Abstract
Chronic hepatitis C virus (HCV) infection is a worldwide health problem causing serious complications, such as liver cirrhosis and hepatoma. Alpha interferon (IFN-α) or its polyethylene glycol-modified form combined with ribavirin is the only recommended therapy. However, an alternative therapy is needed due to the unsatisfactory cure rate of the IFN-based therapy. Using a modified reporter assay based on the HCV subgenomic-replicon system, we found that sodium stibogluconate (SSG), a compound used for leishmania treatment, suppressed HCV replication. We have previously reported that SSG is effective at inhibiting HCV replication in a cell line permissive for HCV infection/replication and in an ex vivo assay using fresh human liver slices obtained from patients infected with HCV (26). In this study, we show that the SSG 50% inhibitory dose for HCV replication is 0.2 to 0.3 mg/ml (equivalent to 345 to 517 μM of Sb) in the HCV subgenomic-replicon system. We also found that SSG and IFN-α exert a strong synergistic anti-HCV effect in both the traditional isobologram analysis and the median effect principle (CalcuSyn analysis). The combination of SSG and IFN-α could sustain the antiviral response better than SSG or IFN-α alone. The results suggest that SSG may be a good drug candidate for use in combination with other therapeutics, such as IFN-α and ribavirin, to treat HCV infection.
Hepatitis C virus (HCV) causes a serious health problem worldwide (23). According to an estimate from the World Health Organization, approximately 3% of the world's population is infected by HCV (24). Although acute HCV infection is commonly associated with mild symptoms or is sometimes asymptomatic, most patients acquire a persistent infection that leads to severe chronic liver diseases, such as cirrhosis and hepatocellular carcinoma (13). Combination of ribavirin with alpha interferon (IFN-α) (or its polyethylene glycol-modified form) is the only recommended therapy. However, this treatment achieves only an approximate 50% response rate to HCV genotype 1, the most prevalent genotype in HCV infections. Moreover, IFN-α and ribavirin combination therapy is expensive and often causes severe side effects (18). Given the high prevalence and severe clinical sequelae of HCV infection, there is an urgent need for the discovery and development of novel agents for HCV therapy.
HCV represents the only genus (Hepacivirus) of the Flaviviridae virus family. HCV contains a single-stranded, positive-sense RNA genome of 9.6 kb, which encodes a unique polyprotein of approximately 3,000 amino acids (5). The polyprotein is co- and posttranslationally processed by cellular and viral proteases to produce the mature structural and nonstructural viral proteins. The order of these proteins is NH2-C-E1-E2-P7-NS2-NS3-NS4A-NS4B-NS5A-NS5B-COOH. Some HCV-encoded proteins can also be produced from alternate reading frames through ribosomal frameshifting (22, 25). One major obstacle impeding the development of HCV therapeutic strategies is the lack of a reproducible in vitro virus culture system. The recent development of a subgenomic-replicon system in Huh-7 cells (4, 17) provides a powerful tool for studying virus replication and for screening anti-HCV drugs.
By screening a set of marketed drugs, we have discovered that arsenic trioxide (As2O3) is a potent HCV inhibitor (10). Compounds identified through this approach should be considered promising candidates for drug development because the toxicity and pharmacokinetic properties of these marketed drugs are well documented. In this study, we evaluated sodium stibogluconate (SSG) (an old drug containing antimony used in leishmania treatment) for its anti-HCV potential. Both antimony and arsenic belong to group 15 (the nitrogen family) in the periodic table. We found that SSG, along with several other antimonial compounds, including Sb2O3 and SbCl3, was able to exert potent anti-HCV activity at concentrations that did not affect cell viability. When SSG was combined with IFN-α, these two drugs acted synergistically to suppress HCV replication and to prolong antiviral activity.
MATERIALS AND METHODS
Cell culture.
