Abstract
An insertion in the rasA gene entirely blocked developmental aggregation and sporulation in Myxococcus xanthus while also reducing swarm expansion on a 0.3% agar surface. Data presented here demonstrate that rasA is required for extracellular fibril formation and social gliding motility.
Myxococcus xanthus is an unflagellated gram-negative soil bacterium that associates in mobile, expanding swarms during vegetative growth. The mode of motility employed by the individual cells as they move across solid surfaces is termed “gliding,” which is characterized by a smooth, nonflagellar motion in the direction of the cell's long axis (12). Motility is integral to the ability of M. xanthus to forage for food, which includes predation of other soil-resident microorganisms (24). When nutrients become depleted, cells within the M. xanthus colony collaborate to construct a multicellular fruiting body within which a fraction of the constituent cells differentiate into resistant myxospores (for a review, see reference 10). As with nutrient foraging, motility is important for M. xanthus to carry out this developmental process, because the complex movements required for fruiting body morphogenesis depend on functional motility apparatuses (for a review, see reference 29).
M. xanthus possesses separate multigene systems for gliding by two distinct mechanisms called adventurous (A) motility and social (S) motility (13, 14, 29). M. xanthus does not appear to have any additional means of spatial translocation, because mutants defective for both the A and S systems are entirely unable to swarm (14). This observation has in fact been used as the basis for categorizing motility genes into either system. Hence, if a gene is required for S-motility, then a mutation of that gene in an A− strain abolishes swarming in the double mutant and vice versa.
Adventurous motility is required for single cells to move in isolation from the colony, although cells within a colonial swarm may also translocate by means of the A system (13). Adventurous motility is thought to be similar to gliding in cyanobacteria, where forward propulsion by the cell is coupled to backward extrusion of a polyelectrolyte gel through specialized nozzles situated at the cell poles (15). Bolstering this hypothesis is the recent discovery by Wolgemuth et al. (36) that M. xanthus possesses similar, albeit smaller, nozzle-like structures that are localized at the poles; these are the regions from which ribbons of slime are ejected. Mathematical models predict that the hydration of the cationic polyelectrolyte slime provides sufficient force to propel the M. xanthus cell forward at observed velocities (36).
In contrast to A-motility, the S-motility system is a contact-dependent mode of movement that requires cells to be in close proximity to one another, i.e., within a distance of about 2 μm (30). This is about the length of the thin, unipolar organelles called type IV pili (TFP), which are absolutely essential for movement by the S system (16, 37). TFP-mediated gliding appears to involve anchoring the pili onto a solid substratum (which may be a neighboring cell) located ahead, followed by pilus retraction, which has the effect of pulling the cell forward (23). In M. xanthus, the pil cluster of genes encodes structural and regulatory proteins that are required for TFP assembly and disassembly (37-40).
In addition to the TFP, other cell surface components that appear to mediate the intercellular contacts required for S-motility have been identified. These include the cell envelope-localized, mixed protein-polysaccharide structures called fibrils that are important for cell-to-cell cohesion and interactions within a swarm (5). Recent evidence indicates that fibrils may serve as TFP attachment points (21), which would explain the S− phenotype of fibril-deficient strains, such as the dsp mutants (2, 9, 28). Another cell surface component important for S-motility is the outer membrane O antigen; however, its role in motility has not been clarified (7).
The S-motility components appear to be regulated by signal transduction pathways that are homologous to the chemosensory system of enteric bacteria. One, the Frz system, appears to govern cell reversal frequency at least partly by regulating the switching of TFP bundles from one pole to the other (32). A second, the Dif system, has been shown to be required for fibril biogenesis (41). Recent evidence indicates that some dsp mutations are linked to the dif locus (19).
Here, we describe the phenotypes of a strain with a mutation in rasA, a gene required for both developmental aggregation and social motility. The rasA gene, identified as MXAN4150 by The Institute for Genomic Research (TIGR), is predicted to be 1,131 bp in length and to encode a 40.8-kDa protein. We identified the rasA gene as part of the characterization of the neighboring brgE gene, which is required for fruiting body morphogenesis and sporulation (25). Both rasA and brgE are predicted to be transcribed in the opposite direction from that of the downstream frzS gene (34) (Fig. 1). The rasA gene does not possess any conserved motifs (e.g., signal sequence or transmembrane regions) to aid in its functional prediction, nor does it bear sequence homology to any known genes on public databases as assessed by PSI-BLAST, Pfam, and SMART analyses (1, 4, 20).
