Abstract
Upon the exposure of Escherichia coli to high temperature (heat shock), cellular levels of the transcription factor σ32 rise greatly, resulting in the increased formation of the σ32 holoenzyme, which is capable of transcription initiation at heat shock promoters. Higher levels of heat shock proteins render the cell better able to cope with the effects of higher temperatures. To conduct structure-function studies on σ32 in vivo, we have carried out site-directed mutagenesis and employed a previously developed system involving σ32 expression from one plasmid and a β-galactosidase reporter gene driven by the σ32-dependent groE promoter on another in order to monitor the effects of single amino acid substitutions on σ32 activity. It was found that the recognition of the −35 region involves similar amino acid residues in regions 4.2 of E. coli σ32 and σ70. Three conserved amino acids in region 2.3 of σ32 were found to be only marginally important in determining activity in vivo. Differences between σ32 and σ70 in the effects of mutation in region 2.4 on the activities of the two sigma factors are consistent with the pronounced differences between both the amino acid sequences in this region and the recognized promoter DNA sequences.
The master regulator for the heat shock response in Escherichia coli, now usually referred to as σ32, was identified as a sigma factor over 20 years ago (11, 18). When E. coli cells are exposed to high temperatures (e.g., 42°C), the levels of σ32 first rise steeply by a variety of different mechanisms and then level off at about twice the initial level (6, 10, 25). σ32 binds to core RNA polymerase (RNAP) and directs the RNAP to the heat shock promoters, for which the consensus sequence differs from those utilized by RNAP containing the housekeeping sigma factor, σ70 (4, 10, 35, 36, 38). The difference is pronounced in the −10 region, but in the −35 region the two classes of promoters have a 4-base-pair sequence in common. In view of the homology between the two proteins in region 4.2 (see Fig. 1A), it had been postulated early on that the recognition of the −35 regions would involve similar amino acids of σ32 and σ70 (30).
FIG. 1.
Sequences of σ32 and the groE promoter. (A) CLUSTAL alignment of E. coli sigma factors σ32 and σ70. Sequences are presented in the one-letter code; identical residues are shown in bold. Numbers on the left of each lane indicate amino acid positions relative to the start of each protein sequence. Region 2.3 is thought to be involved in promoter DNA melting, region 2.4 in the recognition of the −10 promoter element, and region 4.2 in the recognition of the −35 promoter element. (B) The groE promoter recognized by holoenzyme containing σ32, used here to drive transcription of the β-galactosidase reporter gene. The −10 and −35 regions are indicated in bold lettering.
While the most evident role of σ32 is in directing the expression of genes allowing the cell to deal with the consequences of heat shock, it is an essential protein at physiological (37°C) temperatures (37). Despite its importance to the survival of the cell, few studies have addressed the roles of particular amino acids. Amino acids in region 2.1 were found to markedly affect the stability of σ32 in vivo (12); a stretch of amino acids between regions 2 and 3 of σ32 is known to participate in the binding of DnaK (21), which functions as an anti-sigma factor of σ32; and amino acids that play a role in the interaction of σ32 with the core have been identified (13, 14). In addition, random linker insertion mutagenesis has provided information concerning the roles of various regions of σ32 in determining its activity (21). Here we report the results of targeted mutagenesis of amino acids in various regions of σ32, including 4.2 and 2.4, that are involved in the recognition of the −35 and −10 elements, respectively. We demonstrate that the recognition of the −35 element indeed involves similar amino acid residues for σ32 and σ70 and identify other amino acid residues important to the proper function of σ32.
MATERIALS AND METHODS
Chemicals and enzymes.
Oligodeoxyribonucleotide primers were synthesized by Invitrogen. Materials for plasmid purification were purchased from QIAGEN. Site-directed mutagenesis was carried out using a QuikChange kit (Stratagene) according to the manufacturer's instructions. Conjugated antibodies and a chemiluminescent substrate were obtained from Pierce. Media reagents were purchased from Gibco-BRL, and other chemicals were purchased from Sigma or Fisher.
