Abstract
We describe Tn5386, a novel ca.-29-kb Tn916-like mobile element discovered to occur in ampicillin-resistant, Tn916-containing Enterococcus faecium D344R. PCR amplification experiments after overnight growth with or without tetracycline revealed “joint” regions of circularized Tn5386 composed of 6-bp sequences linking different transposon termini. In one case (no tetracycline), the termini were consistent with those derived by target site analysis of the integrated element. In the other case, the termini were virtually identical in distance from the integrase binding regions, as seen with Tn916. These data are consistent with a model in which one PCR product results from the action of Tn5386 integrase, whereas the other results from the action of the Tn916 integrase on Tn5386. Spontaneous conversion of D344R to an ampicillin-susceptible phenotype (D344SRF) was associated with a 178-kb deletion extending from the left end of Tn5386 to the left end of Tn916. Examination of the Tn5386 junction after the large deletion event suggests that the deletion resulted from an interaction between the nonintegrase ends of Tn5386 and Tn916. The terminus of Tn5386 identified in this reaction suggested that it may have resulted from the activity of the Tn916 integrase (IntTn916). The “joint” of the circular element resulting from this excision was amplifiable from D344R, the sequence of which revealed a heteroduplex consistent with IntTn916-mediated excision. In contrast, Tn5386 joints amplified from ampicillin-susceptible D344SRF revealed ends consistent with Tn5386 integrase activity, reflecting the absence of Tn916 from this strain. Tn5386 represents a new member of the Tn916 transposon family. Our data suggest that excision of Tn5386 can be catalyzed by the Tn916 integrase and that large genomic deletions may result from the interaction between these heterologous elements.
Conjugative transposons are important vehicles for disseminating antimicrobial resistance in both gram-positive and gram-negative bacteria (2, 15). The prototype gram-positive conjugative transposon is Tn916, an 18-kb element that encodes tet(M)-mediated resistance to tetracycline and minocycline (6). Tn916 transposition begins with excision of the element from its site of integration (generally but not exclusively in the bacterial chromosome) and circularization by formation of a “joint” region between transposon ends, composed of a heteroduplex of six base strands from the former chromosomal junction sequences frequently referred to as “coupling sequences” (3). Excision and subsequent integration of Tn916 result from the actions of the transposon-encoded integrase (IntTn916). The N terminus of IntTn916 binds to short direct repeats (DR2) located near the termini of Tn916, while the C terminus binds to the ends of the transposon and the flanking sequences, facilitating strand breakage and exchange (12). Tn916 also encodes an excisase (XisTn916) that binds to the ends of the transposon at a location between DR2 and the termini, facilitating (but not required for) excision (22). Transfer to recipient cells is encoded by Tn916, presumably through single-strand transfer after nicking at the origin of transfer within the transposon (11, 24). Excision and transfer are linked in that the transcription of transfer genes is promoted by joint formation, allowing continuation of excisase-integrase transcripts into the other end of Tn916, where transfer functions are encoded (5). Both are linked to tetracycline exposure, which induces increased transcription of tet(M) and the downstream excision genes (5). IntTn916 has also been shown to bind to the Tn916 origin of transfer, which may also link excision and transfer (10).
Conjugative transposons insert and excise by first generating staggered nicks of 5 or 6 bp (23). Ligating the ends of these staggered nicks yields a heteroduplex in both target and circular intermediate. The heteroduplex is resolved in the target site by replication. The joint of the nonreplicating circular excised transposon remains a heteroduplex in Escherichia coli (3) but appears to be resolved by unclear mechanisms in Enterococcus faecalis (13).
Tn916-like elements are widespread in gram-positive bacteria, and most contain tet(M) (15). In some instances, Tn916-like elements have acquired additional resistance determinants, as seen with pneumococcal conjugative transposon Tn1545, which encodes kanamycin and erythromycin resistance in addition to tetracycline resistance (7). In other instances, Tn916-like elements have been discovered incorporated within larger multiresistance elements. Tn5385 is a ca.-60-kb mobile element found in E. faecalis that encodes resistance to erythromycin, gentamicin, streptomycin, tetracycline, and penicillin (18), whereas Tn5253 is a large pneumococcal element encoding both tetracycline and chloramphenicol resistance (1). In both Tn5385 and Tn5252, tetracycline resistance is encoded by Tn916-like elements. More recently, an element with significant similarity to Tn916 that does not encode tetracycline resistance has been described. Tn5382 and Tn1549 are ca.-33-kb transposable elements encoding VanB-type glycopeptide resistance (4, 9). The ends and integrase/excisase genes of Tn5382 and Tn1549 exhibit significant local homology to those of Tn916, and the vanB2 resistance operon is located in the same relative position to the integrase as is tet(M) in Tn916.