High-glucose Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), G418 (Geneticin), and blasticidin were purchased from Invitrogen (Carlsbad, CA). Human hepatoma cells (Huh-7) and HCV subgenomic-replicon cells (Ava5) were kindly provided by Apath, LLC (St. Louis, MO) (4). A reporter-based cell line, Ava5-EG(Δ4AB)SEAP (14, 15), for HCV drug screening was derived from HCV replicon cells (Ava5). EG(Δ4AB)SEAP is a reporter gene expressing enhanced green fluorescent protein (EG), the NS3-NS4A protease decapeptide recognition sequence (Δ4AB), and secreted alkaline phosphatase (SEAP). In the reporter cell line, a reporter gene, the EG(Δ4AB)SEAP gene, was stably integrated in the Ava5 cells to generate Ava5-EG(Δ4AB)SEAP cells. In Ava5-EG(Δ4AB)SEAP cells, SEAP activity in the culture medium can be used to reflect anti-HCV activity (14). Cells were maintained in a humidified atmosphere containing 5% CO2. For Ava5 and Ava5-EG(Δ4AB)SEAP cells, the culture medium was additionally supplemented with 500 μg/ml G418 and 500 μg/ml G418 plus 10 μg/ml blasticidin, respectively, to maintain selection pressures for sustaining the expression of exogenous genes.
Assay for inhibition of HCV subgenomic RNA.
Ava5-EG(Δ4AB)SEAP cells were seeded in 96-well plates at a density of 5 × 103 per well. After incubation for 1 day, cells were treated with the drugs for 48 h. At the end of incubation, the culture medium was replaced with fresh phenol red-free DMEM containing 10% FBS and the same concentration of drugs. Incubation was continued for one additional day. Culture media were collected, and SEAP activities were measured using the Phospha-Light assay kit (Tropix, Foster City, CA) according to the supplier's instructions. IFN-α, Sb2O3, SbCl5, and As2O3 were from Sigma-Aldrich (St. Louis, MO), and SSG (21% [wt/wt] SbV) was from Wuhan Shengmao (Hubei, China).
Cytotoxicity assay.
Cell viability was determined by the MTS assay essentially as described previously (10). In short, for a 96-well microtiter plate, we used 10 ml of reagent containing phenol red-free DMEM, MTS (tetrazolium compound [3-(4,5-dimethylthiozol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt]; Promega, Madison, WI), and phenazine methosulfate (Sigma, St. Louis, MO) in a ratio of 80:20:1, respectively. The mixed reagent was distributed to cells (100 μl/well). The plates were incubated for 1 to 4 h at 37°C in a humidified 5% CO2 atmosphere, and the absorbance was then recorded at 490 nm. Compounds were also analyzed for their effects on cell cycle distribution. In this assay, cells were seeded in six-well plates at the density of 1 × 106 cells per well and incubated in various concentrations of SSG for 24 h. After drug treatment, adherent cells were harvested by trypsin digestion. Cells were then centrifuged, washed once in phosphate-buffered saline (PBS), and resuspended in 200 μl PBS. Cells were then slowly added to 5 ml of ice-cold 70% ethanol and stored at −20°C until analysis. Fixed cells were collected by centrifugation, washed twice with PBS, resuspended in 1 ml of a solution containing 3.4 mM sodium citrate, 20 μg/ml propidium iodide, and 100 μg/ml RNase A, and stored in the dark for 1 h. Cells were analyzed using a FACSVantage flow cytometer (Becton Dickinson Labware, Franklin Lakes, NJ). Cell cycle analysis was performed according to the mathematical model of Jett (12).
Western blotting.
Western blotting was performed essentially as described previously (14). Anti-HCV NS3 and antiactin antibodies were obtained from LTK Biolaboratories (Taipei, Taiwan) and CHEMICON International Inc. (Temecula, CA), respectively (10). Signals were revealed by the ECL Western blotting system (Amersham Biosciences, Piscataway, NJ).
Northern blotting.
Total RNA was extracted from cells using TRIZOL reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA was isolated, and the concentration was determined spectrophotometrically. RNA samples (10 μg per well) were loaded onto a 1% Tris-borate-EDTA agarose gel and were separated by electrophoresis at 10 V/cm for 1.5 h as described previously (10, 16). RNA in the gel was transferred to a positively charged nylon membrane (BrightStar-Plus; Ambion, Austin, TX) by the Speed Vacuum transfer apparatus (Amersham Bioscience, Buckinghamshire, England). After drying, RNA was cross-linked to the membrane by UV irradiation (Stratagene, La Jolla, CA). The membrane was probed separately with the NS5B gene fragment of HCV and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) fragments labeled with [α-32P]dCTP by the Rediprime II random prime labeling system (Pharmacia) in accordance with the manufacturer's instructions. Hybridization was carried out with denatured probes in Rapid-hyb hybridization buffer (Pharmacia) for 2 h at 65°C. After hybridization, the membrane was washed once in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.2% sodium dodecyl sulfate (SDS) for 20 min at 60°C, once in 1× SSC-0.2% SDS for 20 min at 60°C, and twice in 0.5× SSC-0.2% SDS for 15 min at 65°C. The results were visualized by autoradiography.