Plasmids and strains.
Plasmid pLC412 carrying N-terminally six-His-tagged σ32 was obtained from Cathy Chang and Carol Gross. The plasmid pSAKT32 (35) was used as an extrachromosomal, intracellular source of σ32. It is a derivative of pSAK15-70/32 (16), a pACYC-derived vector with a p15A origin of replication. It was modified by the insertion of an ampicillin resistance cassette into the chloramphenicol resistance gene. pSAKT32 carries the σ32 gene (without the six-His tag) under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Plac promoter as well as the lac repressor gene under the control of a mutant promoter (iq) stronger than the wild-type (wt) promoter.
pQF50KgroE (35) was used to compare the activities of wt and mutant σ32 in RNAP holoenzyme. It contains positions −47 to −9 of the σ32-dependent groE promoter (Fig. 1B), either of the wild type or with substitutions as indicated in the text, driving the expression of a β-galactosidase reporter gene. Strain BB1556 (MC4100 ΔdnaK52::Cmr) (2) was obtained from B. Bukau and M. P. Mayer. Its dnaK gene is inactivated by the insertion of DNA containing a chloramphenicol resistance cassette (22). The sidB3 mutation is a frameshift mutation in the σ32 coding region extending the N-terminal region of the protein by 38 amino acids and rendering it more sensitive to proteolysis (2). The result is a much reduced intracellular σ32 activity. To select for various constructs, ampicillin was added to the growth medium at 50 μg/ml, chloramphenicol at 30 μg/ml, and kanamycin at 15 μg/ml.
groE promoter activity driven by plasmid-encoded σ32.
pQF50KgroE and pSAKT32 were both transformed into the ΔdnaK sidB3 mutant strain BB1556; in this strain, the dnaK gene had been inactivated by cassette mutagenesis, and the σ32 protein had been rendered more labile due to a spontaneous DNA insertion in the gene (2). Cells harboring both plasmids were selected with three different antibiotics: chloramphenicol, kanamycin, and ampicillin. This served to ensure the growth of only those cells carrying the transposon that inactivates the dnaK gene by insertion and to select the pQF50KgroE (kanamycin) and pSAKT32 (ampicillin) plasmids. Cells were grown at 32°C with extensive aeration. An aliquot (250 to 350 μl) of an overnight culture grown in M9 medium supplemented with 0.2% Casamino Acids and 1% glucose was diluted in 5 ml of fresh M9 medium to an optical density at 600 nm (OD600) of 0.1, and at an OD600 of 0.3 the synthesis of σ32 was induced by the addition of IPTG to 1 mM. Cell growth was stopped 3 h later, and β-galactosidase assays were carried out as described previously (20). Each mutant was subjected to five or six independent assays.
Determination of the relative cellular levels of σ32: Western blotting.
The level of σ32 in the cells was examined by Western immunoblot analysis. Cells were grown and induced as described above. Aliquots (2 to 4 ml) containing equal numbers of cells (determined by measuring OD600s) were centrifuged to pellet the cells, which were resuspended in equal volumes (100 to 200 μl) of sodium dodecyl sulfate (SDS) buffer (62.5 mM Tris, pH 6.8; 1.75% SDS; 10% glycerol; 0.01% bromophenol blue). Equal volumes of cell lysates were electrophoresed by the method of Laemmli (17) in 12% polyacrylamide gels. Proteins were transferred from the gels to nitrocellulose membranes as described in the work of Towbin et al. (33) using a Mini Trans-Blot cell apparatus (Bio-Rad). The membranes were treated with 1% nonfat dry milk (Bio-Rad) in TTBS buffer (50 mM Tris; 150 mM NaCl; 0.1% Tween 20) for 1 h to block nonspecific binding of antibodies. They were then incubated with 1:2,500 dilutions of the anti-σ32 polyclonal antibodies (generous gifts from M. P. Mayer and B. Bukau and from C. Chan and C. Gross) overnight at 4°C, washed in TTBS buffer, and subsequently exposed for 1 h to a 1:5,000 dilution of goat anti-rabbit immunoglobulin G conjugated to alkaline phosphatase (Pierce). The membranes were washed in TTBS buffer and developed using Lumi-Phos WB substrate. The amounts of protein migrating at the position of the σ32 marker were quantified using a ChemiGenius gel documentation and analysis system (Syngene). Band intensities were corrected for the background signal exhibited by extract from cells not bearing a σ32 expression plasmid and normalized to the intensities of a low-molecular-mass E. coli protein (∼17 kDa) with which the antibody cross-reacts, in order to compensate for variations in the total amounts of cellular protein loaded.