To our knowledge, multiple copies of Tn916 within clinical enterococcal isolates are uncommon. In contrast, in vitro mating experiments often yield transconjugants containing several copies of the transferred Tn916-like elements (20). Subsequent transfer from transconjugant strains containing multiple Tn916 copies often occurs at a higher rate than the original transfer, suggesting an interaction between different transposons that promotes excision and transfer (8). In this paper, we identify and partially characterize Tn5386, a Tn916 family mobile element in Enterococcus faecium D344R. Tn5386 can excise to form two species of circular intermediates, in one case with the ends closely approximating the length of Tn916 termini relative to the positions of the DR2 sequences. Interactions between the ends of Tn5386 and a copy of Tn916 also present in the D344R genome result in an excision event in which a large segment of the genome is deleted. Our data suggest that Tn916-like integrases have broader substrate specificities than previously suspected and can interact with the termini of heterologous Tn916-like elements to facilitate excision of large chromosomal regions, thereby exerting a significant impact on bacterial genome evolution.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. faecium D344R (26) is a penicillin-resistant clinical strain isolated in France. Strain D344S (16) is a penicillin-sensitive derivative of D344R resulting from spontaneous loss of pbp5 (both kindly provided by Laurent Gutmann). For these experiments, we employed D344SRF, a rifampin- and fusidic acid-resistant variant of D344S selected by sequential passage on inhibitory concentrations of these antibiotics (19). E. coli DH10B was purchased from a commercial source (Invitrogen, Carlsbad, Calif.). Specific plasmids created and used in these experiments are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in these experiments
| Bacterial strain or plasmid | Resistance phenotype or genotypea | Description (reference or source) |
|---|---|---|
| Strains | ||
| E. faecium | ||
| D344R | Apr Eryr Tcr | High-level ampicillin-resistant clinical isolate (26) |
| D344SRF | Aps Eryr Tcr Fusr Rifr | pbp5-negative, ampicillin-susceptible derivative of D344R (16) |
| C68 | Apr Eryr Tcr Vmr | Clinical isolate initially used to define sequences downstream of pbp5 (4) |
| GE-1 | Aps Kanr Fusr Rifr | pbp5-negative recipient for enterococcal mating experiments (4) |
| E. coli DH10B | ΔM15 ΔlacX74 deoR recA1 ara Δ139 Δ(ara leu)7697 galU galK λ-rpsL endA1 nupG | Transformation-competent E. coli (Invitrogen, Carlsbad, Calif.) |
| Plasmids | ||
| pIndigoBAC-5 | Cmr | Cloning vector for large DNA fragments (Epicentre Technologies, Madison, Wis.) |
| pBCSK(−) | Cmr | Cloning vector for smaller DNA fragments (Stratagene, La Jolla, Calif.) |
| pCR-XL-Topo | Cmr | Linearized vector with TA overhang for cloning PCR products (Invitrogen, Carlsbad, Calif.) |
| pACYC184 | Cmr Tcr | Cloning vector for smaller fragments (21) |
| pCWR796 | Cmr | Ca.-40-kb BglII fragment from D344R containing intact Tn5386 cloned into BamHI site of pIndigoBAC-5 (this study) |
| pCWR808 | Cmr | Ca.-40-kb BgIII fragment from D344SRF containing intact Tn5386 cloned into BamHI site of pIndigoBAC-5 (this study) |
| pCWR845 | Cmr Tcr | Ca.-25 to -30-kb BgIII fragment from D344R containing intact Tn916 cloned into BamHI site of pIndigoBAC-5 (this study) |
| pCWR850 | Cmr | 1.2-kb HincII fragment containing the left junction of Tn916 in D344R cloned into pBCSK(−) (this study) |
| pCWR854 | Cmr | 3.5-kb HincII fragment containing the right junction of Tn916 cloned into pACYC184 (this study) |
| pCWR815 | Cmr | 6-kb BglII/HincII fragment containing the left junction of Tn5386 cloned into pBCSK(−)—4.4 kb of Tn5386 (this study) |
| pCWR869 | Cmr | 3.5-kb EcoRV fragment of PCR product containing the right junction of Tn5386 in D344R (identical to fragment from D344S)—2.7 kb of Tn5386 cloned into pBCSK(−) (this study) |
| pCWR822 | Cmr | 4.7-kb HincII fragment containing the left junction of Tn5386 in D344SRF—4.4 kb of Tn5386 (this study) |
Ap, ampicillin; Cm, chloramphenicol; Ery, erythromycin; Fus, fusidic acid; Kan, kanamycin; Rif, rifampin; Tc, tetracycline; and Vm, vancomycin.
Pulsed-field gel electrophoresis.
Lysis of E. faecium cells was performed as previously described (4). Digestion of genomic DNA with restriction enzyme SmaI (Promega, Madison, Wis.) used 40 U of restriction enzyme in 400 μl of designated restriction buffer. The program for separation of SmaI restriction digests used the auto-algorithm function (Bio-Rad, Hercules, Calif.) with the following details: separation was set for a low of 20 kb and a high of 500 kb to run over 24 h, the calibration factor was 1.0, gels consisted of 1% PFC agarose and were run in a bath of 0.5× Tris-borate-EDTA at 14°C with a gradient of 6.0 V/cm, the included angle was 120°, the initial switch time was 2.98 s, and the final switch time was 44.69 s with linear ramping.
Molecular techniques.
Isolation of genomic DNA, restriction digestion, separation on agarose gels, Southern transfer, hybridization using digoxigenin-labeled probes, and detection with chemiluminescent assays were performed by standard techniques as previously described (4). Cloning of large chromosomal fragments (>10 kb) was accomplished using commercially purchased HindIII- or BamHI-digested cloning vector pIndigoBAC (Epicentre Technologies, Madison, Wis.). Smaller fragments were cloned using vector pBCSK(−) (Stratagene, La Jolla, Calif.). Fragments to be cloned were originally identified by Southern hybridization of digested genomic DNA. Fragments in the region of the expected size were then cut from a subsequent agarose gel. The excised segment with the greatest quantity of the desired sequence was then identified by an additional Southern hybridization. Target fragments were then ligated to cloning vectors at 4°C overnight, followed by electroporation into commercially purchased competent E. coli DH10B (Invitrogen). Transformant colonies were selected using antibiotics designed to select for resistance encoded by the cloning vector, and colonies containing the appropriate insert were identified by colony hybridization. Inserts were confirmed as the appropriate size by restriction digestion and agarose separation, along with PCR amplification of the region used as a probe in the colony hybridizations.
DNA sequencing.
DNA sequencing was performed in our laboratory using an A.L.F. automated sequencer (Pharmacia, Piscataway, N.J.) as previously described (4), or sequencing services were commercially purchased from Cleveland Genomics (Cleveland, Ohio). We also used the available E. faecium partial genome sequences (Baylor College of Medicine [ftp://ftp.hgsc.bcm.tmc.edu/pub/data/Efaecium/] and Joint Genome Institute [http://genome.jgi-psf.org/draft_microbes/entfa/entfa.home.html]) to facilitate our cloning efforts as we “walked” along the chromosome downstream of pbp5 in E. faecium D344R.
Excision experiments.
