Abstract
Escherichia coli excretes the catecholate siderophore enterobactin in response to iron deprivation. While the mechanisms underlying enterobactin biosynthesis and ferric enterobactin uptake and utilization are widely understood, nearly nothing is known about how enterobactin is exported from the cell. Mutant and high-performance liquid chromatography analyses demonstrated that the outer membrane channel tunnel protein TolC but none of the respective seven resistance nodulation cell division (RND) proteins CusA, AcrB, AcrD, AcrF, MdtF (YhiV), or the twin RND MdtBC (YegNO) was essential for enterobactin export across the outer membrane. Mutant E. coli strains with additional deletion of tolC or the major facilitator entS were growth deficient in iron-depleted medium. Strains with deletion of tolC or entS, but not with deletion of genes encoding RND transporters, excreted very little enterobactin into the growth medium. Enterobactin excretion in E. coli is thus probably a two-step process involving the major facilitator EntS and the outer membrane channel tunnel protein TolC. Quantitative reverse transcription-PCR analysis of gene-specific transcripts showed no significant changes in tolC expression upon iron depletion. However, iron starvation led to increased expression of the RND gene mdtF and a decrease in acrD.
Enterobactin (33), also known as enterochelin (30), is the catecholate-type siderophore of Escherichia coli and of several other bacteria. Enterobactin, a cyclic triester of 2,3-dihydroxybenzoylserine (DHBS), is one of the most effective ferric iron chelating compounds known (1, 36). While the molecular processes involved in ferric enterobactin uptake by TonB-energized outer membrane receptor proteins such as FepA (recently reviewed in reference 15) were studied exhaustively over the last decades, a mechanism for enterobactin efflux across the cytoplasmic membrane was discovered only recently. EntS (the ybdA gene product) (5), a member of the vast major facilitator superfamily (MFS) of membrane-bound transporters, was shown to be necessary for effective enterobactin export in E. coli (9). Cells with entS deleted excreted very little enterobactin into the surrounding medium, but degradation products of enterobactin were released into supernatants. Since those degradation products are themselves efficient siderophores and were still exported, strains lacking entS suffered no iron depletion (9).
Because enterobactin is too big to diffuse freely through the porins of the outer membrane, transport from the periplasm to the outside has to be accomplished by another still unknown transport system. Previously, we and others (18, 20, 26, 27) could demonstrate that transport systems of the resistance nodulation cell division (RND) type (38) may transport their substrates from the periplasm (or from the cytoplasmic membrane in the case of hydrophobic substances) rather than from the cytoplasm to the outside. At least for copper and cobalt, we provided evidence that efflux is probably a two-step process involving a transporter of the cytoplasmic membrane and a resistance nodulation cell division protein complex for detoxification of the periplasmic metal cations (23, 37).
Resistance nodulation cell division proteins are typical transporters of gram-negative bacteria and function as a huge protein complex spanning from the cytoplasmic membrane to the outer membrane. These protein complexes are composed of an RND protein, a membrane fusion protein (MFP), and an outer membrane channel tunnel protein (17), also termed outer membrane factor (32). Recently, the complete three-dimensional structure of the whole efflux complex MexAB-OprM was assembled (14). Resistance nodulation cell division-type transport complexes transport a very broad variety of substances, including antibiotics, dyes, detergents, or heavy metal cations (24, 25). Thus, this would make resistance nodulation cell division complexes ideal candidates for enterobactin export from the periplasm to the outside.
While there are seven resistance nodulation cell division transport proteins in E. coli (AcrB, AcrD, AcrF, MdtB [YegN], MdtC [YegO], MdtF [YhiV], and CusA [YbdE]) and a plethora of known and putative membrane fusion proteins, only two outer membrane factors, TolC (43) and CusC, have been shown to be involved in efflux in E. coli. CusC is the exclusive outer membrane channel tunnel protein for the Cus system (8). The CusC(F)BA transporter was shown to be responsible for copper efflux, probably from the periplasm, and contributes some resistance to the antibiotic fosfomycin (28). The cus determinant is located near the enterobactin biosynthesis gene cluster on the chromosome and was initially annotated as a putative iron transport system (accession no. BAB34036).
The second outer membrane channel tunnel protein of E. coli, TolC, is needed for transport mediated by the other six resistance nodulation cell division transporters (28) but not for Cus (8). In order to identify the transporter responsible for periplasmic enterobactin efflux, tolC or all seven resistance nodulation cell division genes, respectively, were deleted from the chromosome of E. coli, and their effects on enterobactin excretion were determined. This demonstrated that the outer membrane factor TolC was necessary for enterobactin export beyond the outer membrane.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli strains were grown in Luria-Bertani (LB) medium or Tris-buffered mineral salts medium (22) with or without iron, containing 2 ml glycerol and 3 g Casamino Acids per liter. The strains used in this study are listed in Table 1. Antibiotics (chloramphenicol, 15 to 20 μg/ml; kanamycin, 25 μg/ml), FeCl3, or 2,2′-dipyridyl (DIP) were added where appropriate. Cellular dry weights were determined by conversion of absorbances at 600 nm from a previously derived calibration curve of dried cells (11).