Effect of substitutions on σ32-core interaction: coimmunoprecipitation assay.
Sigma-core interactions were monitored by selective immunoprecipitation of core RNAP with specific antibodies and subsequent Western blotting to determine the amounts of coprecipitated σ32. Cells were grown, and the expression of wt or mutated σ32 was induced as described above. For all samples, aliquots (2 to 4 ml) containing equal numbers of cells (determined by measuring OD600) were pelleted by centrifugation. The pelleted cells were subjected to several freeze-thaw cycles, and whole-cell extracts were prepared by resuspending pellets in native lysis buffer (50 mM Tris, pH 7.5; 150 mM NaCl; 1% Nonidet P-40; 0.5% sodium deoxycholate; 1 mM phenylmethylsulfonyl fluoride). Following the addition of lysozyme (0.2 mg/ml), extracts were incubated for 10 min at room temperature followed by DNase (10 μg/ml) treatment in the presence of Mg2+ (10 mM) for 30 min on ice. The lysates were cleared from cell debris by centrifugation at 14,000 rpm in a microcentrifuge for 10 min.
Protein A-agarose beads (Roche) were equilibrated in W buffer (50 mM Tris, pH 7.5; 150 mM NaCl; 0.1% Nonidet P-40). Equal volumes of monoclonal antibodies against the β and β′ subunits of E. coli RNA polymerase (Neoclone) were added to the prepared beads, which were then rotated at 4°C overnight. Following washing in W buffer, the complexes of beads and antibodies were incubated with native cell lysates in IP buffer (50 mM Tris, pH 7.5; 150 mM NaCl) with mixing at 4°C overnight. Unbound proteins were removed by washing with W buffer, while bound proteins were eluted by boiling the beads in SDS buffer. To determine the distribution between free and holoenzyme-bound σ32, the bound and unbound fractions were run on 10% SDS-polyacrylamide gel electrophoresis (PAGE) gels and analyzed by quantitative Western blotting with anti-σ32 polyclonal antibody as described in the previous section.
RESULTS AND DISCUSSION
To assess the importance of particular amino acid residues for σ32 function, a collection of variants was constructed and expressed from pSAKT32, which results in approximately physiological intracellular levels of σ32 protein upon IPTG induction (35). The selection of amino acids for substitution was based on a comparison of the σ70 and σ32 sequences (Fig. 1A) and on the available sigma factor structures (3, 19). We were also guided by the results of a study on the effects of insertional mutagenesis (21), which had left uncovered most of regions 4.2 and 2.4, shown for other sigma factors to be involved in recognizing the −35 and −10 promoter regions, respectively (9). Our study targeted the above two regions as well as others possibly involved in DNA strand separation during open complex formation (region 2.3) (3, 7, 19, 32), interaction with DNA phosphates (32), and protein-protein interaction with the α subunit of RNAP (28).
Comparison of wt and mutant σ32 protein: expression levels and core RNAP binding.