Synergistic analyses.
The isobologram analysis was used to evaluate the effects of combined drug treatments (10, 16). Traditional isobologram analysis is a frequently used method for analyzing the effects of multiple drugs and for determining their additivity, synergism, or antagonism. Various doses of SSG and IFN-α were combined in a checkerboard manner to generate dose-response curves (isoboles) of 50% and 80% inhibition of HCV replication to evaluate the effects of the drug combinations. The synergism between two drugs was quantified by combination indices (CI) using the CalcuSyn for Windows computer program (Biosoft, Cambridge, United Kingdom) (6). CI values of <1 indicate a synergistic effect, a CI value of 1 an additive effect, and a CI of >1 an antagonistic effect. The evaluation of drug combinations based on a median effect equation has been widely employed in the literature.
Sustained anti-HCV response after drug removal.
Ava5 cells containing HCV subgenomic-replicon RNA were plated in 15-cm culture dishes at a density of 2.0 × 106 cells per dish. During this experiment, cells were treated with the indicated drugs for 5 days. The culture medium containing the drugs was subsequently removed, and the cells were cultured in fresh medium containing 10% FBS and 1 mg/ml of G418 without drugs. Cells were then allowed to grow for 1 month. Surviving cells were visualized by staining with crystal violet.
RESULTS
Effects of antimonial compounds on HCV replication and cellular toxicity.
We had previously found that arsenic trioxide (As2O3), which is clinically used to treat acute promyelocytic leukemia (2), could suppress HCV replication in the HCV subgenomic RNA system (replicon) at doses that did not cause cellular toxicity (10). Since antimony (Sb) and arsenic belong to the same group in the periodic table, we decided to evaluate whether antimonial compounds also possess anti-HCV activities. In this study, we found that several antimony-containing compounds, including Sb2O3, SbCl3, and SSG, could inhibit HCV replication in Ava5-EG(Δ4AB)SEAP cells (Table 1). Ava5-EG(Δ4AB)SEAP cells were treated with 5 μM of Sb2O3, 5 μM of SbCl3, or 0.5 mg/ml of SSG (equivalent to 0.105 mg/ml or 860 μM of pentavalent antimony [SbV], when considering that the percentage [by weight] of Sb in SSG is approximately 21%) for 72 h. Compared to that of untreated control cells, the remaining SEAP activity of cells treated with Sb2O3, SbCl3, or SSG was 18, 31, or 38%, respectively. The remaining SEAP activity was 19 or 23% when cells were treated with 100 IU/ml of IFN-α or 1 μM of As2O3, respectively. There was no observed cellular toxicity, as revealed by MTS analysis, when cells were treated with these agents for up to 72 h (data not shown).
TABLE 1.
Inhibition of HCV replication by antimony in reporter-based cellsa
| Control value | Mean SEAP activity ± SD (% of control) with:
|
||||
|---|---|---|---|---|---|
| IFN-α | As2O3 | Sb2O3 | SbCl3 | SSG | |
| 100.0 ± 5.2 | 19.2 ± 5.2 | 22.5 ± 1.6 | 18.1 ± 2.6 | 31.5 ± 3.6 | 38.2 ± 4.8 |
Ava5-EG(Δ4AB)SEAP cells were treated with IFN-α (100 IU/ml), As2O3 (1 μM), Sb2O3 (5 μM), SbCl3 (5 μM), and SSG (0.5 mg/ml; 864 μm) for 72 h. The intracellular HCV subgenomic-replicon copy number was determined by measuring SEAP activities. All measurements were performed in triplicate.
