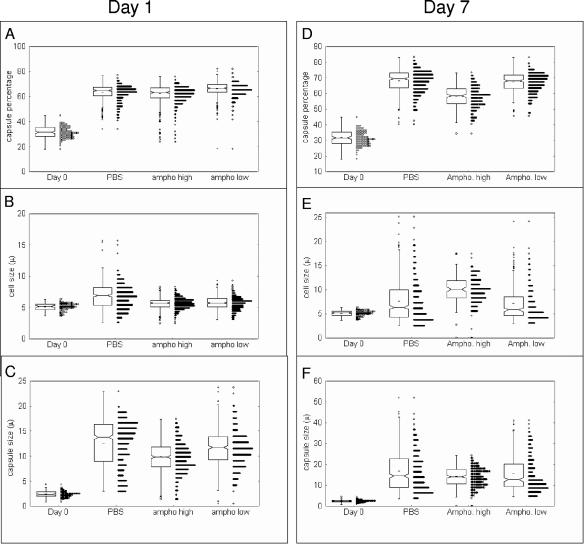
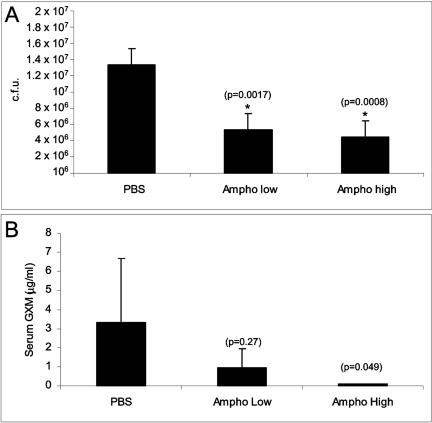
During pulmonary infection, the capsule of C. neoformans dramatically increases in size (8) in as little as 5 min after infection and is most pronounced after 24 h of infection. This increase in capsule size is thought to be important in pathogenesis (13). The CFU at day 1 after infection were similar in the three groups of mice, and propidium iodide staining showed a viability of >97% (data not shown). After 1 and 7 days of infection, C. neoformans isolated from control mice showed a significant increase in capsule size (Fig. 1). In contrast, C. neoformans isolated at day 7 after infection from mice treated with AMB (1 mg/kg) had bigger cell body sizes and smaller capsules (Fig. 1). This result suggests that AMB arrests the cells at a stage where the cells are bigger but the capsule is proportionally smaller. Although the cell size at day 1 was more homogeneous than for the control, the 0.25-mg/kg dose did not result in a significant morphological effect (Fig. 1). Homogeneity may benefit the host immune response, since heterogeneity of C. neoformans cells has been linked to dissemination (3). We examined the effect of the antifungals relative to the fungal burden in the lung at day 7 after infection. At the concentrations of drugs examined, treatment with AMB was associated with a significant reduction in the number of yeast cells present in the lung (Fig. 2A). Measurement of GXM concentration in serum revealed that the higher dose of AMB significantly reduced the capsular polysaccharide levels (Fig. 2B). There was a trend toward a reduction in serum GXM levels with the lower dose. Hence, AMB can alter C. neoformans morphology and reduce the CFU in the lung and the GXM levels in serum.
FIG. 1.
Effect of amphotericin B on cell and capsule size during murine infection. C57BL/6J mice were infected with the H99 strain (106 cells) and treated with different doses of amphotericin (1 mg/kg, high dose, and 0.25 mg/kg, low dose). Control mice received PBS. The mice were sacrificed after 1 or 7 days of infection. The yeast cells were isolated as described in the text, and the relative size of the capsule (A and D), the cell body size (B and E), and the capsule diameter (C and F) were calculated at the days indicated and compared to the size before infection (day 0). At least 200 cells per sample were measured, and all the cells are presented using a box-whisker plot.
FIG. 2.
Effect of amphotericin B on CFU and GXM levels during C. neoformans murine infection. Mice were infected and treated as described in the text. After 7 days of infection, the lungs were extracted and homogenized in PBS. The extracts were plated on Sabouraud plates, and CFU per total lung were calculated (A). Serum from the same animals was obtained, and GXM levels in serum were measured by capture ELISA (B). The means and the standard deviations were plotted, and significance (P values) was calculated with the Kruskal-Wallis test.
Our results suggest that, in addition to the direct effects of AMB on the fungal cell membrane, it can significantly affect the development and release of polysaccharide capsule. To our knowledge this finding is the first evidence that AMB alters C. neoformans cellular characteristics in vivo. This issue is relevant to the mechanism of AMB antifungal action, since this drug has a large volume of distribution and its concentration in tissue may be below the fungicidal threshold (5-7, 12, 18). In this regard AMB has been shown to be relatively ineffective against C. neoformans in nude mice, suggesting that its direct antifungal effects alone are insufficient to modify infection in severely immunocompromised hosts (9). Our results showing a change in cryptococcal cellular morphology imply that indirect mechanisms are important factors in the activity of AMB.
Although the benefits of a large capsule have not been well characterized in vivo, it is reasonable to consider that this increase confers a selective advantage during infection, such as a mechanism to avoid phagocytosis (11). Furthermore, the polysaccharide capsule has potent anti-inflammatory activities (10, 20). However, the data should be carefully interpreted, since capsule growth occurs in the first hours of infection (8). We interpret our results as a consequence of a lower yeast growth rate due to the presence of the antifungal drug, which would result in cells with larger cell bodies. In summary, we investigated the effects of AMB on the pathogenic fungus C. neoformans during murine infection and show changes in some of the parameters related to infection, such as CFU, GXM levels, and capsule and cell size. In addition to the purported direct effects of AMB on the fungal cell membrane, the alteration of cellular morphological characteristics and the reduction of GXM in the serum of infected animals may facilitate a more effective host response against the fungus. The findings of this study provide fertile avenues for future research into the effects of antifungals on the pathogenesis of cryptococcosis.