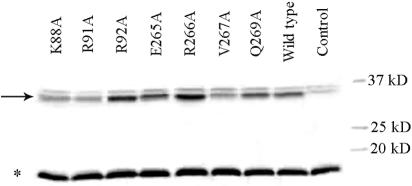
Interpretation of the in vivo activities of the mutant sigma factors requires information concerning both their relative amounts in the cell and their abilities to bind core RNAP. The relative cellular levels of σ32 mutants were determined by separating the proteins contained in cell lysates with SDS-PAGE, a procedure followed by Western blotting to identify the band corresponding to σ32 protein. An example is shown in Fig. 2. The relative amounts of σ32 (calculated as described in Materials and Methods) for the mutants shown varied from 70% of wt for V267A to 145% for R92A. To facilitate the quantitative comparison of σ32 function, all values obtained in β-galactosidase assays were first normalized to the relative amounts of σ32 and then renormalized to a β-galactosidase activity of 100% for wt σ32 (see Fig. 3). For the 12 σ32 variants displaying the lowest levels of β-galactosidase activity (under 40% of wt activity), it was checked whether the substitutions impaired the ability of σ32 to bind core RNAP, which would impede the determination of their effect on open complex formation at promoters. Towards this goal, we developed a coimmunoprecipitation assay for monitoring the interaction between σ32 and RNAP core in whole-cell lysates (see Materials and Methods).
FIG. 2.
Determination of in vivo levels of σ32. Relative levels of cellular σ32 were determined by Western blotting using polyclonal anti-σ32 antibodies as described in Materials and Methods. The position of the σ32 band is indicated with an arrow, and the band used as an internal standard (to correct for protein loading) is labeled with an asterisk. The identities of the σ32 substitutions are indicated above the lanes. In the control lane, an extract was loaded from a cell not containing a σ32 expression plasmid.
FIG. 3.
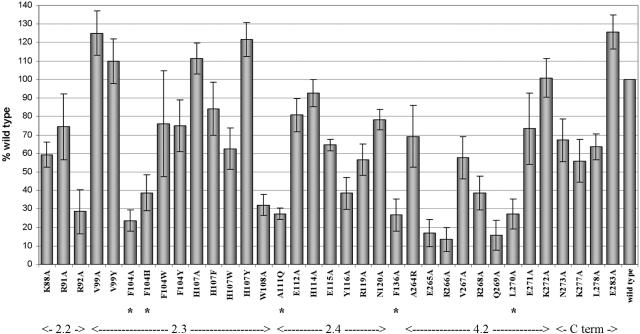
Effect of single amino acid mutation on σ32-dependent reporter gene expression. For each mutant, β-galactosidase activity was normalized to the total detectable amount of mutant σ32 protein in cell lysates relative to the amount of wt σ32, as described in Results. Then, all activities were normalized to that observed in wt cell extracts. All values are averages of five to six independent experiments, with the error bars representing the standard deviations. The functional regions of σ32 protein as deduced from the sequence are indicated. Substitutions that reduce interaction with RNAP core enzyme to less than 50% of wt σ32 (Fig. 4 and Table 1) are indicated by asterisks (see text).
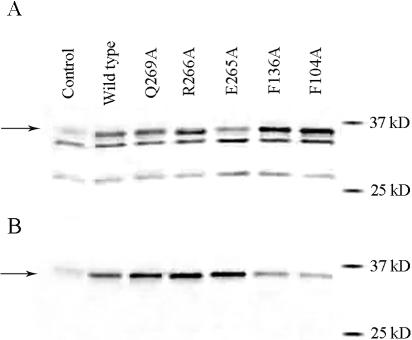
The RNAP from lysates prepared under nondenaturing conditions was first captured by a mixture of β and β′ antibodies attached to protein A-agarose beads. The latter were then separated from the rest of the solution by centrifugation. The entire pellet, subsequent to extraction by SDS-containing solution, and an aliquot from the supernatant were analyzed by SDS-PAGE and then subjected to Western probing to evaluate the fraction of σ32 bound to the core RNAP. The results of one such experiment are shown in Fig. 4A (supernatant) and 4B (pellet). In comparing the gel patterns shown in Fig. 2 and Fig. 4A, it is seen that both are doublets, presumably due to the presence of contaminating antibodies in the serum. Curiously, σ32 is the bottom band shown in Fig. 2 but the top band shown in Fig. 4A (compare with control lanes lacking the σ32 expression plasmid). These locations correlate with lysis under denaturing and nondenaturing conditions, respectively.