FIG. 1.
Physical organization of the M. xanthus chromosome in the rasA region. Open reading frames and predicted directions of transcription are represented by arrows.
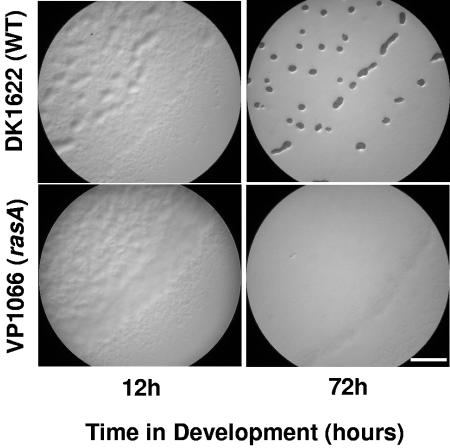
A rasA insertion mutation abolishes fruiting and sporulation.
A rasA insertion mutant was constructed by insertion mutagenesis of plasmid pDV678 (Table 1) into the rasA open reading frame using previously described methods (26). Southern blot analyses were used to confirm the insertion in rasA within colony-purified cells, which were grown on selective media to prevent loss of the inserted plasmid. The developmental defects of the resultant strain, VP1066, were assessed on TPM (Tris-phosphate-magnesium) starvation media by previously described methods (18, 33). As shown in Fig. 2, the rasA mutant is unable to form the multicellular assemblages elaborated by wild-type cells over a developmental time course. In addition, it produced fewer than 10 viable spores from an initial input of 5 × 108 cells, compared to wild-type cells, which produce 1 × 107 to 2 × 107 spores from the same number of input cells.
TABLE 1.
M. xanthus strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| DK1622 | Wild type | 16 |
| DK1217 | aglB1 | 14 |
| DK1250 | aglB1 tgl-1 | 14 |
| DK3468 | dsp-1680 | D. Morandi, Stanford University |
| DK10410 | ΔpilA | 40 |
| VP1066 | rasA::pDV678 | This study |
| VP1145 | rasA::pDV678 ΔpilA | This study |
| VP1146 | rasA::pDV678 aglB1 | This study |
| Plasmids | ||
| pBGS18 | nptII (Kmr) | 31 |
| pDV678 | RasA-F/Raamplicon in pBGS18 | This study |
PCR primers amplifying a 661-bp region of rasA between base pairs 277 and 938; RasA-F sequence, CTGAAGCTTGAAGAGGTACAGCCGCAGGT (the underlined sequence is an engineered site for restriction by HindIII); RasA-R sequence, TTCGAATTCCACTCGCGGTTGCTGTAGATG (the underlined sequence is an engineered site for restriction by EcoRI).
FIG. 2.
Light microscopy images showing developmental progression of DK1622 (wild type) and VP1066 (rasA) cells on TPM (starvation) agar. Mid-exponential-phase cells were concentrated 10-fold to a density of 5 × 109 CFU/ml and spotted onto TPM agar to induce development. Bar = 1 mm.
rasA is required for S-motility.
M. xanthus cells swarm fastest when spotted on a rich medium containing a low percentage of agar (27). The S-motility system operates most efficiently when the substrate is soft and wet, while the A-motility system appears to require a firmer surface over which to power gliding. Thus, the motility phenotype of S− mutants, which can move only by the A gliding apparatus, is most apparent when cells are placed on a 0.3% agar surface, while A− mutants display their phenotype most prominently on 1.5% agar media.