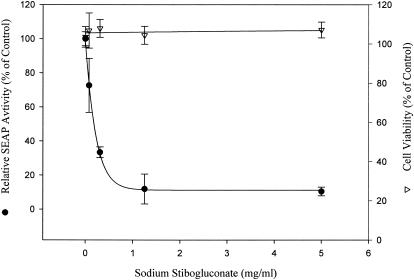
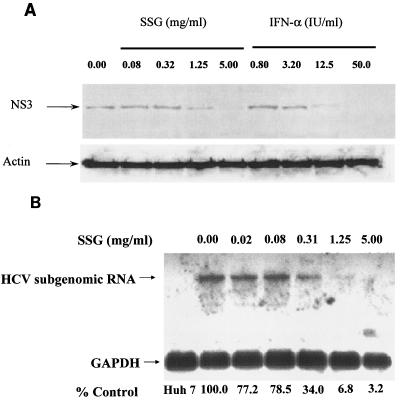
Because SSG is an existing drug, the anti-HCV effect of SSG was analyzed in more detail. Ava5-EG(Δ4AB)SEAP cells were treated with serially diluted SSG for 72 h, and SEAP activities in culture media were analyzed to measure the anti-HCV activities (Fig. 1). SSG reduced SEAP activities in a dose-dependent manner. Activity was reduced to ∼10% of that in the control group, and there was no cellular toxicity, as revealed by MTS analysis, when the cells were treated with up to 5 mg/ml (8.6 mM) of SSG. The 50% effective concentration (EC50) of SSG for HCV inhibition was 0.2 to 0.3 mg/ml (equivalent to 0.042 to 0.063 mg/ml [345 to 517 μM] of Sb). Western blotting analysis was also performed to confirm the results in the Ava5-EG(Δ4AB)SEAP reporter cell system. Ava5 cells were treated with various doses of IFN-α and SSG for 72 h, and cell lysates were collected and analyzed with anti-HCV NS3 antibody. As shown in Fig. 2A, the level of HCV NS3 protein decreased upon SSG treatment in a dose-dependent manner, which correlated with the results shown in Fig. 1. In addition, results from Northern blotting indicated that SSG at 1.15 or 5 mg/ml effectively reduced the level of HCV RNA in a dose-dependent manner (Fig. 2B). The mRNA level of GAPDH remained unchanged under SSG treatment for up to 5 mg/ml, and no HCV signal could be detected in the parental Huh-7 cells because of the lack of HCV subgenomic RNA in Huh-7 cells. Under the same conditions, no difference in cell cycle distribution was observed when Ava5 cells were treated with various concentrations of SSG for 24 h and analyzed for propidium iodide-stained DNA content by flow cytometry (results not shown).
FIG. 1.
Inhibition of HCV replication by sodium stibogluconate in the reporter-based cells. Ava5-EG(Δ4AB)SEAP cells were treated with serially diluted sodium stibogluconate for 72 h. SEAP activities were measured to indicate the anti-HCV activity (filled circles), and cell toxicity was evaluated by MTS assay (open triangles).
FIG. 2.
Inhibition of HCV replication by sodium stibogluconate in Ava5 cells. (A) Ava5 cells were treated with SSG in a dose-dependent manner for 72 h. Cellular lysates were extracted and analyzed using Western blotting. (B) Ava5 cells were treated with SSG in a dose-dependent manner for 24 h. Total cellular RNA was purified and analyzed by Northern blotting.
Synergistic anti-HCV activity of SSG and IFN-α combination.
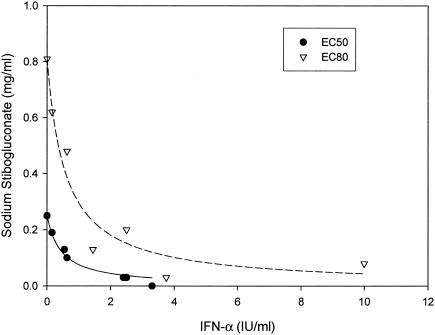
We then assessed whether the combination of SSG and IFN-α exerts synergistic, additive, or antagonistic anti-HCV effects, using the isobologram method and the CalcuSyn Computer program as described in Materials and Methods. In the isobologram method, synergism, additivity, or antagonism are represented by concave, linear, or convex isoeffective curves (isoboles), respectively. Inhibition of HCV replication was evaluated in Ava5-EG(Δ4AB)SEAP reporter cells treated with various doses of SSG (0, 0.03, 0.13, 0.5, and 2 mg/ml) in combination with various doses of IFN-α (0, 0.16, 0.63, 2.5, and 10 IU/ml) (Table 2). The results presented in Table 2 were used to generate isoboles of 50% and 80% inhibition of HCV replication (Fig. 3). SSG and IFN-α exerted strong synergistic anti-HCV activities, as revealed by the sharp concave isobole plots and by the CI values of <1 (range, 0.26 to 0.60), calculated using CalcuSyn analysis (Table 3).