FIG. 4.
Determination of free and core-bound σ32 by coimmunoprecipitation. Core RNAP was captured by bead-immobilized anti-β and -β′ antibodies. SDS-PAGE gels were run on fractions containing free (A) and bead-immobilized (B) σ32 (indicated by arrows). The identities of the σ32 are shown above each lane. After transferring the proteins to nitrocellulose membranes, these were probed with polyclonal anti-σ32 antibodies to reveal the presence of σ32. See Materials and Methods for details.
For each mutant, the quantification of the amounts σ32 in the bound and free fractions enabled a calculation of the fraction of σ32 bound to core RNAP. To correct for a significant day-to-day variability in the assay (the percentage of wt σ32 in the pellet varied from 10% to 30% of the total), all values were normalized to the total amount of wt σ32 in the pellet. The thus-normalized values are shown in Table 1. The F136A substitution previously reported to weaken the RNAP-σ32 interaction (14) was included as a control. It was indeed found to reduce the amount of core-bound σ32 by 58% compared to the wt σ32 levels. Another substitution (L278A) was at a residue previously implicated in the σ32-core interaction (14). However, it had relatively high levels of reporter activity and was not further considered. Among the substitutions studied in this work, F104A, F104H, A111Q, and L270A negatively affected the ability of σ32 to bind to core RNAP, reducing the amount of bound σ32 to less than 50% of wt levels. This precluded the drawing of firm conclusions concerning the effects of the substitutions on the ability of RNAP holoenzyme containing these σ32 mutants to form transcription-competent RNAP-promoter complexes. As one substitution (R268A) had lesser effects on the RNAP-core interaction and the six others had essentially no effects thereon, decreases in β-galactosidase expression for these mutant σ32s could be interpreted as being due to defects in the formation of transcription-competent promoter complexes. In the following discussion, the results of mutagenesis in the various regions of σ32, summarized in Fig. 3, will be treated separately.
TABLE 1.
Effects of substitutions in σ32 on its ability to bind core RNAP
| Substitution in σ32 | Bound σ32 (% of wt)a |
|---|---|
| None (wt) | 100 |
| R92A | 166 ± 16 |
| F104A | 20 ± 8 |
| F104H | 20 ± 3 |
| W108A | 92 ± 6 |
| A111Q | 42 ± 6 |
| Y116A | 115 ± 12 |
| F136A | 42 ± 2 |
| E265A | 170 ± 15 |
| R266A | 89 ± 27 |
| R268A | 62 ± 2 |
| Q269A | 102 |
| L270A | 33 ± 4 |
All values have been normalized to the extent bound for wt σ32 (10% to 30% of total detectable σ32 in different experiments). Averages of duplicates are shown ± half the spread; values of the duplicates were the same for Q269A.
Substitutions in region 4.2.
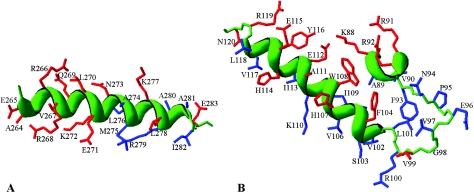
Four of the nine substitutions in region 4.2 had pronounced effects. Based on the model proposed by Siegele et al. (30), it was expected that the recognitions of similar −35 sequences (TTGA) in σ70- and σ32-dependent promoters would involve similar amino acid residues as well. This hypothesis was borne out: the data in Fig. 3 show that substitutions E265A, R266A, and Q269A reduce β-galactosidase expression to less than 20% of wt levels (the L270A substitution is ignored due to its core-binding defect). Substitutions of three other amino acids (V267, R268, and E271) had less drastic effects on groE promoter activity (the A264R substitution is a special case and is discussed below). Such a pattern is consistent with the α-helical structure shown in Fig. 5A, where the amino acid sequence of part of σ32 region 4.2 is superimposed on the structure of the homologous region of the Thermus aquaticus RNAP (3). The amino acid residues at which we have introduced substitutions are shown in red. It is seen that the substitutions for residues on the top face of the helix, as shown in the figure, had the greatest effect. This is the side of closest approach to the DNA in the T. aquaticus structure (3), where residues identical in sequence to E265, R266, and Q269 are seen to participate in contacting the −35 sequence; in E. coli σ70, the homologous amino acids (E585, R586, and Q589) are envisaged to be involved in −35 recognition as well.