Evidence for excision of Tn916, Tn5386, and the large region between these elements was sought by PCR amplification using primers designed to amplify products representing either circular forms of excised regions or religated “target” regions after excision from the genome of E. faecium D344R. We used a Light Cycler Fast Start DNA Master SYBR Green kit to perform amplifications (Roche Diagnostics, Indianapolis, Ind.). Amplification conditions varied depending on the primer sets being used. Amplifications were performed on genomic DNA extracted from cultures grown overnight in brain heart infusion (BHI) broth either with or without tetracycline (10 μg/ml). The locations of the primers used for these experiments are indicated in Fig. 1, and the sequences of the different primers are listed in Table 2.
FIG. 1.
Proposed map of E. faecium D344R and D344S in the areas of the excised segment. The positions of Tn916, Tn5386, pbp5, and the SmaI site downstream of pbp5 are marked. The large plasmid clones containing either Tn916 or Tn5386 from the different strains are marked by lines terminating in filled circles. The regions referred to in the text as left and right junctions of the two mobile elements are marked above the transposons. Arrows above marked genes represent direction of transcription. Regions for which probes were generated for use in the Southern hybridization in Fig. 2 are marked by single and double asterisks. pbp5, penicillin binding protein 5 gene; int, integrase genes.
TABLE 2.
Primers used in these experiments
| Name | Sequence | Purpose |
|---|---|---|
| 101 | 5′-TGAACCCTTATAAAAGCGAATACAGCTAGG-3′ | Amplification of joint region from circularized Tn5386 and of left junction of Tn5386 |
| 102 | 5′-GCTAAACTGACATTTAAGAAGTTATGAAGAGATAAGTGG-3′ | Amplification of joint region from circularized Tn5386 and of right junction of Tn5386 |
| 103 | 5′-CTGTTATGCGTATTCAGGAATTCG-3′ | Amplification of left Tn5386 junction in D344R and of regenerated Tn5386 target sequence after excision |
| 104 | 5′-GATCATTGCATAAGCGATACTTG-3′ | Amplification of right junction of Tn5386 in D344R and D344SRF and of regenerated target sequence of D344R after Tn5386 excision |
| 105 | 5′-GAAGCTATCAATATTCAGAAGCAATACC-3′ | Amplification of integrase and excisase of Tn5386 and of right junctions in D344R and D344SRF |
| 201 | 5′-GTTTTGACCTTGATAAAGTGTGATAAGTCC-3′ | Amplification of joint region of Tn916 from D344R |
| 202 | 5′-CACTTCTGACAGCTAAGACATGAG-3′ | Amplification of joint region of Tn916 from D344R |
| 418-1 | 5′-AACAGATGGTGATCGAGAAG-3′ | Creation of probe flanking Tn5386 in D344R and D344SRFa |
| 418-2 | 5′-GGAATCCGTTCTTGGATAGC-3′ | Creation of probe flanking Tn5386 in D344R and D344SRFa |
| 837-RV-1 | 5′-GCTAAGATCAGCACAGCAG-3′ | Creation of probe flanking Tn916 in D344Rb |
| 822rev-1 | 5′-TACGTTGTTTTGCGATATC-3′ | Creation of probe flanking Tn916 in D344Rb |
| 939 R | 5′-TTGCAAATGTCAGCAAGGCTTC-3′ | Amplification of Tn5386 integrase message |
| 735 F | 5′-TATCCGCATGAGCGAAAACG-3′ | Amplification of Tn5386 integrase message |
| 916F | 5′-CCAAAAGTGGCGAACGTCAA-3′ | Amplification of Tn916 integrase message |
| 916R | 5′-AAGACCTTTCATCATGCCGTTG-3′ | Amplification of Tn916 integrase message |
Tetracycline induction of Tn5386 and Tn916 integrase transcription.
Total cellular RNA was extracted from E. faecium D344R using an RNeasy mini kit (QIAGEN, Valencia, Calif.) after overnight growth in BHI broth alone or containing tetracycline (10 μg/ml). DNA was removed with DNase, and the absence of contaminating DNA was confirmed by PCR without reverse transcriptase. Tn5386 integrase mRNA was converted to DNA and amplified using a LightCycler RNA Master SYBR Green kit (Roche Diagnostics) with primers 939 R and 735 F (Table 2). These primers amplified a 204-bp internal product of the putative Tn5386 integrase mRNA. Primers used to amplify Tn916 integrase message were 916F and 916R (Table 2). These primers yielded a 186-bp product. PCR conditions for both reactions were as follows: 1 cycle of 61°C for 20 min and 95°C for 30 s, followed by 40 cycles of 95°C for 1 s, 60°C for 5 s, and 72°C for 12 s. Transcripts were detected using a LightCycler instrument (Roche Applied Sciences). Equal starting quantities of total RNA were verified by determining optical density at 260 nm.
Nucleotide sequence accession numbers.
The sequences of the two ends of Tn5386 and the putative integrase and excisase have been submitted to GenBank under the accession numbers AY928173 and AY928174.
RESULTS
Discovery of Tn5386.