TABLE 1.
E. coli strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| W3110 | Wild type | 4 |
| ECA263 | ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| ECA264 | ΔtolC::kan ΔfecABCDE ΔzupT::cat ΔmntH ΔfeoABC | This study |
| ECA265 | ΔacrB::cat ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| ECA266 | ΔacrD::cat ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| ECA267 | ΔacrF::cat ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| ECA268 | ΔmdtBC::cat ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| ECA269 | ΔmdtF::cat ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| ECA270 | ΔcusCFBA::cat ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| ECA271 | ΔentS::cat ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| ECA272 | ΔentC::cat ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| ECA296 | ΔtonB::cat ΔfecABCDE ΔzupT ΔmntH ΔfeoABC | This study |
| GG199 | Δfur::cat | 12 |
| ECA273 | ΔtolC::kan Δfur | This study |
| ECA274 | ΔacrB::cat Δfur | This study |
| ECA275 | ΔacrD::cat Δfur | This study |
| ECA276 | ΔacrF::cat Δfur | This study |
| ECA277 | ΔmdtBC::cat Δfur | This study |
| ECA278 | ΔmdtF::cat Δfur | This study |
| ECA279 | ΔcusCFBA::cat Δfur | This study |
| ECA280 | ΔentS::cat Δfur | This study |
| GG213 | ΔentC::cat Δfur | 12 |
Genetic techniques.
Standard molecular genetic techniques were used (39). PCR was performed with Taq, Pwo, or Tgo DNA polymerase (Roche, Mannheim, Germany).
Gene deletions.
Genes were deleted by the insertion of Camr or Kanr cassettes, employing a protocol developed in the laboratory of B. Wanner which is based on the λ Red-recombinase system, as described previously (6). Initial deletions comprising a chloramphenicol (cat) or kanamycin (kan) resistance cassette were transduced by general transduction with phage P1 into E. coli strain W3110. Multiple deletions were constructed by elimination of the respective resistance cassette (6) and subsequent general phage P1 transduction.
Extraction of enterobactin and its degradation products.
E. coli Δfur derivatives are deregulated in enterobactin biosynthesis (40), which leads to overexpression and secretion of enterobactin. Overnight cultures of Δfur strains grown in LB broth were diluted 1:400 into Tris minimal medium and grown overnight at 37°C with shaking. Cultures were diluted 1:1,000 into Tris minimal medium without iron, and after 16 h of growth (dry weight of approximately 0.1 mg/ml), cells were separated from the growth medium by centrifugation (two times at 7,650 × g at 4°C for 15 min). Supernatants were acidified with 50 μl 37% HCl per 10 ml and extracted at 4°C twice with 1.5 ml ethyl acetate. After centrifugation (2 min, 7,650 × g, 4°C), the ethyl acetate phase was evaporated. Dried residues were resuspended in 150 μl methanol and used immediately for high-performance liquid chromatography (HPLC) analysis.
RNA isolation and preparation of cDNA.
E. coli wild-type (W3110) cells were inoculated either with 25 μM 2′2′-dipyridyl or with 100 μM FeCl3 in Tris minimal medium and harvested at 100 Klett units at the early logarithmic growth phase. At this DIP concentration, growth was not affected. Total RNA was isolated as described previously (13). After RNA isolation, DNase treatment was performed with 1 U RQ1 RNase-free DNase (Promega GmbH, Mannheim, Germany) per 10 μg RNA followed by purification with RNeasy columns (QIAGEN, Hilden, Germany). To exclude experimental artifacts resulting from DNA contaminations, only RNA preparation were used that did not generate PCR fragments in a PCR performed in the same PCR cycler used for real-time PCR with chromosomal primers without a previously performed reverse transcription (RT) reaction. RNA concentration was determined photometrically, and RNA quality was checked on formamide gels (39). In an RT reaction, 2 μg of total RNA was transcribed in a 20-μl reaction mixture using 0.1 μg hexamer primers, 0.25 mM each of dATP, dGTP, dTTP and dCTP, 10 mM dithiothreitol, and 200 U reverse transcriptase (Superscript II) in reaction buffer (Invitrogen, Karlsruhe, Germany). Primers and RNA were heated to 65°C for 5 min and snap-cooled on ice. Reverse transcription proceeded for 10 min at room temperature, followed by 1 h at 42°C. To inactivate the remaining transcriptase, the reaction mixture was incubated for 15 min at 70°C.
qRT-PCR.