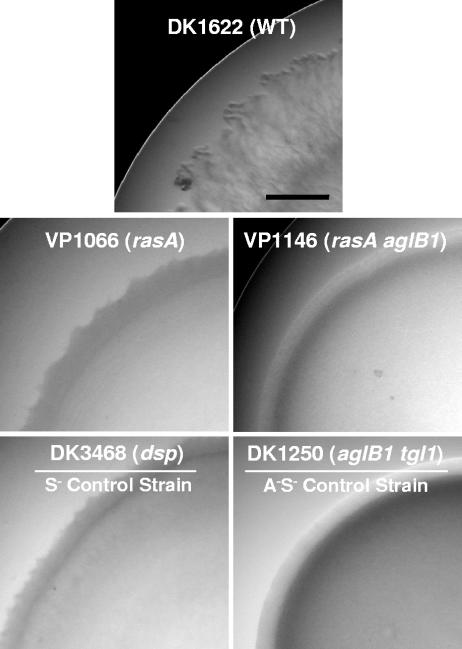
Because many developmental defects are caused by impairments in the social motility system (14, 22), the rasA mutant was assessed for motility phenotypes on both 1.5% and 0.3% agar containing a rich medium using standard methods (27). No differences between the mutant and its wild-type parent were observed on 1.5% agar (data not shown), which is similar to the phenotype of several dif mutants (6). In contrast, on 0.3% agar, the swarm edge of the mutant contained dark, disorganized spikes, in contrast to the translucent “frill” of the wild-type swarm edge (Fig. 3). Moreover, with respect to swarm expansion rates, which are calculated from the difference in swarm radii at 0 and 94 h, the rasA mutant expanded at a rate only one-third that of the wild type, which is comparable to the rates for other S− strains, including DK3468 (dsp) and DK10410 (ΔpilA) (Table 2). This phenotype is not due to growth defects, because there are no differences between the growth rates of VP1066 and the parent wild-type strain DK1622: both show a doubling time of 5.5 ± 0.085 h.
FIG. 3.
Swarm edge morphology of motility mutants on rich (Casitone-yeast extract) medium containing 0.3% agar. The strains and their genotypes are displayed within each light micrograph. See reference 27 for equivalent A− S+ pictures. Bar = 5 mm.
TABLE 2.
Swarm expansion on 0.3% agara
| Strain | Genotype | Swarm expansion (μm/min) |
|---|---|---|
| DK1622 | Wild type | 1.84 ± 0.40 |
| VP1066 | rasA | 0.49 ± 0.17 |
| VP1145 | rasA ΔpilA | 0.50 ± 0.17 |
| VP1146b | rasA aglB1 | 0.07 ± 0.10 |
| DK3468 | dsp | 0.48 ± 0.10 |
| DK10410 | ΔpilA | 0.37 ± 0.12 |
Mid-exponential-phase cells were spotted on a rich medium (Casitone-yeast extract) containing low-percentage (0.3%) agar. The radius of each spot was measured at regular intervals for 94 hours. Results shown are for threefold replicate experiments. See reference 27 for A− S+ swarm expansion data.
The aglB1 point mutation causes an A-motility defect.
In order to categorize rasA more firmly as an A- or S-motility gene, double mutants containing the rasA insertional disruption as well as a mutation in either aglB (an A gene) or pilA (an S gene) were generated and assessed for phenotypes on the two types of agar surfaces. When spotted onto hard and soft agar media, strain VP1145 (rasA ΔpilA) displayed no noticeable phenotype on 1.5% agar (data not shown) but formed stubby, rounded extensions at the swarm edge on 0.3% agar that are similar to those of the ΔpilA strain (data not shown). In contrast, strain VP1146 (rasA aglB) did not swarm on either surface, indicating that both motility systems had been impaired in this strain (Fig. 3). The smooth, nonexpanding swarm edge of strain VP1146 resembled that of the A− S− control strain DK1250 (Fig. 3).
Cell-to-cell cohesion is dependent on RasA.
Close cell-to-cell contact is important for S-motility, and the mediators of such an interaction appear to include cell surface components like TFP and fibrils. Accordingly, mutants lacking these appendages glide in small, loosely cohesive groups or as individuals rather than in the large swarms observed for A+ S+ and A− S+ cells (28). Cell cohesion can be quantified using the agglutination assay, which measures the turbidity of a dispersed cell culture (28). Over time, the cells form large multicellular clumps that drop out of suspension and reduce the culture's turbidity. The fibril-defective strain DK3468 (dsp-1680) was previously shown to fail to agglutinate (28). Strain DK3468 and wild-type strain DK1622 (which is cohesion proficient) were used as controls against which agglutination of strain VP1066 (rasA) was compared. As shown in Table 3, the agglutination property of strain VP1066 is very similar to that of agglutination-defective strain DK3468.