These investigations began as an attempt to determine the mechanism by which pbp5 was deleted from the D344R genome, resulting in ampicillin-susceptible strain D344S. Pulsed-field gel electrophoresis of SmaI-digested genomic DNA from D344R and the fusidic acid- and rifampin-resistant D344S derivative D344SRF revealed that loss of pbp5 from D344R was associated with the disappearance of a ca.-242-kb SmaI fragment in D344SRF along with the appearance of a smaller fragment of 164 kb (Fig. 2). At that time, we did not know whether the lost segment of DNA contained a SmaI restriction site. Cloning and sequencing of fragments downstream of pbp5 in E. faecium C68 (data not shown; results to be reported in a separate manuscript) and comparison with the E. faecium database (Baylor College of Medicine [ftp://ftp.hgsc.bcm.tmc.edu/pub/data/Efaecium/] and Joint Genome Institute [http://genome.jgi-psf.org/draft_microbes/entfa/entfa.home.html]) allowed us to develop an accurate picture of this chromosomal region, including identification of a SmaI restriction site approximately 30 kb downstream of pbp5 (Baylor E. faecium database, 25 July 2001; contig numbers 336, 356, 418, and 422). Using our knowledge of the sequence in the region downstream of pbp5, we used PCR amplification and hybridization to confirm that these sequences were present in the same relative orientation in D344R and absent from D344SRF (data not shown). At a point approximately 70 kb downstream of pbp5, the restriction map of D344R diverged from that predicted by the database. Cloning of the diverging restriction fragment (pCWR796) and subsequent sequencing of one HincII subclone (pCWR815) revealed that the probe binding sequence was present but interrupted by a sequence with nucleic acid similarity to the nonintegrase end of Tn916; in particular, there was significant similarity to the DR2 integrase binding site of Tn916 (Fig. 3A). These data suggested that a Tn916-like element had interrupted the expected sequence. A 20-kb HincII fragment of pCWR796 was then digested with RsaI, yielding many small fragments, some of which were cloned and sequenced to suggest potential templates for future amplification reactions.
FIG. 2.
Pulsed-field gel electrophoresis of SmaI-digested genomic DNA from E. faecium D344R and E. faecium D344SRF. Lane 1, bacteriophage lambda concatemer size standard (sizes are marked to the left); lane 2, D344R digested with SmaI; lane 3, D344SRF digested with SmaI; lanes 4 and 5, SmaI digestions seen in lanes 2 and 3 hybridized with a probe derived from the left flanking region (depicted as single asterisk in Fig. 1); and lanes 6 and 7, SmaI digestions seen in lanes 2 and 3 hybridized with a probe derived from the right flanking region (depicted as double asterisk in Fig. 1). Note that the left and right flanking region probes hybridize with the same fragment in D344SRF, consistent with excision of the region between the left flanking region and Tn5386. The lower hybridizing band in D344R likely represents a doublet in which only one of the bands hybridizes to the probe.
FIG. 3.
Comparison of nucleotides composing the termini of Tn916 and Tn5386. (A) Comparison of the termini at the nonintegrase ends of the transposons. The DR2 integrase binding sites are underlined, and identical nucleotides are marked by vertical lines. The boxed region is the coupling sequence that lies adjacent to the end of Tn5386 in D344R as defined by analysis of the target sites and the circular form resulting in the larger joint PCR product. The arrow reflects the end of Tn5386 as defined in the circular form resulting in the smaller PCR product (presumed to result from the action of IntTn916 on the ends of Tn5386). This also reflects the end of the transposon used to yield the excision of the larger region resulting in the creation of D344S. (B) Comparison of the termini at the integrase ends of the transposons. The DR2 integrase binding sites are underlined, and identical nucleotides are marked by vertical lines. The arrow reflects the end of Tn5386 as defined in the circular form resulting in the smaller PCR product (presumed to result from the action of IntTn916 on the ends of Tn5386). Extending beyond the arrow is the Tn5386 terminus as defined by analysis of the target sites and the circular form resulting in the larger joint PCR product.
We created primers based on the sequences of the RsaI subclones to amplify and clone large segments of pCWR796. One such amplification product using primers 104 and 105 (Table 2 and Fig. 1) was predicted to contain the junction between the other end of the mobile element and the D344R genome: digestion of this product with EcoRV resulted in a 3.5-kb fragment that was cloned, yielding pCWR869. Analysis of the pCWR869 insert revealed the expected database sequence adjacent to the sequence homologous to one end of Tn916, again particularly in the region of the direct repeats (Fig. 3B). Further sequencing of this end of the element revealed open reading frames with significant homology to the Tn916 integrase (66% amino acid identity and 81% similarity over the span of the enzyme) and excisase (83% identical and 91% similar over 67 amino acids), confirming that the DNA (estimated to be about 29 kb) interrupting the sequence found in the database was likely to be a Tn916-like transposon, which we have designated Tn5386. Sequence analysis of the full transposon is in progress.
Characterization of Tn5386 termini.
Analysis of the two junctions in comparison to the target sequence (which was present uninterrupted in the available E. faecium database and shown to be present uninterrupted in other clinical strains in our laboratory) allowed us to make reasonable predictions regarding the Tn5386 termini. The target sequence (Fig. 4B) is interrupted in D344R by a sequence for which the first 6 bp are TATCAC (Fig. 4C). The expected continuation of the target sequence at the other end of the transposon (CATGTT, emphasized by the hatched box in Fig. 4A) is preceded by the sequence ATTGAA. The presence of the expected continuation sequence CATGTT at that end of the transposon suggests that CATGTT represents the 6-bp target sequence for the initial Tn5386 insertion. In this scenario, the ATTGAA sequence found to the left of the target sequence in the integrated state represents the “right” end of Tn5386. The other (“left”) end of the integrated form should then be next to a 6-bp coupling sequence brought with the transposon from its prior integration site. In this model, the TATCAC sequence (boxed in Fig. 4C) represents the 6-bp coupling sequence, and the adjacent AATAAAA sequence represents the left end of Tn5386.
FIG. 4.
Comparison of left and right Tn5386 junction sequences with the target sequence as defined by the E. faecium database and the D344R genome after Tn5386 excision. The ends of Tn5386 are marked by italics. The coupling sequence brought with it by Tn5386 is marked by the solid box adjacent to the left end of Tn5386. The coupling sequence representing the target is marked by the 6-base-pair sequence in the hatched box adjacent to the right end of Tn5386.
Evidence that Tn5386 is a functional transposon.