For quantitative real-time RT-PCR (qRT-PCR), duplicate reactions with 1 μl of template cDNA, 5 pmol primers, and the QuantiTect SYBR Green PCR kit (QIAGEN, Hilden, Germany) were used. Details for PCR protocols and primer sequences are available on request. Fluorescence was measured at the end of each 72°C incubation and analyzed using Rotor-Gene software (version 4.6.94). Melting curve analyses (60 to 99°C, 1.0°C increments) were performed to ensure PCR specificity. For quantification, standard curves of cDNA dilutions (1:10 to 1:1,000) were performed as duplicates and a relative quantitation was done. As an endogenous control, 16S rRNA was used for normalization. The template cDNA for the endogenous control was diluted 1:10 to avoid saturation of fluorescence signals. A no-template control and a no-RT (negative) control were performed under identical conditions as for the target genes. Expression ratios were obtained by dividing normalized expression levels of iron-depleted (2,2′-dipyridyl) cells by iron-replete cells of E. coli wild-type strain W3110. An average of sextuplicates of expression levels from two independent biological samples was calculated.
HPLC analysis.
Standards of enterobactin and its degradation products were obtained from EMC Microcollections GmbH (Tübingen, Germany). Reverse-phase (RP)-HPLC analysis was carried out on a Nucleosil-300 column (C18, 4 × 250 mm, 5 μm; Knauer, Berlin, Germany) using a Merck-Hitachi (Darmstadt, Germany) LaChrom system containing a D-7000 interface, an L-7100 pump, an L-7200 autosampler, and a D-7450 diode array detector. Mobile phases consisted of 0.075% (vol/vol) trifluoroacetic acid in H2O (pH 2, phase A) and acetonitrile (phase B). The flow rate was adjusted to 1 ml min−1, and 10 μl of each supernatant extraction was injected and separated as described by the manufacturer of the standard. Spectral data (220 to 400 nm) were collected each 4 ms during the entire run. Separation of enterobactin and related compounds was controlled at 220 nm.
RESULTS
Expression of resistance nodulation cell division transporter genes and of tolC in response to iron availability. To investigate whether iron deprivation results in increased expression of resistance nodulation cell division transporter genes or tolC, all seven genes for resistance nodulation cell division transporters and for tolC were analyzed using quantitative real-time RT-PCR. DIP was used as an iron-specific chelator to induce iron deficiency, and FeCl3 was used for iron repletion. As an endogenous control, expression of 16S rRNA was used for normalization of all transcript levels (Table 2). For the gene-specific transcripts of acrB, cusA, mdtB, mdtC, and tolC, no significant change in expression in response to iron availability was determined (Table 2). Iron depletion led to a threefold decrease in transcript levels of the acrD gene, whereas mdtF showed a threefold increase in expression. For comparison, two examples of iron deprivation-induced genes, fepA and entC, encoding the outer membrane ferric enterobactin receptor and isochorismate synthase 2, respectively, were also examined. Both genes were highly expressed under iron starvation (fepA, 81-fold; entC, 336-fold; Table 2), mirroring the participation of EntC and FepA in iron homeostasis. Of the RND genes, only acrD (2.8-fold down-regulated under iron starvation) and mdtF (3.3-fold up-regulated) were iron responsive, but on a much lower level than entC or fepA (Table 2).
TABLE 2.
Ratios of gene expression of resistance nodulation cell division transporter genes tolC, fepA, and entC in response to iron deprivation
| Gene | Ratio of transcript levels [+DIP/+Fe(III)]a |
|---|---|
| tolC | 0.99 ± 0.04 |
| acrB | 0.83 ± 0.18 |
| acrD | 0.35 ± 0.10 |
| acrF | 0.65 ± 0.09 |
| mdtB | 0.97 ± 0.28 |
| mdtC | 0.96 ± 0.16 |
| mdtF | 3.34 ± 0.53 |
| cusA | 1.00 ± 0.23 |
| fepA | 81.63 ± 12.41 |
| entC | 331.92 ± 67.00 |
To determine expression levels of the selected genes, cells were cultivated in Tris minimal medium containing 25 μM 2,2′-dipyridyl or 100 μM FeCl3. Levels of gene-specific transcripts were determined by quantitative real-time RT-PCR. The ratios of normalized transcript levels of iron-deprived cells and iron-saturated control cells were calculated. Three replicate experiments per gene were performed as duplicates. The average and standard deviations are shown.
Deletion only of tolC but of neither resistance nodulation cell division gene results in impaired growth of E. coli mutants under iron-depleted conditions.
Growth of E. coli mutant strains with additional deletion of tolC, entS, or the resistance nodulation cell division genes acrB, acrD, acrF, mdtBC, mdtF, and cusA was investigated under iron-depleted conditions.
E. coli strain ECA272, with deletions of the most important iron uptake systems fecABCDE, mntH, zupT, feoABC, and entC (encoding isochorismate-synthase 2, EntC, necessary for enterobactin biosynthesis), was retarded in growth in the presence or absence of iron (10) (Fig. 1, negative control). Recently, it was shown that ZupT and MntH are additional (ferrous) iron transporters in E. coli (10, 21); therefore, the respective genes were also deleted in addition to the genes involved in supplying E. coli with ferrous iron, ferric citrate, or ferric enterobactin. The gene for the magnesium uptake transporter CorA of E. coli was not deleted because recently it was convincingly shown that CorA is not involved in iron uptake (31).