TABLE 3.
Surface properties of the rasA mutant
| Strain | Genotype | Agglutinationa | Surface componentsb
|
Dye binding%c
|
||||
|---|---|---|---|---|---|---|---|---|
| FibA | PilA | O antigen | CW | Congo red | Trypan blue | |||
| DK1622 | Wild type | 0.099 ± 0.062 | + | + | + | + | 63.3 ± 3.5 | 66.6 ± 0.8 |
| VP1066 | rasA | 0.897 ± 0.002 | − | + | + | − | 31.1 ± 2.9 | 2.9 ± 0.7 |
| DK3468d | dsp | 0.887 ± 0.002 | − | + | ND | − | 27.9 ± 1.6 | 6.2 ± 2.3 |
Turbidity of a suspension of cells was monitored at an optical density of 600 nm (OD600) over a 2-hour period at 6-min intervals. Data shown are the relative absorbance readings at the 2-hour endpoint, which were obtained by dividing the sample readings at each time point by the intial absorbance readings.
Production of FibA and PilA was separately assessed by Western blotting using anti-FibA monoclonal antibody 2105 and anti-PilA polyclonal antibodies, respectively. O antigen production was determined by silver staining lipopolysaccharide preparations separated by 13% deoxycholate-polyacrylamide gel electrophoresis (M. Esmaeiliyan, personal communication). ND, not determined.
Calcofluor white (CW) binding was evidenced by the formation of a white halo around an M. xanthus swarm exposed to long-wavelength UV light (365 nm). The Congo red and trypan blue dye binding data shown represent the percentage of bound dye at OD490 and OD585, respectively.
Strain DK3468 was used as the fibril-defective S− control strain against which the rasA mutant strain VP1066 was compared.
A rasA insertion mutation causes defects in extracellular fibrils.
As mentioned above, cell surface components are important for S-motility, including components that contribute to TFP, fibril, and O antigen production, assembly, and transport (2, 7, 37). To determine which component(s) was deficient in a rasA mutant, the mutant's ability to make PilA, FibA, and O antigen was assessed. A Western immunoblot analysis using monoclonal antibody 2105 (11) revealed that strain VP1066 is defective for making the fibril-associated protein FibA (Table 3), which is an autoproteolytic metalloprotease that exists primarily in the 66-kDa form (17). This was similar to the defect of the dsp-1680 mutant strain DK3468, which is known to lack fibril components (8, 28). On the other hand, no defects were observed for the generation of prepilin (PilA) from whole-cell extracts or for extracellular pili from pilus preparations (Table 3), nor was the rasA mutant deficient for O antigen (M. Esmaeiliyan and H. Kaplan, personal communication).
The fibril defect of VP1066 cells was further corroborated by assays that measure the binding of the fibril-targeted dyes calcofluor white M2R (9), Congo red, and trypan blue (3, 6). As shown in Table 3, strain VP1066 is deficient for binding all three dyes, which is similar to the fibril-defective dsp-1680 mutant strain DK3468.
Concluding remarks.
Intriguingly, the rasA open reading frame is adjacent to the frz cluster of genes (Fig. 1), which are hypothesized to participate in S-motility in the capacity of a signal-transducing pathway (for a review, see reference 35). However, the association between RasA and the Frz pathway needs further study, because unlike RasA, the Frz components are not known to be defective for fibrils. Rather, defects in fibril production are more often associated with the Dif system, which forms another putative signal-transducing pathway that is essential for S-motility (41).
Many questions remain concerning how the various components involved in S-motility, including the Frz and Dif systems, are related to each other. Signaling via the Frz and Dif systems, by virtue of their common involvement in S-motility, may be integrated by a regulator that couples cell reversal frequency with the production of fibrils. RasA is a fitting candidate for such a role, given its requirement in fibril biogenesis and its circumstantial relationship, by virtue of proximal location, with the Frz system. Because it lacks any known functional domains, it is not possible to predict whether RasA carries out a regulatory, enzymatic, or structural function as an integral S-motility component. Experiments to determine whether RasA physically interacts with any of the Frz and Dif proteins, whether it regulates their expression, or whether its own expression is regulated by Frz and/or Dif signal transduction activities may help better define its role in S-motility.