TABLE 2.
Effects of combination of SSG and IFN-α on HCV replicationa
| IFN-α concn (IU/ml) | Mean SEAP activity ± SD with a sodium stibogluconate concn (mg/ml) of:
|
||||
|---|---|---|---|---|---|
| 0.00 | 0.03 | 0.13 | 0.50 | 2.00 | |
| 0.00 | 101.7 ± 1.9 | 72.0 ± 6.1 | 70.7 ± 4.0 | 31.6 ± 6.1 | 13.2 ± 2.8 |
| 0.16 | 101.8 ± 7.6 | 67.8 ± 1.6 | 64.9 ± 2.4 | 24.9 ± 2.6 | 12.8 ± 1.7 |
| 0.63 | 75.4 ± 1.0 | 62.0 ± 4.9 | 44.7 ± 2.2 | 22.8 ± 0.8 | 11.5 ± 2.0 |
| 2.50 | 61.8 ± 3.8 | 49.6 ± 4.8 | 26.8 ± 4.2 | 16.5 ± 1.1 | 9.7 ± 3.6 |
| 10.00 | 31.2 ± 1.4 | 26.2 ± 2.4 | 16.4 ± 1.9 | 10.9 ± 1.8 | 9.6 ± 5.5 |
Remaining SEAP activities (% of control) are shown. All measurements were performed in triplicate.
FIG. 3.
Anti-HCV effect of SSG and IFN-α in combination. The synergism, additivity, and antagonism between two drugs were analyzed by traditional isobologram analysis. Isoboles of 50% (EC50; filled circles) and 80% (EC80; open triangles) inhibition of HCV replication by the combination of SSG and IFN-α were shown to indicate that these two drugs exerted a synergistic anti-HCV effect. Each point in the isoboles was derived from calculation of raw data from Table 2.
TABLE 3.
Interaction of SSG with IFN-αa
| IC | CI with the following SSG/IFN-α ratio:
|
|
|---|---|---|
| 1:5 | 1:20 | |
| 50% | 0.60 | 0.60 |
| 75% | 0.55 | 0.39 |
| 90% | 0.52 | 0.26 |
CalcuSyn analysis provides CI values to determine potential drug additivity. The results showed that SSG combined with IFN-α is synergistic, with CI values ranging from 0.32 to 0.60. The CI determines the degree of the interaction of drugs; e.g., CIs of <1, 1, and >1 are indicative of synergistic, additive, and antagonistic effects, respectively.
Sustained anti-HCV response after drug removal.
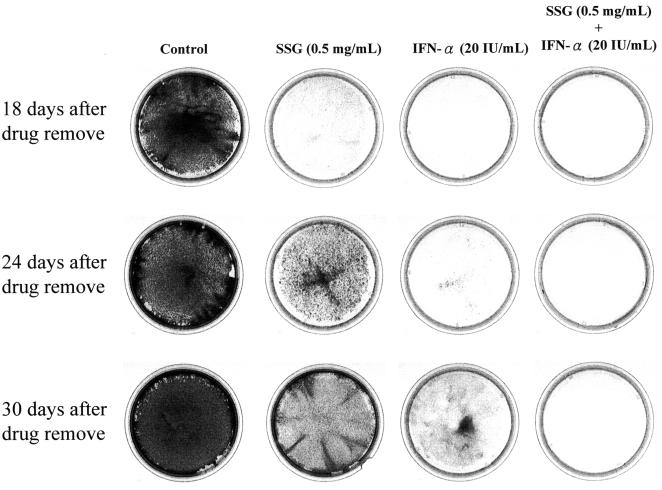
Current IFN-based therapy suffers from an unsatisfactory sustained viral response (SVR) rate. An SVR is defined as a response showing undetectable HCV levels 6 months after the termination of antiviral treatment. In this study, we wished to evaluate if the addition of SSG might benefit IFN-based treatment by enhancing the sustained anti-HCV response using Ava5 subgenomic cells. Toward this end, Ava5 cells were first treated with drugs to suppress the replication of HCV subgenomic RNA. This was performed in the absence of G418 to remove the selection pressure in the culture medium, because the HCV subgenomic RNA in those cells contains a G418-resistant gene. Then, the drugs were removed and cells were allowed to recover in the presence of G418, and only those cells with sufficient HCV subgenomic RNA content survived. If the HCV subgenomic RNA in drug-treated Ava5 cells “rebounded” after drug removal, then the cells would become resistant to G418 selection. In this experiment, Ava5 cells were treated with SSG (0.5 mg/ml), IFN-α (20 U/ml), or the combination of both for 5 days in the absence of G418. The drugs were removed and fresh culture medium containing 500 μg/ml of G418 was added. After cells were cultured for up to 30 days after drug removal, only control cells without drug treatment exhibited a G418-resistant phenotype by forming colonies that can be observed under crystal violet staining (Fig. 4). At day 18 after drug removal, no drug-treated cells could be observed when allowed to grow under G418 selection. At day 24, no cell seemed to survive under G418 selection if cells were treated with the combination of IFN-α and SSG. However, a small