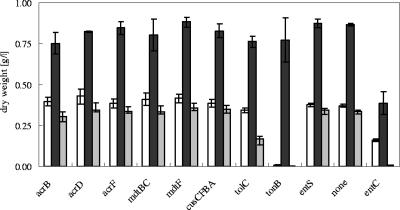
FIG. 1.
Growth of E. coli mutants in the presence or absence of iron. Cultures grown overnight in Tris minimal medium were diluted 1:400 into fresh medium for 2 h and diluted 1:400 into fresh iron-free minimal medium with 50 μM FeCl3 (black bars), 75 μM DIP (gray bars), or no additives (white bars). Cultures were cultivated for 16 h at 37°C with shaking, and the dry weight was calculated. The strains tested were derivatives of ECA263 (ΔfecABCDE ΔzupT ΔmntH ΔfeoABC; positive control), with additional deletion of the indicated genes: entC (negative control, no production of catecholate sideropohores); acrB, acrD, acrF, mdtB, mdtC, mdtF, and cusCFBA (encoding resistance nodulation cell division proteins); tolC (outer membrane channel tunnel protein); and entS (cytoplasmic membrane enterobactin transporter). Shown are the averages and standard deviations of three independent experiments.
Strain ECA263 with deletion of only feoABC, fecABCDE, mntH, and zupT, but not of entC, is able to synthesize and export enterobactin, but the other iron uptake systems are inactivated. Consequently, it can utilize iron even in the presence of iron chelators due to the higher affinity of enterobactin and its linear degradation products toward iron (Fig. 1, positive control). This strain was useful to investigate siderophore export when additional genes were deleted.
Resistance nodulation cell division- driven efflux complexes require a channel tunnel protein within the outer membrane for transport of substrates across the outer membrane. If resistance nodulation cell division proteins are responsible for periplasmic export of this siderophore, deletion of the genes for the channel tunnel protein TolC or the second channel tunnel protein, CusC, from E. coli should therefore lead to decreased export of enterobactin. Diminished excretion of enterobactin would accordingly result in loss of one of the major cellular iron acquisition systems.
For comparison, the gene tonB or entS was also deleted. Strains with deletion of tonB secrete enterobactin but are unable to take up ferric enterobactin since the FepA, Cir, and Fiu receptors are no longer energized through TonB (42). Consequently, strain ECA296 (ΔtonB::cat ΔfecABCDE ΔmntH ΔzupT ΔfeoABC) was only able to grow when iron was added to cultures (Fig. 1). Almost no growth was observed when either no additional iron was present or iron was depleted by DIP. Mutants of the major facilitator gene entS were previously shown to secrete only a little enterobactin but were not growth impaired (9). When entS was deleted in strain ECA271 (ΔfecABCDE ΔmntH ΔzupT ΔfeoABC), growth of this strain was indistinguishable from that of the positive control parental strain ECA263 under the conditions tested (Fig. 1).
Conversely, deletion of the tolC gene in this strain (leading to strain ECA264) resulted in diminished growth in the presence of the iron chelator DIP (Fig. 1). In contrast, deletion of the cus determinant that codes for another channel tunnel protein, CusC, did not influence growth in the presence of DIP (Fig. 1). This indicated that TolC but not CusC might be involved in periplasmic enterobactin efflux. Thus, in the presence of DIP, export of enterobactin across the outer membrane by TolC was the limiting step for growth rather than enterobactin transport across the cytoplasmic membrane by the MFS protein EntS.
Since only the tolC deletion strain showed an iron-dependent phenotype and resistance nodulation cell division transporters AcrB, AcrD, AcrF, MdtB, MdtC, and MdtF only function in cooperation with TolC, the respective resistance nodulation cell division-encoding genes were deleted from E. coli strain ECA263 (ΔfecABCDE ΔmntH ΔzupT ΔfeoABC). When growth of the resulting six mutant strains was investigated under low- and high-iron conditions, unexpectedly, deletion of none of the resistance nodulation cell division genes resulted in a similar growth defect as observed for the tolC mutant strain (Fig. 1).
Thus, Cus is not responsible for enterobactin transport, but TolC might partake in enterobactin efflux. However, the TolC-dependent efflux probably does not involve one of the resistance nodulation cell division transporters (AcrB, AcrD, AcrF, MdtB, MdtC, or MdtF) alone.
Growth of a tolC deletion strain in the presence of 2,2′-dipyridyl.
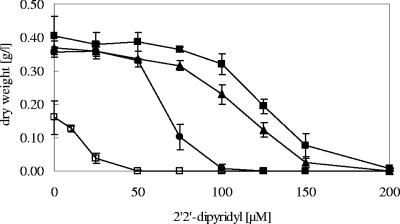
Strains with tolC (ECA272) or entS (ECA271) deleted were analyzed in dose-response experiments. Increasing concentrations of DIP were used in these experiments to increase iron depletion. This should yield information on how effective the remaining siderophore export machinery in the respective strains was.