Acknowledgments
We thank Y. Cheng and D. Kaiser for generously providing strains and the anti-PilA antibody. We also thank M. Esmaeiliyan and H. Kaplan for sharing unpublished data and for sending us the anti-FibA antibody originally constructed by M. Dworkin.
This work was supported, in part, by the Floyd and Mary Schwall Medical Research Fellowship and Public Health Service grant T32GM0737 to V.D.P. and by Public Health Service grant GM54592 to M.S.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold, J. W., and L. J. Shimkets. 1988. Cell surface properties correlated with cohesion in Myxococcus xanthus. J. Bacteriol. 170:5771-5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold, J. W., and L. J. Shimkets. 1988. Inhibition of cell-cell interactions in Myxococcus xanthus by Congo red. J. Bacteriol. 170:5765-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman, A., L. Coin, R. Durbin, R. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. Sonnhammer, D. Studholme, C. Yeats, and S. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138-D141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behmlander, R. M., and M. Dworkin. 1994. Biochemical and structural analyses of the extracellular matrix fibrils of Myxococcus xanthus. J. Bacteriol. 176:6295-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, W. P., and Z. Yang. 2004. Myxococcus xanthus chemotaxis homologs DifD and DifG negatively regulate fibril polysaccharide production. J. Bacteriol. 186:1001-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowden, M. G., and H. B. Kaplan. 1998. The Myxococcus xanthus lipopolysaccharide O-antigen is required for social motility and multicellular development. Mol. Microbiol. 30:275-284. [DOI] [PubMed] [Google Scholar]
- 8.Chang, B. Y., and M. Dworkin. 1994. Isolated fibrils rescue cohesion and development in the Dsp mutant of Myxococcus xanthus. J. Bacteriol. 176:7190-7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dana, J. R., and L. J. Shimkets. 1993. Regulation of cohesion-dependent cell interactions in Myxococcus xanthus. J. Bacteriol. 175:3636-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dworkin, M. 1996. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 60:70-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill, J., E. Stellwag, and M. Dworkin. 1985. Monoclonal antibodies against cell-surface antigens of developing cells of Myxococcus xanthus. Ann. Inst. Pasteur Microbiol. 136A:11-18. [DOI] [PubMed] [Google Scholar]
- 12.Henrichsen, J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36:478-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgkin, J., and D. Kaiser. 1979. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): genes controlling movement of single cells. Mol. Gen. Genet. 171:167-176. [Google Scholar]
- 14.Hodgkin, J., and D. Kaiser. 1979. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol. Gen. Genet. 171:177-191. [Google Scholar]
- 15.Hoiczyk, E., and W. Baumeister. 1998. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr. Biol. 8:1161-1168. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser, D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 76:5952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearns, D. B., P. J. Bonner, D. R. Smith, and L. J. Shimkets. 2002. An extracellular matrix-associated zinc metalloprotease is required for dilauroyl phosphatidylethanolamine chemotactic excitation in Myxococcus xanthus. J. Bacteriol. 184:1678-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117:252-266. [DOI] [PubMed] [Google Scholar]
- 19.Lancero, H., J. E. Brofft, J. Downard, B. W. Birren, C. Nusbaum, J. Naylor, W. Shi, and L. J. Shimkets. 2002. Mapping of Myxococcus xanthus social motility dsp mutations to the dif genes. J. Bacteriol. 184:1462-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letunic, I., R. R. Copley, S. Schmidt, F. Ciccarelli, T. Doerks, J. Schultz, C. P. Ponting, and P. Bork. 2004. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32:D142-D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, Y., H. Sun, X. Ma, A. Lu, R. Lux, D. Zusman, and W. Shi. 2003. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 100:5443-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacNeil, S. D., A. Mouzeyan, and P. L. Hartzell. 1994. Genes required for both gliding motility and development in Myxococcus xanthus. Mol. Microbiol. 14:785-795. [DOI] [PubMed] [Google Scholar]