In an effort to determine whether Tn5386 can function as a transposon, we used primers designed to amplify the “joint” (primers 101 and 102) and the putative “target” (primers 103 and 104) of the transposon after excision from the chromosome of D344R (Table 2 and Fig. 1). Amplification utilized genomic DNA isolated after overnight growth of D344R in BHI broth either with or without tetracycline (10 μg/ml). A joint region PCR product of Tn5386 of the anticipated size was amplifiable in greater quantities after overnight growth in tetracycline (Fig. 5, lane B). Amplification products were cloned into pCR-XL-TOPO, and four individual clones were sequenced. The sequences of the amplification products revealed Tn5386 ends that were consistent with ends deduced by comparison of the target sequence with the integrated form (Fig. 6). The two ends were separated by 6 bp, which in all four clones contained TATCAC that was present adjacent to the left end of Tn5386 in the integrated state. Products from a separate PCR amplification of the Tn5386 joint were sequenced directly on both strands, each of which contained the TATCAC 6-nucleotide coupling sequence between the transposon ends, with no suggestion of ambiguity in the region of the joint (data not shown). The site representing the target after excision was also amplifiable. Four such clones were sequenced, and each revealed regeneration of the original target site (CATGTT) (Fig. 6). These data suggest that the mechanism for Tn5386 excision is similar to that described for Tn916; however, our identification of only one coupling sequence in the circular-form clones and the other on regeneration of the original target sequence in four of four such clones suggests that the mechanisms for excision may differ in some ways from that of Tn916.
FIG. 5.
PCR amplification of the Tn5386 joint after growth of D344R with tetracycline (lane B) and without tetracycline (lane C). Size markers are shown at the left. Identical primers (primers 101 and 102) were used to generate the products in both instances. A small amount of the smaller product is visible in lane B (arrow), but the larger product is absent from lane C.
FIG. 6.
Tn5386 joints and regenerated target sequences generated from amplification performed on DNA extracted from D344R grown in the presence of tetracycline (10 μg/ml). In four of four (4/4) clones, the joint sequence of the circular form was identical to the coupling sequence (TATCAC) found at the left junction of Tn5386. Similarly, in four of four clones the coupling sequence (CATGTT) found at the right junction of Tn5386 is present in the regenerated target sequence.
Amplification of the Tn5386 joint after growth without tetracycline yielded a product that was smaller than the expected size (Fig. 5, lane C). This product was cloned and sequenced and found to reflect a joint amplification product that had different Tn5386 termini than the joint product described above. The integrase (right) end of Tn5386 in these products was 70 bp shorter than the terminus deduced by target sequence comparison and analysis of the larger joint products. The location of this terminus is indicated by the arrow in Fig. 3B. Alignment of the integrase ends of Tn916 and Tn5386 using the DR2-R integrase binding site as the start shows that the new terminus of Tn5386 matches almost precisely the length of Tn916 extending from the direct repeat to the end. The other end of Tn5386 in this smaller joint amplification product also differs from our previously determined end, this time being 8 bp longer. Again, lining up the nonintegrase ends of Tn916 and Tn5386 by the DR2-L integrase binding site, the “new” end is within 2 nucleotides of the length between the direct repeat at this end of Tn916 and its terminus (Fig. 3A). These findings raise the intriguing possibility that the smaller amplification product that predominates after growth without tetracycline results from the action of the Tn916 integrase on Tn5386 and that the larger PCR product is the result of the activity of the Tn5386 integrase. We were readily able to amplify an appropriately sized joint product from Tn916 (data not shown), suggesting that IntTn916 present in D344R was functional. Despite several attempts and under various conditions, we were unable to amplify a religated chromosomal segment corresponding to excision of Tn5386 that generated the smaller PCR product. In each instance, only regenerated target sequences corresponding to the larger excision product were amplified.
Tetracycline induction of Tn5386 excision and integrase transcription.
Using reverse transcriptase PCR analysis, we calculated transcript quantities from the putative Tn5386 integrase after overnight growth in broth with or without tetracycline (10 μg/ml). intTn5386 transcript quantities were virtually identical after growth either with or without tetracycline (data not shown), indicating that the appearance of one joint versus the other was not the result of tetracycline-induced intTn5386 transcription. PCR products from these reactions were cloned and sequenced, confirming that they represented Tn5386 integrase transcripts (data not shown). Prior studies have indicated that exposure to tetracycline increases tet(M) transcription in Tn916 (25). Consistent with this, intTn916 transcript quantities were increased greater than 10-fold after incubation with tetracycline (data not shown).
Identification of the other end of the larger excised pbp5 region.
Hybridization of genomic DNA from D344SRF using an amplified probe for the Tn5386 integrase confirmed that Tn5386 was present in this strain (data not shown). The entirety of Tn5386 was cloned from the D344SRF chromosome (yielding pCWR808), which was subsequently restriction digested and subcloned. Sequence analysis of these smaller fragments revealed that the “outside” Tn5386-genome junction was identical in D344R and D344SRF. The sequence adjacent to the left end of Tn5386 in D344SRF differed from that observed for D344R and was not found in the E. faecium database. These data suggested that the deleted pbp5 segment stretched from this new sequence (hereafter referred to as the “flanking sequence”) to the left end of Tn5386 in D344R.
We used the D344SRF flanking sequence as a probe (denoted by single asterisk in Fig. 1) of BglII-digested genomic DNA from D344R, identifying a ca.-30-kb restriction fragment that was cloned into BamHI-digested pIndigoBAC-5. The plasmid was designated pCWR845. A 1.2-kb HincII subfragment of the pCWR845 insert that hybridized to the probe was cloned and sequenced. Sequence analysis revealed an insert in which the probe sequence (which was adjacent to the left end of Tn5386 in D344SRF) was adjacent to one end of Tn916 in D344R. This finding suggested a structure as depicted in Fig. 1, in which the large pbp5 region was flanked by Tn916 and Tn5386, with the deletion event resulting in D344S occurring between the left end of Tn5386 and the left end of Tn916. HincII digestion of pCWR845 revealed fragments consistent in size with known internal HincII fragments of Tn916 (data not shown), suggesting that an intact copy of Tn916 was present in that clone.