The negative control strain ECA272 (ΔentC ΔfecABCDE ΔmntH ΔzupT ΔfeoABC) was unable to produce any enterobactin and did not grow even with very small amounts of DIP added. Growth was completely inhibited at 50 μM DIP (Fig. 2). Similarly, growth of the tolC mutant strain was severely affected at low DIP concentrations and completely inhibited at 100 μM DIP. In contrast, the entS deletion strain exhibited a phenotype similar to that of the positive control strain ECA263 (ΔfecABCDE ΔmntH ΔzupT ΔfeoABC), and the dry weight after 16 h of growth was only a little reduced over the full span of DIP concentrations used (Fig. 2). The MIC of strain ECA264 (ΔtolC) was only 50% of that of strain ECA271 (ΔentS) or the positive control strain, ECA263. This also suggested that TolC was important for siderophore export across the outer membrane.
FIG. 2.
Dose-response curves of E. coli mutants under iron limitation. Cultures were grown as described for Fig. 1, except increasing concentrations of DIP were added to induce iron depletion. E. coli strains were derivatives of ECA263 (ΔfecABCDE ΔfeoABC ΔmntH ΔzupT) (▪) with additional deletion of tolC (•), entS (▴), or entC (□). Shown are the averages and standard deviations of three independent experiments.
RP-HPLC analysis of enterobactin and its degradation products excreted by E. coli mutant strains.
To investigate whether strains with deletion of tolC or resistance nodulation cell division genes differ in their ability to release enterobactin or its degradation products, HPLC was performed. Strains with fur deleted, which encodes the global regulator of iron homeostasis, were used throughout. Deletion of fur resulted in constitutive production of enterobactin; thus, all strains investigated should produce similar basal enterobactin levels. The growth yields of all strains were comparable at approximately 0.1 mg/ml dry weight.
E. coli strains with fur cus, fur acrB fur acrD, fur acrF, fur mdtBC, or fur mdtF deleted, respectively, secreted enterobactin and oligomers of DHBS to a similar extent as a Δfur single-deletion parent strain that served as the positive control (Fig. 3). In contrast, deletion of entC, entS, mdtBC, or tolC from the Δfur mutant strain led to different patterns (Fig. 3). Since the Δfur ΔentC strain had deletion of the entC gene, required for enterobactin synthesis, it could not produce any enterobactin or DHBS oligomers. Consequently, these substances did not appear in the growth medium (Fig. 3), allowing usage of the Δfur ΔentC strain as a negative control in this HPLC analysis. We also tried to investigate a fur tonB double-deletion strain. This Δfur ΔtonB strain was not able to take up ferric enterobactin and is at the same time deregulated for enterobactin biosynthesis. However, this strain reached only one-third of the dry weight value of all other strains (data not shown) and was therefore omitted from HPLC analysis.
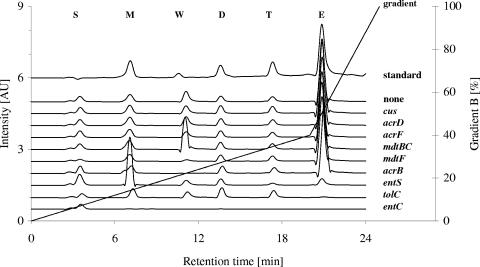
FIG. 3.
RP-HPLC analysis of enterobactin and its degradation products synthesized from several E. coli strains. Enterobactin and its degradation products were extracted from acidified supernatants from cultures of derivatives of E. coli strain GG199 (Δfur::cat), with additional deletion of the indicated genes, grown overnight in minimal medium at 37°C for 16 h, and subjected to HPLC analysis. Shown are panels of individual chromatograms. Peaks of enterobactin (E) and its degradation products DHBS triester (T), DHBS diester (D), and DHBS (M), tryptophan (W), and the injection peak (S) from the standard are indicated. Values of a medium control were subtracted from all chromatograms. The concentration profile of the gradient of acetonitrile is indicated.
As published previously (9), the strain with entS deleted excretes only minor amounts of enterobactin but larger amounts of DHBS and its di- and triester. The concentration of monomeric DHBS was appreciably higher, though, than that of the Δfur parent strain. Conversely, the strain with fur and tolC deleted did not export detectable amounts of enterobactin. However, the amounts of DHBS and its oligomers excreted by the Δfur ΔtolC mutant cells into the growth medium were similar to those produced by the Δfur parent strain (Fig. 3).
The strain with mdtBC deleted was found to excrete tryptophan in larger amounts than all other strains, while the pattern of siderophore excretion is very similar to that of the other RND gene deletion strains. The reason for this remains unknown.
Taken together, (i) lack of TolC resulted in specific absence of excreted enterobactin, while DHBS oligomers were slightly increased; (ii) deletion of the MFS-encoding gene entS led to a diminished concentration of enterobactin and DHBS triester but to an increase of monomeric DHBS; and (iii) deletion of mdtBC from the genome of a Δfur mutant strain led to no change in excretion of enterobactin or DHBS derivatives but did lead to increased production of tryptophan. This indicated that EntS and TolC are required for export of enterobactin into the growth medium, but they have different effects on release of these various compounds.