- 23.Maier, B., L. Potter, M. So, C. D. Long, H. S. Seifert, and M. P. Sheetz. 2002. Single pilus motor forces exceed 100 pN. Proc. Natl. Acad. Sci. USA 99:16012-16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pham, V. D., C. W. Shebelut, M. E. Diodati, C. T. Bull, and M. Singer. 2005. Mutations affecting predation ability of the soil bacterium Myxococcus xanthus. Microbiology 151:1865-1874. [DOI] [PubMed] [Google Scholar]
- 25.Pham, V. D., C. W. Shebelut, E. J. Zumstein, and M. Singer. 2005. BrgE is a regulator of Myxococcus xanthus development. Mol. Microbiol. 57:762-773. [DOI] [PubMed] [Google Scholar]
- 26.Plamann, L., J. M. Davis, B. Cantwell, and J. Mayor. 1994. Evidence that asgB encodes a DNA-binding protein essential for growth and development of Myxococcus xanthus. J. Bacteriol. 176:2013-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi, W., and D. R. Zusman. 1993. The two motility systems of Myxococcus xanthus show different selective advantages on various surfaces. Proc. Natl. Acad. Sci. USA 90:3378-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimkets, L. J. 1986. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J. Bacteriol. 166:837-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spormann, A. M. 1999. Gliding motility in bacteria: insights from studies of Myxococcus xanthus. Microbiol. Mol. Biol. Rev. 63:621-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spormann, A. M., and D. Kaiser. 1999. Gliding mutants of Myxococcus xanthus with high reversal frequencies and small displacements. J. Bacteriol. 181:2593-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spratt, B. G., P. J. Hedge, S. te Heesen, A. Edelman, and J. K. Broome-Smith. 1986. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene 41:337-342. [DOI] [PubMed] [Google Scholar]
- 32.Sun, H., D. R. Zusman, and W. Shi. 2000. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr. Biol. 10:1143-1146. [DOI] [PubMed] [Google Scholar]
- 33.Thöny-Meyer, L., and D. Kaiser. 1993. devRS, an autoregulated and essential genetic locus for fruiting body development in Myxococcus xanthus. J. Bacteriol. 175:7450-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward, M. J., H. Lew, and D. R. Zusman. 2000. Social motility in Myxococcus xanthus requires FrzS, a protein with an extensive coiled-coil domain. Mol. Microbiol. 37:1357-1371. [DOI] [PubMed] [Google Scholar]
- 35.Ward, M. J., and D. R. Zusman. 2000. Developmental aggregation and fruiting body formation in the gliding bacterium Myxococcus xanthus, p. 243-262. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 36.Wolgemuth, C., E. Hoiczyk, D. Kaiser, and G. Oster. 2002. How myxobacteria glide. Curr. Biol. 12:369-377. [DOI] [PubMed] [Google Scholar]
- 37.Wu, S. S., and D. Kaiser. 1995. Genetic and functional evidence that type IV pili are required for social gliding motility in Myxococcus xanthus. Mol. Microbiol. 18:547-558. [DOI] [PubMed] [Google Scholar]
- 38.Wu, S. S., and D. Kaiser. 1997. Regulation of expression of the pilA gene in Myxococcus xanthus. J. Bacteriol. 179:7748-7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, S. S., J. Wu, Y. L. Cheng, and D. Kaiser. 1998. The pilH gene encodes an ABC transporter homologue required for type IV pilus biogenesis and social gliding motility in Myxococcus xanthus. Mol. Microbiol. 29:1249-1261. [DOI] [PubMed] [Google Scholar]
- 40.Wu, S. S., J. Wu, and D. Kaiser. 1997. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol. Microbiol. 23:109-121. [DOI] [PubMed] [Google Scholar]
- 41.Yang, Z., X. Ma, L. Tong, H. B. Kaplan, L. J. Shimkets, and W. Shi. 2000. Myxococcus xanthus dif genes are required for biogenesis of cell surface fibrils essential for social gliding motility. J. Bacteriol. 182:5793-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]