Acknowledgments
J.D.N. is supported by NIH AI056070-01A2 and AI52733, an Albert Einstein College of Medicine Center for AIDS Research grant, and an Infectious Disease Society of America Wyeth Vaccine Young Investigator Research Award. A.C. is supported by NIH AI52733, AI33774, AI13342, and HL59842.
REFERENCES
- 1.Brajtburg, J., W. G. Powderly, G. Kobayashi, and G. Medoff. 1990. Amphotericin B: current understanding of mechanism of action. Antimicrob. Agents Chemother. 34:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall, A., J. Mukherjee, and M. D. Scharff. 1992. Monoclonal antibody based ELISA for cryptococcal polysaccharide. J. Immunol. Methods 154:27-35. [DOI] [PubMed] [Google Scholar]
- 3.Charlier, C., F. Chretien, M. Baudrimont, E. Mordelet, O. Lortholary, and F. Dromer. 2005. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am. J. Pathol. 166:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherniak, M. R., and J. B. Sundstrom. 1994. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect. Immun. 62:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christiansen, K. J., E. M. Bernard, J. W. Gold, and D. Armstrong. 1985. Distribution and activity of amphotericin B in humans. J. Infect. Dis. 152:1037-1043. [DOI] [PubMed] [Google Scholar]
- 6.Collette, N., P. Van der Auwera, F. Meunier, C. Lambert, J. P. Sculier, and A. Coune. 1991. Tissue distribution and bioactivity of amphotericin B administered in liposomes to cancer patients. J. Antimicrob. Chemother. 27:535-548. [DOI] [PubMed] [Google Scholar]
- 7.Dugoni, B. M., B. J. Guglielmo, and H. Hollander. 1989. Amphotericin B concentrations in cerebral spinal fluid in patients with AIDS and cryptococcal meningitis. Clin. Pharmacol. 8:220-221. [PubMed] [Google Scholar]
- 8.Feldmesser, M., Y. Kress, and A. Casadevall. 2001. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 147:2355-2365. [DOI] [PubMed] [Google Scholar]
- 9.Graybill, J. R., P. C. Craven, L. F. Mitchell, and D. J. Drutz. 1978. Interaction of chemotherapy and immune defenses in experimental murine cryptococcosis. Antimicrob. Agents Chemother. 14:659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson, D. K., J. E. Bennett, and M. A. Huber. 1982. Long-lasting, specific immunologic unresponsiveness associated with cryptococcal meningitis. J. Clin. Investig. 69:1185-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozel, T. R., and E. C. Gotschlich. 1982. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 129:1675-1680. [PubMed] [Google Scholar]
- 12.Louria, D. B. 1958. Some aspects of the absorption, distribution, and excretion of amphotericin B in man. Antibiot. Med. Clin. Ther. 5:295-301. [PubMed] [Google Scholar]
- 13.McFadden, D. C., and A. Casadevall. 2001. Capsule and melanin synthesis in Cryptococcus neoformans. Med. Mycol. 39:19-30. [PubMed] [Google Scholar]
- 14.Mozaffarian, N., J. W. Berman, and A. Casadevall. 1997. Enhancement of nitric oxide synthesis by macrophages represents an additional mechanism of action for amphotericin B. Antimicrob. Agents Chemother. 41:1825-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nosanchuk, J. D., W. Cleare, S. P. Franzot, and A. Casadevall. 1999. Amphotericin B and fluconazole affect cellular charge, macrophage phagocytosis, and cellular morphology of Cryptococcus neoformans at subinhibitory concentrations. Antimicrob. Agents Chemother. 43:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saag, M. S., R. J. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, W. E. Dismukes, et al. 2000. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 17.Sau, K., S. S. Mambula, E. Latz, P. Henneke, D. T. Golenbock, and S. M. Levitz. 2003. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J. Biol. Chem. 278:37561-37568. [DOI] [PubMed] [Google Scholar]
- 18.Shadkchan, Y., Z. Zaslavsky, and E. Segal. 2003. Pharmacokinetics of amphotericin B in serum and tissues in mice treated with amphotericin B-Intralipid. Med. Mycol. 41:15-19. [DOI] [PubMed] [Google Scholar]
- 19.Tohyama, M., K. Kawakami, and A. Saito. 1996. Anticryptococcal effect of amphotericin B is mediated through macrophage production of nitric oxide. Antimicrob. Agents Chemother. 40:1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vecchiarelli, A. 2000. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 38:407-417. [DOI] [PubMed] [Google Scholar]
- 21.Wilson, E., L. Thorson, and D. P. Speert. 1991. Enhancement of macrophage superoxide anion production by amphotericin B. Antimicrob. Agents Chemother. 35:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf, J. E., and S. E. Massof. 1990. In vivo activation of macrophage oxidative burst activity by cytokines and amphotericin B. Infect. Immun. 58:1296-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi, H., S. Abe, and Y. Tokuda. 1993. Immunomodulating activity of antifungal drugs. Ann. N. Y. Acad. Sci. 685:447-457. [DOI] [PubMed] [Google Scholar]