DISCUSSION
More than a decade ago, it was suggested that pyoverdine, the major siderophore of Pseudomonas aeruginosa, is exported by the MexA-MexB-OprK resistance nodulation cell division complex and it was speculated that AcrA and AcrB of E. coli were involved in the secretion of enterobactin and/or its metabolites (35). Also, in the study on the major facilitator of E. coli, EntS, which is involved in transport of enterobactin across the cytoplasmic membrane (9), it was postulated that AcrAB or the major facilitator EmrB and its associated membrane fusion protein, EmrA (19), might pump out excess enterobactin under stress conditions. These authors recognized TolC as the Achilles' heel of transenvelope efflux (38), since a variety of exporters share TolC as the outer membrane channel tunnel protein of an energy-driven efflux complex. Thus, deletion of the tolC gene is an ideal access point for dissecting enterobactin export from the periplasm. Since TolC is utilized by several resistance nodulation cell division proteins, deletion of the tolC gene would render all of these systems nonfunctional.
Resistance nodulation cell division pumps are involved in efflux of antibiotics or heavy metal cations, and the export goes probably from the periplasm directly into the growth medium surrounding the cell (18, 20, 26, 27). Consequently, resistance nodulation cell division proteins might also be required for excretion of siderophores. Three further observations pointed in this direction. First, in Acinetobacter baumannii 8399, a gene for a resistance nodulation cell division transporter related to MexB from P. aeruginosa or AcrB and an EntS ortholog were found to be part of the bacterium's locus for siderophore biosynthesis and transport (7). Second, mutants of P. aeruginosa defective in mexA-mexB-oprK showed a decreased ability to grow in the presence of the iron chelator 2,2′-dipyridyl (34), marking this transenvelope efflux system as a likely candidate for siderophore export. Third, the cusA (ybdE) gene of E. coli was initially and misleadingly annotated as an iron efflux system (accession no. BAB34036) and is located close to entS (ybdA) (5) on the chromosome. This genetic arrangement is somewhat reminiscent of the situation in A. baumanii 8399 (7).
This study, however, suggests that for enterobactin efflux TolC does probably not interact with one RND transporter alone when it comes to enterobactin export. It cannot be completely ruled out that several RNDs together contribute to enterobactin efflux and deletion of a single system can be compensated for by the others. However, neither the double deletion of the twin RND system mdtBC (Fig. 3) nor the deletion of the gene of AcrA, the membrane fusion protein partner of AcrB and AcrD, led to diminished enterobactin excretion (data not shown). Interaction of TolC with other transporters such as the ATP-driven ABC system MacAB (16, 19, 29) or the major facilitator drug efflux system EmrAB or EmrKY appears unlikely in the light that these transporters transport their substrates directly from the cytoplasm to the outside. Therefore, we agree with McIntosh and coworkers (9) that TolC may interact with an uncharacterized MFP and predict that an unknown MFS protein as part of an MFS-MFP-TolC complex transports enterobactin from the cytoplasm to the outside.
Contrary to the prediction (9) that deletion of the other components of the enterobactin efflux system would produce a phenotype almost identical to the entS mutation, introduction of a ΔentS or a ΔtolC mutation in the genome of a mutant strain that can use only enterobactin or its derivatives for iron acquisition led to different phenotypes (Fig. 1 to 3). Since the tolC mutant was growth deficient and secreted no detectable amounts of enterobactin, EntS is probably not able to recruit a membrane fusion protein such as YhcQ or YiaY (9) to constitute an EntS-MFP-TolC tripartite transenvelope efflux system. If such a system exists, the entS and the tolC deletion mutant should exhibit a very similar phenotype, which was not the case. Thus, EntS is not identical to the hypothetical MFS protein of the suggested MFS-MFP-TolC enterobactin export pathway.
While the entS deletion strain still secreted some enterobactin, the tolC deletion strain did not. This is again evidence for the existence of a suggested MFS-MFP-TolC pathway that can compensate for a missing EntS. On the other hand it seems that TolC forms the only pathway for enterobactin to leave the periplasm to the outside. Since EntS probably transports enterobactin to the periplasm and might not interact with MFPs for further export and the suggested MFS-MFP-TolC pathway should export cytoplasmic substrates directly to the outside like other TolC-ABC-type or TolC-MFS-type efflux complexes, such as that for Mac or Emr (16, 19), do, a second route may exist for export of cytoplasmic enterobactin to the periplasm.
So far, only RND-type efflux complexes have been discussed as being able to transport substrates from the periplasm to the outside. Therefore, to explain the data presented in this study, the existence of two enterobactin export pathways should be considered: an MFS-MFP-TolC efflux system for a one-step extrusion of enterobactin from the cytoplasm directly to the outside and a two-step export via EntS to the periplasm and further on via an RND-MFP-TolC pump also to the outside (Fig. 4). A candidate for this RND protein is MdtF, since this is the only RND protein induced by iron starvation (Table 2). However, involvement of this RND system in enterobactin export can only be investigated when the identity (and existence) of the hypothetical MFS protein has been uncovered.