number of cells became stainable by crystal violet when cells were treated with 20 U/ml of IFN-α alone. Many more cells were stainable by crystal violet when cells were treated with 0.5 mg/ml of SSG alone. At day 30, more crystal violet-stainable cells were observed when cells were treated with either IFN-α or SSG alone. No crystal violet-stainable cells could be observed for those cells treated with the combination of IFN-α and SSG. These results indicated that the suppression of HCV subgenomic RNA in Ava5 cells could be sustained longer by treatment with the combination of IFN-α and SSG.
FIG. 4.
Survival of HCV subgenomic-replicon cells after a 5-day treatment with drugs. Ava5 cells were treated with 0.5 mg/ml of SSG, 20 IU/ml of IFN-α, or the combination of both. After 5 days of drug treatment, the compounds were withdrawn and 500 μg/ml of G418 was added to enrich the remaining HCV replicon-positive cells that are capable of growing in the presence of G418. The cells were stained with crystal violet after incubation for another 18, 24, and 30 days in the presence of G418.
DISCUSSION
Currently, the only therapeutic option for treating chronic HCV infection is the IFN-based treatment. However, this therapy is often accompanied by severe side effects, and the response rate of approximately 50% is not satisfactory. Thus, new and more-effective therapeutic agents are needed to combat HCV infection. To shorten an otherwise lengthy process for drug discovery and development, we chose to screen drugs that are already in use for the treatment of other human diseases. Using an ex vivo system (liver biopsy samples), we have previously shown that SSG is effective at reducing HCV replication (26). SSG is a compound containing antimony. In this study, we examined the effect of SSG and other antimonial compounds on HCV replication using the subgenomic-replicon system. We showed that several compounds containing Sb, such as Sb2O3 and SbCl3, also possess potent anti-HCV activities.
Given the absence of potent individual agents against HCV other than combinations of IFN-α with ribavirin that possess potential antiviral effects, it is important to explore the potential benefit of combination therapy with other new anti-HCV agents. The importance of combination therapy in the treatment of HCV infection has been demonstrated by the fact that combined IFN-α and ribavirin gave rise to an SVR of 38 to 49% in patients with chronic HCV infections, compared with a 5 to 13% SVR with IFN-α monotherapy (8, 19). In this study, we showed that the combination of SSG with IFN-α has a strong synergistic inhibitory effect on HCV replication. Moreover, the sustained antiviral effect and the benefit of combination treatments were examined using Ava5 cells harboring the HCV subgenomic replicon. We show herein that the combination of SSG and IFN-α could sustain the antiviral response in the subgenomic-replicon system. Therefore, results from this study suggest that SSG should be considered for evaluation of its clinical efficacy.
The mechanism by which SSG inhibits the replication of HCV subgenomic RNA is not known. SSG has been shown to enhance the anticancer activities of IFNs in IFN-resistant cancer cells by inhibiting protein tyrosine phosphatase inhibitory activity and therefore inducing protein tyrosine phosphorylation (27). Whether inhibition of protein tyrosine phosphatase is involved in the anti-HCV activity of SSG requires further study.