To provide supportive evidence that the excised segment extended from the left end of Tn916 to the left end of Tn5386, we took advantage of the SmaI site within the excised segment. Using probes for DNA flanking either Tn916 or Tn5386 (single and double asterisks, respectively, in Fig. 1), we hybridized SmaI digests of genomic DNA from D344R and D344SRF separated by pulsed-field gel electrophoresis. The anticipated result was that the probes would hybridize to separate fragments in D344R but, with loss of the SmaI site, to the same fragment in D344SRF. The results of these hybridizations are shown in Fig. 2. Consistent with our hypothesis, loss of the pbp5 segment resulted in the two probes hybridizing to the same fragment in D344SRF. These hybridization studies also allowed us to derive a reasonably precise estimate of the total size of the excised DNA, which we estimate to be approximately 178 kb (including Tn916) [(242 + 100) − 164].
Tn5386 joint products generated in D344SRF.
If our hypothesis regarding the use of either the Tn5386 or the Tn916 integrase to form the different joints observed above is correct, then Tn5386 joints amplifiable from D344SRF should be exclusively of the larger variety. Amplification of Tn5386 joints from D344SRF after overnight growth without tetracycline (the conditions under which the smaller amplification product was found in D344R) yielded only the larger PCR product, which on direct sequencing yielded the expected sequence, again with solely TATCAC serving as the 6-bp joint (data not shown). These data are consistent with the smaller joint product seen only in D344R being the result of Tn916 integrase activity on the ends of Tn5386.
Comparison of Tn916 and Tn5386 junctions.
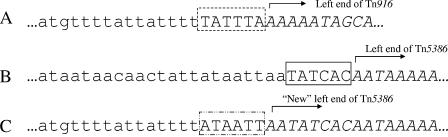
One hypothesis generated by these findings is that the excision event resulted from (Tn916 or Tn5386) integrase-mediated interaction between the ends of heterologous but related Tn916-like elements. If that is the case, the nucleotides adjacent to the left Tn5386 terminus in D344SRF should have a characteristic footprint, with the sequence adjacent to the Tn916 outside junction in D344R found immediately adjacent to either the Tn5386 coupling sequence from D344R or the Tn916 coupling sequence, which itself should then be adjacent to the inside Tn5386 end. In Fig. 7, the left junction of Tn916 in D344R is compared with the left junctions of Tn5386 in D344R and D344SRF. In D344R, the AAAAA representing the left end of Tn916 is flanked by a 6-bp sequence (TATTTA [Fig. 7A]) representing the coupling sequence for this end of the element. The sequence adjacent to the left end of Tn5386 in D344SRF is identical to the Tn916 flanking sequence through TTTT that lies immediately adjacent to this TATTTA coupling sequence in D344R. If the excision reaction represented a strict interaction between the left end of Tn916 and the left end of Tn5386 (as defined above), it would be anticipated that the TTTT sequence would be located either immediately adjacent to the TATCAC Tn5386 coupling sequence or next to the TATTTA sequence from D344R, which would be linked directly to the end of Tn5386 as defined in Fig. 4. Instead, TTTT is found 8 bp upstream of this location, separated from the TATCAC Tn5386 coupling sequence by an 8-bp sequence (ATAATTAA) that is identical to the sequence found next to the TATCAC sequence in D344R.
FIG. 7.
Comparison of left junction of Tn916 in D344R with the left junctions of Tn5386 in D344R and D344SRF. (A) Left Tn916 junction within the D344R genome. The genome sequence is presented in lowercase, while the transposon sequence is in italics. The hatched box represents the 6-bp coupling sequence between Tn916 and the flanking sequence. (B) Left junction sequence of Tn5386 in D344R. As above, the genomic sequence is in lowercase, and the transposon is italicized. The 6-bp coupling sequence is boxed. (C) Left junction sequence of Tn5386 in D344SRF, after excision of the pbp5 region. The putative new coupling sequence is now in the hatched and dotted box, and the “new” end of Tn5386 is incorporated into the italicized sequence representing the transposon.
Keeping in mind the conservative mechanism of Tn916 transposition, the most plausible explanation for these findings is that the excision event did indeed proceed by an interaction between the ends of Tn916 and Tn5386 in which the integrase utilized a Tn5386 terminus located 8 bp to the left of where our Tn5386 excision data indicate that it should be located (Fig. 7). In other words, in the event that led to excision of the larger region, AATATCAC served as the end of Tn5386, and ATAATT served as the coupling sequence between this end of Tn5386 and the newly joining Tn916 flanking region. As noted above in our discussion of the smaller joint products of Tn5386, this “end” very closely approximates the distance between the direct repeat of Tn916 and its terminus and is in agreement with the ends as defined by analysis of the smaller joint products. As such, these data suggest that this excision event, if it was mediated by one of the transposon integrases, was mediated by IntTn916.
Excision corresponding to the event resulting in D344S.
In an effort to accumulate evidence that the deletion event resulting in D344S was an integrase-mediated event, we developed primers to amplify PCR products from the joint region of the excised segment in D344R. These primers yielded product only after incubation with tetracycline (10 μg/ml). The amplification products were cloned, and four clones were sequenced. Results are shown in Fig. 8. In all four cases, the end of Tn916 was separated from the sequence adjacent to the “new” coupling sequence seen in D344SRF by 6 bp. In three of the four clones, those 6 bp (ATAATT) reflected the coupling sequence adjacent to the “new” left end of Tn5386 as described above. In the other instance, the 6 bp (TATTTA) reflected the coupling sequence adjacent to the left end of Tn916 in D344R. These data support a model in which this excision event was mediated by an integrase-type molecule, one that proceeds through formation of a heteroduplex joint. For the reasons stated above, we believe that the most likely integrase to have facilitated this excision was that from Tn916.
FIG. 8.
Proposed mechanism for excision of the large pbp5-containing region from D344R. The orientation of the two transposons in D344R and the junction sequences are depicted at the top of the figure (A). We propose that the transposons align as depicted in panel B, with strand exchange occurring between the regions denoted by the X. This yields two products: a circularized form that contains both pbp5 and Tn916 (C) and a regenerated chromosomal region that contains Tn5386 linked to the region that flanked Tn916 in D344R (D). The asterisks denote regions of newly joined DNA. Below the circularized form in panel C are depicted the two different joints found in the PCR products, with their relative prevalences among the four inserts sequenced. i, integrase.