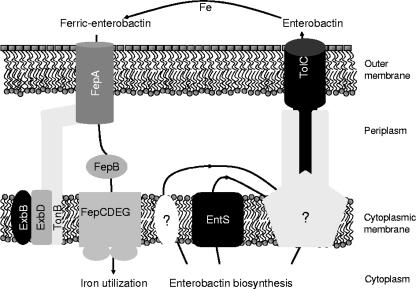
FIG. 4.
Proposed model of enterobactin transport in E. coli. After biosynthesis, enterobactin is translocated by the major facilitator EntS and to a lesser extent by other unknown mechanisms across the cytoplasmic membrane. There enterobactin is probably accepted by an efflux complex or complexes comprising the outer membrane factor TolC because for function TolC needs other transport proteins that are energized (e.g., by the proton motive force). After export by TolC, enterobactin scavenges ferric iron from the surrounding medium and is subsequently recognized and taken up as ferric enterobactin from the outer membrane receptor FepA, which is energized by the TonB/ExbB/ExbB machinery. FepB delivers ferric enterobactin from the periplasm to the ATP-driven membrane-bound ABC transporter FepCDEG. Within the cytoplasm, ferric enterobactin is degraded, aided by the Fes protein circumventing the high affinity of enterobactin toward iron, and the freed iron can be utilized.
Because the entS and the tolC mutants still secreted enterobactin breakdown products, other transporter(s) might be able to export dihydroxybenzoylserine and its di- or triester, which in turn serve as siderophores. Alternatively, the smaller degradation products might leave the cell through porins. In E. coli there are a plethora of uncharacterized genes encoding proteins of the major facilitator family present on the chromosome that could be accountable for the export of enterobactin or its degradation products across the cytoplasmic membrane.
While entS (positions 621187 to 622437 in the E. coli K-12 genome) is part of the enterobactin biosynthesis and transport gene cluster in E. coli and is thus regulated by iron deprivation (41), the gene for TolC that is carried elsewhere on the chromosome (positions 3177624 to 0.3179111 in the E. coli K-12 genome) was nonresponsive to iron availability. Previously, tolC and acrAB were identified as part of the mar-sox regulon that is involved in oxidative stress response (2, 3). On the other hand, acrD or mdtF expression is not dependent on mar-sox and was altered by the iron status of the cells. For the time being, no explanation can be given for the observation that acrD is down-regulated threefold by iron.
Taken together, identification of TolC as being necessary for enterobactin export beyond the outer membrane closed a gap in our knowledge about cellular iron acquisition by enterobactin. While the modes of uptake of ferric enterobactin and the pathways of enterobactin synthesis have been known for some time, the proteins required for enterobactin excretion had been unknown until now, and the crossing of the periplasm still is!
Acknowledgments
This work was supported by grants Ni262/3 and GR2061/1-1 of the Deutsche Forschungsgemeinschaft (DFG) and Fonds der Chemischen Industrie to D.H.N. and to G.G. Moreover, this work was part of the Graduiertenkolleg “Adaptive physiological biochemical reactions to ecologically important agents” of the DFG, which contributed funding to G.J.K, C.G., J.S., and D.H.N. HWP funds of the “Land Sachsen-Anhalt” were endowed for C.G. and D.H.N.
We thank Grit Schleuder for skillful technical assistance. Thanks are due Sylvia Franke (Tucson, Arizona) for critical reading of the manuscript.
REFERENCES
- 1.Albrecht-Gary, A. M., and A. L. Crumbliss. 1998. Coordination chemistry of siderophores: thermodynamics and kinetics of iron chelation and release. Met. Ions Biol. Syst. 35:239-327. [PubMed] [Google Scholar]
- 2.Aono, R. 1998. Improvement of organic solvent tolerance level of Escherichia coli by overexpression of stress-responsive genes. Extremophiles 2:239-248. [DOI] [PubMed] [Google Scholar]
- 3.Aono, R., N. Tsukagoshi, and M. Yamamoto. 1998. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J. Bacteriol. 180:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann, B. J. 1972. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 36:525-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chenault, S. S., and C. F. Earhart. 1991. Organization of genes encoding membrane proteins of the Escherichia coli ferrienterobactin permease. Mol. Microbiol. 5:1405-1413. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorsey, C. W., M. E. Tolmasky, J. H. Crosa, and L. A. Actis. 2003. Genetic organization of an Acinetobacter baumannii chromosomal region harbouring genes related to siderophore biosynthesis and transport. Microbiology 149:1227-1238. [DOI] [PubMed] [Google Scholar]
- 8.Franke, S., G. Grass, C. Rensing, and D. H. Nies. 2003. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J. Bacteriol. 185:3804-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furrer, J. L., D. N. Sanders, I. G. Hook-Barnard, and M. A. McIntosh. 2002. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol. Microbiol. 44:1225-1234. [DOI] [PubMed] [Google Scholar]