Even though SSG has been used in the treatment of leishmaniasis for more than half a century, there is a limited knowledge of its pharmacokinetic properties in humans. SSG consists of Sb conjugated with glucose. After administration to humans, the peak concentration of Sb in plasma is approximately 10 μg/ml (∼82 μM) (1, 7, 11). Though such a peak Sb concentration seems relatively low compared to the SSG EC50 for HCV inhibition found in this study (0.2 to 0.3 mg/ml of SSG or 345 to 517 μM of Sb), several factors should to be taken into consideration. First, at ∼40 μg/ml (equivalent to the clinically achievable Sb levels in humans), SSG is capable of inhibiting HCV replication by ∼30% and inducing a synergistic anti-HCV activity with IFN-α. These results are similar to a recent report by Tanabe et al. (21). They have shown that, using HCV replicon cells, the EC50 value of ribavirin alone was 126 μM, far above the concentrations achievable (∼10 μM) in plasma after the administration of standard doses, while ribavirin at 10 μM in combination with IFN-α showed strong synergistic inhibitory effects on HCV replication. Second, if the peak Sb concentration (∼82 μM) in plasma was all derived from Sb in its free form but not in the glucose-conjugated form in plasma, this Sb concentration would be well above the effective concentration of free Sb (<10 μM). In Table 1, we showed that 5 μM of SbCl3 could inhibit the replication of HCV by 68%, as revealed in the Ava5-EG(Δ4AB)SEAP system. However, it is unknown whether free Sb can be released from SSG after it is administered to humans. Finally, the concentration of Sb in the liver (one of the main target tissues for anti-HCV indication) after administration of SSG is not known. Several studies have indicated that high concentration of Sb in the liver could be achieved by modifying the delivery methods of SSG (3, 9). Nieto et al. have also shown that improved pharmacokinetic properties, including prolonged half-life and increased volume of distribution at steady state, could be achieved with a new formulation of SSG (20). Thus, future investigations are warranted to develop novel formulations of SSG for the treatment of HCV infection. In conclusion, we believe that SSG, in its current dosage form or in a new formulation, is a promising candidate drug to be used in combination therapy for treating HCV infection.
Acknowledgments
We thank Charles Rice at Rockefeller University and Apath LLC for providing the Huh-7 and Ava5 cells.
This work was supported by the National Health Research Institutes at Taiwan (grant no. BP-094-PP-14).
REFERENCES
- 1.al Jaser, M., A. el-Yazigi, M. Kojan, and S. L. Croft. 1995. Skin uptake, distribution, and elimination of antimony following administration of sodium stibogluconate to patients with cutaneous leishmaniasis. Antimicrob. Agents Chemother. 39:516-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachleitner-Hofmann, T., M. Kees, and H. Gisslinger. 2002. Arsenic trioxide: acute promyelocytic leukemia and beyond. Leuk. Lymphoma 43:1535-1540. [DOI] [PubMed] [Google Scholar]
- 3.Baillie, A. J., G. H. Coombs, T. F. Dolan, and J. Laurie. 1986. Non-ionic surfactant vesicles, niosomes, as a delivery system for the anti-leishmanial drug, sodium stibogluconate. J. Pharm. Pharmacol. 38:502-505. [DOI] [PubMed] [Google Scholar]
- 4.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 5.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 6.Chou, T.-C. 1991. The median effect principle and combination index for quantitation of synergism and antagonism, p. 61-102. In T.-C. Chou and D. C. Rideout (ed.), Synergism and antagonism in chemotherapy. Academic Press, San Diego, Calif.