DISCUSSION
The data we present in this paper make a compelling case for Tn5386 being a functional mobile element. The similarities between the ends of this element and the ends of Tn916, along with the high degree of homology between its putative integrase and excisase and those of Tn916, are striking. The ability to form a circular intermediate, as evidenced by our ability to amplify a joint region of the expected 6-bp length, is supportive of a Tn916-like process responsible for excision. We cannot at this point conclude with certainty that it is a transposon, since we have not demonstrated movement in a recombination-deficient environment. Nor can we conclude that its mechanism of excision is identical to that of Tn916, since we were unable to demonstrate the presence of a heteroduplex in its joint. We and others (3, 17) have readily identified heteroduplexes in joints amplified from Tn916-like elements, using the same techniques employed in this report. It is possible that we did not sample enough joints and rejoined target regions to reveal the other strand (four joints and four religated target region clones). However, the failure to observe any ambiguity in the joint region on direct sequencing of PCR products is supportive of the absence of a heteroduplex. Whether this finding represents a different mechanism of excision for Tn5386 or some postcircularization processing of the joint within E. faecium is unclear at present and will be the subject of future investigations.
We have not demonstrated that Tn5386 is conjugative. To date, our sequencing of the element has not revealed the presence of any open reading frame that would be predicted to encode antimicrobial resistance, so tracing its movement is challenging. There does appear to be genetic material encoding production of a bacteriocin within the transposon (data not shown). In addition, we have been able to transfer ampicillin resistance from D344R into E. faecium GE-1, which lacks pbp5 (4; data not shown). Transconjugants resulting from these matings varied in their contents, but one contained both Tn916 and Tn5386. These data suggest that the larger region is transferable and suggest that in some instances Tn5386 may be part of this transfer. Studies designed to examine the content and nature of the transfer event are ongoing.
Our observation that two distinct PCR products were amplified from circularized Tn5386 in D344R is unique and interesting. The larger of the two PCR products, present only after incubation with tetracycline, has Tn5386 termini that are consistent with ends deduced based on a comparison between the target sequence prior to insertion (available in the E. faecium genome sequence database) and the inserted Tn5386. The smaller product, present in much greater quantities after incubation without antibiotic selection, had termini that were 70 bp shorter on one end and 8 bp longer on the other. These lengths result in distances from the direct repeat integrase binding sites that are virtually identical to the distances within the termini of Tn916, raising the possibility that the shorter PCR products represent a Tn5386 excision that is catalyzed by the Tn916 integrase. Potentially inconsistent with this scenario is the fact that direct sequencing of the joint regions from these smaller PCR products did not reveal any ambiguity in the joint region, indicating the absence of a heteroduplex. On the other hand, all PCR products obtained from Tn5386 excision in D344SRF (which lacks Tn916) were of the larger variety. These findings are consistent with the Tn5386 integrase catalyzing the excision event resulting in the larger product and the Tn916 integrase catalyzing the event resulting in the smaller PCR product.
Previous work by Jia and Churchward (12) indicated that the maltose binding protein-linked C terminus of IntTn916 binds specifically to the ends of Tn916. In these experiments, excesses of unlabeled left and right Tn916 ends effectively displaced radiolabeled ends, whereas nonspecific unlabeled oligonucleotides did not, suggesting that binding of the C-terminal portion of IntTn916 was specific for the ends of Tn916. Changes in coupling sequences, however, do not impact IntTn916 binding to the ends of Tn916 and flanking sequences (14). Moreover, the variety of sequences found adjacent to Tn916 integration sites argues for some flexibility in binding sequences for the C-terminal portion of IntTn916. Our data suggest that IntTn916 may not be completely specific for sequences found within the Tn916 ends, since it appears to be able to catalyze circularization of Tn5386 by using ends that do not exhibit any obvious homology to the ends of Tn916 (Fig. 3A and B). Instead, it would appear that the physical distance from the N-terminal integrase binding site may be critical for the heterologous cleavage reaction. It is intriguing that we were unable to identify a PCR amplification product reflecting the religated target sequence that would correspond to the smaller PCR product resulting from amplification of Tn5386 joints. This may reflect a failure of the Tn916 integrase to religate the target site after excision, perhaps because IntTn916 action on heterologous substrates is inefficient or incomplete. Alternatively, the reaction could have involved exchange of only a single strand with the “target” resolved by replication, leaving an intact copy of Tn5386 in the genome and negating our ability to amplify the target sequence.
Our data also suggest that IntTn916 can act on ends from heterologous transposons to facilitate excision of large segments of chromosomal DNA. Analysis of the Tn5386-chromosomal junction after loss of the larger pbp5 region from D344R reveals that the excised segment of DNA extended from the junction at the left end of Tn916 to the left end of Tn5386. We were able to amplify a joint region from a circular form that results from this interaction. This joint is a heteroduplex in which the joint sequences represent one or the other of coupling sequences found adjacent to the respective ends of Tn916 or Tn5386. The terminus of Tn5386 employed for this excision corresponds to the terminus present in the smaller PCR product described above, that is, the terminus that we hypothesize results from excision of Tn5386 catalyzed by IntTn916. The use of this terminus and the presence of a heteroduplex within the circularized form both argue that IntTn916, rather than IntTn5386, catalyzes this excision event.
The importance of conjugative transposons in the dissemination of antimicrobial resistance has generally been ascribed to the resistance genes encoded by the transposons themselves or to the larger mobile elements within which the conjugative transposons have been located. Our data expand the potential involvement of Tn916-like transposons in mobile resistance by implicating these elements in large genomic excisions and potentially in transfer of resistance determinants within the excised regions. There are undoubtedly a variety of Tn916-like transposons present in gram-positive bacteria. The possibility that even distantly related elements (or perhaps even randomly distributed direct repeat integrase binding sequences) can cooperate to facilitate mobilization of large segments of DNA suggests that these elements may also play an important role in genome evolution in the species they frequent.