- 10.Grass, G., S. Franke, N. Taudte, D. H. Nies, L. M. Kucharski, M. E. Maguire, and C. Rensing. 2005. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J. Bacteriol. 187:1604-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grass, G., and C. Rensing. 2001. Genes involved in copper homeostasis in Escherichia coli. J. Bacteriol. 183:2145-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grass, G., K. Thakali, P. E. Klebba, D. Thieme, A. Müller, G. F. Wildner, and C. Rensing. 2004. Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. J. Bacteriol. 186:5826-5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groβe, C., G. Grass, A. Anton, S. Franke, A. N. Santos, B. Lawley, N. L. Brown, and D. H. Nies. 1999. Transcriptional organization of the czc heavy-metal homeostasis determinant from Alcaligenes eutrophus. J. Bacteriol. 181:2385-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins, M. K., E. Bokma, E. Koronakis, C. Hughes, and V. Koronakis. 2004. Structure of the periplasmic component of a bacterial drug efflux pump. Proc. Natl. Acad. Sci. USA 101:9994-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klebba, P. E. 2003. Three paradoxes of ferric enterobactin uptake. Front. Biosci. 8:1422-1436. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, N., K. Nishino, and A. Yamaguchi. 2001. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 183:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koronakis, V., C. Andersen, and C. Hughes. 2001. Channel-tunnels. Curr. Opin. Struct. Biol. 11:403-407. [DOI] [PubMed] [Google Scholar]
- 18.Legatzki, A., G. Grass, A. Anton, C. Rensing, and D. H. Nies. 2003. Interplay of the Czc system and two P-type ATPases in conferring metal resistance to Ralstonia metallidurans. J. Bacteriol. 185:4354-4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomovskaya, O., and K. Lewis. 1992. Emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 89:8938-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lomovskaya, O., H. I. Zgurskaya, and H. Nikaido. 2002. It takes three to tango. Nat. Biotechnol. 20:1210-1212. [DOI] [PubMed] [Google Scholar]
- 21.Makui, H., E. Roig, S. T. Cole, J. D. Helmann, P. Gros, and M. F. Cellier. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol. Microbiol. 35:1065-1078. [DOI] [PubMed] [Google Scholar]
- 22.Mergeay, M., D. Nies, H. G. Schlegel, J. Gerits, P. Charles, and F. Van Gijsegem. 1985. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munkelt, D., G. Grass, and D. H. Nies. 2004. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 186:8036-8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nies, D. H. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27:313-339. [DOI] [PubMed] [Google Scholar]
- 25.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikaido, H., M. Basina, V. Nguyen, and E. Y. Rosenberg. 1998. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those β-lactam antibiotics containing lipophilic side chains. J. Bacteriol. 180:4686-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikaido, H., and H. I. Zgurskaya. 2001. AcrAB and related multidrug efflux pumps of Escherichia coli. J. Mol. Microbiol. Biotechnol. 3:215-218. [PubMed] [Google Scholar]
- 28.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishino, K., and A. Yamaguchi. 2002. EvgA of the two-component signal transduction system modulates production of the YhiUV multidrug transporter in Escherichia coli. J. Bacteriol. 184:2319-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien, I. G., and F. Gibson. 1970. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim. Biophys. Acta 215:393-402. [DOI] [PubMed] [Google Scholar]
- 31.Papp, K. M., and M. E. Maguire. 2004. The CorA Mg2+ transporter does not transport Fe2+. J. Bacteriol. 186:7653-7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulsen, I. T., J. H. Park, P. S. Choi, and M. H. Saier, Jr. 1997. A family of gram-negative bacterial outer membrane factors that function in the export of proteins, carbohydrates, drugs and heavy metals from gram-negative bacteria. FEMS Microbiol. Lett. 156:1-8. [DOI] [PubMed] [Google Scholar]
- 33.Pollack, J. R., and J. B. Neilands. 1970. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem. Biophys. Res. Commun. 38:989-992. [DOI] [PubMed] [Google Scholar]
- 34.Poole, K., D. E. Heinrichs, and S. Neshat. 1993. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol. Microbiol. 10:529-544. [DOI] [PubMed] [Google Scholar]
- 35.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raymond, K. N., E. A. Dertz, and S. S. Kim. 2003. Enterobactin: an archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 100:3584-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rensing, C., and G. Grass. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27:197-213. [DOI] [PubMed] [Google Scholar]
- 38.Saier, M. H., Jr., R. Tam, A. Reizer, and J. Reizer. 1994. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol. Microbiol. 11:841-847. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 41.Shea, C. M., and M. A. McIntosh. 1991. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol. Microbiol. 5:1415-1428. [DOI] [PubMed] [Google Scholar]
- 42.Skare, J. T., B. M. Ahmer, C. L. Seachord, R. P. Darveau, and K. Postle. 1993. Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J. Biol. Chem. 268:16302-16308. [PubMed] [Google Scholar]
- 43.Whitney, E. N. 1971. The tolC locus in Escherichia coli K12. Genetics 67:39-53. [DOI] [PMC free article] [PubMed] [Google Scholar]