- 7.Chulay, J. D., L. Fleckenstein, and D. H. Smith. 1988. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans. R. Soc. Trop. Med. Hyg. 82:69-72. [PubMed] [Google Scholar]
- 8.Davis, G. L., R. Esteban-Mur, V. Rustgi, J. Hoefs, S. C. Gordon, C. Trepo, M. L. Shiffman, S. Zeuzem, A. Craxi, M. H. Ling, J. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N. Engl. J. Med. 339:1493-1499. [DOI] [PubMed] [Google Scholar]
- 9.Hunter, C. A., T. F. Dolan, G. H. Coombs, and A. J. Baillie. 1988. Vesicular systems (niosomes and liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J. Pharm. Pharmacol. 40:161-165. [DOI] [PubMed] [Google Scholar]
- 10.Hwang, D.-R., Y.-C. Tsai, J.-C. Lee, K.-K. Huang, R.-K. Lin, C.-H. Ho, J.-M. Chiou, Y.-T. Lin, J. T. A. Hsu, and C.-T. Yeh. 2004. Inhibition of hepatitis C virus replication by arsenic trioxide. Antimicrob. Agents Chemother. 48:2876-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaser, M. A., A. el-Yazigi, and S. L. Croft. 1995. Pharmacokinetics of antimony in patients treated with sodium stibogluconate for cutaneous leishmaniasis. Pharm. Res. 12:113-116. [DOI] [PubMed] [Google Scholar]
- 12.Jett, J. H. 1978. Mathematical analysis of DNA: histograms from asynchronous and synchronous cell populations, p. 93-102. In D. Lutz (ed.), Pulse cytophotometry. European Press, Brussels, Belgium.
- 13.Lauer, G. M., and B. D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41-52. [DOI] [PubMed] [Google Scholar]
- 14.Lee, J. C., C. F. Chang, Y. H. Chi, D. R. Hwang, and J. T. Hsu. 2004. A reporter-based assay for identifying hepatitis C virus inhibitors based on subgenomic replicon cells. J. Virol. Methods 116:27-33. [DOI] [PubMed] [Google Scholar]
- 15.Lee, J. C., Y. F. Shih, S. P. Hsu, T. Y. Chang, L. H. Chen, and J. T. Hsu. 2003. Development of a cell-based assay for monitoring specific hepatitis C virus NS3/4A protease activity in mammalian cells. Anal. Biochem. 316:162-170. [DOI] [PubMed] [Google Scholar]
- 16.Leu, G. Z., T. Y. Lin, and J. T. Hsu. 2004. Anti-HCV activities of selective polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 318:275-280. [DOI] [PubMed] [Google Scholar]
- 17.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 18.McHutchison, J. G., and M. W. Fried. 2003. Current therapy for hepatitis C: pegylated interferon and ribavirin. Clin. Liver Dis. 7:149-161. [DOI] [PubMed] [Google Scholar]
- 19.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, J. K. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 20.Nieto, J., J. Alvar, A. B. Mullen, K. C. Carter, C. Rodríguez, M. I. San Andrés, M. D. San Andrés, A. J. Baillie, and F. González. 2003. Pharmacokinetics, toxicities, and efficacies of sodium stibogluconate formulations after intravenous administration in animals. Antimicrob. Agents Chemother. 47:2781-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanabe, Y., N. Sakamoto, N. Enomoto, M. Kurosaki, E. Ueda, S. Maekawa, T. Yamashiro, M. Nakagawa, C. H. Chen, N. Kanazawa, S. Kakinuma, and M. Watanabe. 2004. Synergistic inhibition of intracellular hepatitis C virus replication by combination of ribavirin and interferon-alpha. J. Infect. Dis. 189:1129-1139. [DOI] [PubMed] [Google Scholar]
- 22.Walewski, J. L., T. R. Keller, D. D. Stump, and A. D. Branch. 2001. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA 7:710-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasley, A., and M. J. Alter. 2000. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin. Liver Dis. 20:1-16. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 1997. Hepatitis C: global prevalence. Wkly. Epidemiol. Rec. 72:341-344. [PubMed] [Google Scholar]
- 25.Xu, Z., J. Choi, T. S. Yen, W. Lu, A. Strohecker, S. Govindarajan, D. Chien, M. J. Selby, and J. Ou. 2001. Synthesis of a novel hepatitis C virus protein by ribosomal frameshift. EMBO J. 20:3840-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh, C. T., D. R. Hwang, H. Y. Lai, and J. T. Hsu. 2003. Inhibition of authentic hepatitis C virus replication by sodium stibogluconate. Biochem. Biophys. Res. Commun. 310:537-541. [DOI] [PubMed] [Google Scholar]
- 27.Yi, T., M. K. Pathak, D. J. Lindner, M. E. Ketterer, C. Farver, and E. C. Borden. 2002. Anticancer activity of sodium stibogluconate in synergy with IFNs. J. Immunol. 169:5978-5985. [DOI] [PubMed] [Google Scholar]