Acknowledgments
This work was supported by a Department of Veterans Affairs Merit Review and a grant from the National Institute of Allergy and Infectious Diseases (R01 AI045626-04) (both to L.B.R.).
REFERENCES
- 1.Ayoubi, P., A. O. Kilic, and M. N. Vijayakumar. 1991. Tn5253, the pneumococcal Ω (cat tet) BM6001 element, is a composite structure of two conjugative transposons, Tn5251 and Tn5252. J. Bacteriol. 173:1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrus, V., G. Pavlovic, B. Decaris, and G. Guedon. 2002. Conjugative transposons: the tip of the iceberg. Mol. Microbiol. 46:601-610. [DOI] [PubMed] [Google Scholar]
- 3.Caparon, M. G., and J. R. Scott. 1989. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell 59:1027-1034. [DOI] [PubMed] [Google Scholar]
- 4.Carias, L. L., S. D. Rudin, C. J. Donskey, and L. B. Rice. 1998. Genetic linkage and cotransfer of a novel, vanB-containing transposon (Tn5382) and a low-affinity penicillin-binding protein 5 gene in a clinical vancomycin-resistant Enterococcus faecium isolate. J. Bacteriol. 180:4426-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celli, J., and P. Trieu-Cuot. 1998. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol. Microbiol. 28:103-117. [DOI] [PubMed] [Google Scholar]
- 6.Clewell, D. B., S. E. Flannagan, L. O. Zitzow, Y. A. Su, P. He, E. Senghas, and K. E. Weaver. 1991. Properties of conjugative transposon Tn916, p. 39-44. In G. M. Dunny, P. Patrick, and L. L. Cleary (ed.), Genetics and molecular biology of streptococci, lactococci, and enterococci. American Society for Microbiology, Washington, D.C.
- 7.Courvalin, P., and C. Carlier. 1987. Tn1545: a conjugative shuttle transposon. Mol. Gen. Genet. 206:259-264. [DOI] [PubMed] [Google Scholar]
- 8.Flannagan, S. E., and D. B. Clewell. 1991. Conjugative transfer of Tn916 in Enterococcus faecalis: trans activation of homologous transposons. J. Bacteriol. 173:7136-7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garnier, F., S. Taourit, P. Glaser, P. Courvalin, and M. Galimand. 2000. Characterization of transposon Tn1549, conferring VanB-type resistance in Enterococcus spp. Microbiology 146:1481-1489. [DOI] [PubMed] [Google Scholar]
- 10.Hinerfeld, D., and G. Churchward. 2001. Specific binding of integrase to the origin of transfer (oriT) of the conjugative transposon Tn916. J. Bacteriol. 183:2947-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaworski, D. D., and D. B. Clewell. 1995. A functional origin of transfer (oriT) on the conjugative transposon Tn916. J. Bacteriol. 177:6644-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia, Y., and G. Churchward. 1999. Interactions of the integrase protein of the conjugative transposon Tn916 with its specific DNA binding sites. J. Bacteriol. 181:6114-6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manganelli, R., S. Ricci, and G. Pozzi. 1997. The joint of Tn916 circular intermediates is a homoduplex in Enterococcus faecalis. Plasmid 38:71-78. [DOI] [PubMed] [Google Scholar]
- 14.Pethel, B., and G. Churchward. 2000. Coupling sequences flanking Tn916 do not determine the affinity of binding of integrase to the transposon ends and adjacent bacterial DNA. Plasmid 43:123-129. [DOI] [PubMed] [Google Scholar]
- 15.Rice, L. B. 1998. Tn916 family conjugative transposons and dissemination of antimicrobial resistance determinants. Antimicrob. Agents Chemother. 42:1871-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice, L. B., S. Bellais, L. L. Carias, R. Hutton-Thomas, R. A. Bonomo, P. Caspers, M. G. Page, and L. Gutmann. 2004. Impact of specific pbp5 mutations on expression of beta-lactam resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 48:3028-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice, L. B., and L. L. Carias. 1994. Studies on excision of conjugative transposons in enterococci: evidence for joint sequences composed of strands with unequal numbers of nucleotides. Plasmid 31:312-316. [DOI] [PubMed] [Google Scholar]
- 18.Rice, L. B., and L. L. Carias. 1998. Transfer of Tn5385, a composite, multiresistance element from Enterococcus faecalis. J. Bacteriol. 180:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice, L. B., L. L. Carias, R. Hutton-Thomas, F. Sifaoui, L. Gutmann, and S. D. Rudin. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 45:1480-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice, L. B., S. H. Marshall, and L. L. Carias. 1992. Tn5381, a conjugative transposon identifiable as a circular form in Enterococcus faecalis. J. Bacteriol. 174:7308-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose, R. E. 1988. The nucleotide sequence of pACYC184. Nucleic Acids Res. 16:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudy, C. K., J. R. Scott, and G. Churchward. 1997. DNA binding by the Xis protein of the conjugative transposon Tn916. J. Bacteriol. 179:2567-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott, J. R. 1992. Sex and the single circle: conjugative transposition. J. Bacteriol. 174:6005-6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott, J. R., F. Bringel, D. Marra, G. Van Alstine, and C. K. Rudy. 1994. Conjugative transposition of Tn916: preferred targets and evidence for conjugative transfer of a single strand and for a double stranded circular intermediate. Mol. Microbiol. 11:1099-1108. [DOI] [PubMed] [Google Scholar]
- 25.Su, Y. A., P. He, and D. B. Clewell. 1992. Characterization of the tet(M) determinant of Tn916: evidence for regulation by transcription attenuation. Antimicrob. Agents Chemother. 36:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorzi, W., X. Y. Zhou, O. Dardenne, J. Lamotte, D. Raze, J. Pierre, L. Gutmann, and J. Coyette. 1996. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J. Bacteriol. 178:4948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]