FIG. 5.
Structural models for σ32 residues 264 to 283 of region 4.2 (A) and for residues 88 to 120 of regions 2.2 to 2.4 (B). These models were rendered using DeepView-Swiss PDB Viewer version 3.7 by superimposing the sequences of E. coli σ32 onto the experimentally determined structures of T. aquaticus σA (3), as indicated on the figures. The justification for this simplified approach is derived from the observation that structure is remarkably well conserved among the σ70-like sigma factors. The side chains for which substitutions were introduced are colored red, and other residues are blue. The protein backbone is green, and for α-helices it is shown as a green ribbon.
Identification of the contact between a region 4.2 amino acid and a −35 base pair.
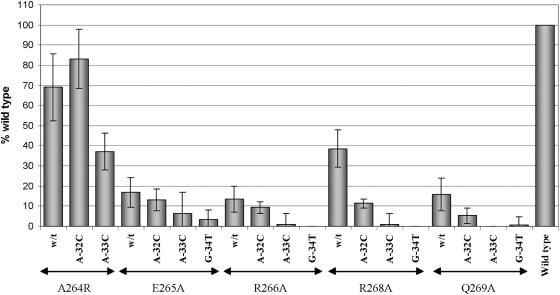
In an attempt to identify interacting partners, we have searched for substitutions in σ32 that either reduced the specificity of promoter recognition, as evidenced by indifference to substitutions at particular positions in the promoter DNA, or altered the specificity, resulting in improved recognition of particular mutant promoters by mutant sigma factors compared to that by wt σ32 (see Fig. 6). By identifying a mutation that affected specificity, we would be able to assign the particular base pair of the −35 sequence with which the substituted residue in σ32 interacts (e.g., references 30, 34, and 39). Of the five σ32 mutants tested, one (A264R) was found to exhibit such behavior. The A264R substitution reduced the activity of σ32 with the wt promoter to only 70% that of the wt σ32, despite the fact that the substituted arginine side chain was much larger than the alanine it replaced. The A-32C promoter substitution had previously been shown to reduce the activity seen with wt σ32 to 65% of that obtained with the wt promoter (35). However, when combined, the A264R substitution in σ32 and the A-32C substitution in the promoter resulted in an activity of 83% of that seen with wt σ32 and the promoter (Fig. 6), even though the expected activity if the sigma factor and promoter mutations were acting independently would be 46% of that of wt (70% of 65%). No such rescue was seen with the A264R σ32 for the A-33C promoter substitution or with the R268A and Q269A σ32 for the A-32C promoter variant, while the very low activity of sigma factors with the E265A and R266A substitutions precluded their evaluation in this regard (Fig. 6).
FIG. 6.
Search for altered specificity mutations in region 4.2 of σ32 protein. BB1556 (DnaK−) cells were cotransformed with the pSAKT32 and pQF50KgroE plasmids bearing, respectively, the gene encoding wt σ32 or that with the indicated substitutions in region 4.2 and the wt groE promoter or that with the indicated substitutions in the −35 promoter element. β-Galactosidase activities were normalized to those of cells with wt RNAP and wt promoter as described in the text. All values are averages of five to six independent experiments, with the error bars representing the standard deviations.
Our results are consistent with A264 of wt σ32 being a surface residue (Fig. 5A) interacting with the methyl group of −32T on the template strand, as proposed over 15 years ago by Siegele et al. (30). The substituted arginine side chain sticks out into the solution, but its β-CH2 group still might be able to interact with the −32T. The A264R substitution likely changes the specificity due to the arginine interacting with the −32G on the template strand of the A-32C promoter, resulting in the observed second site suppression: the mutant −35 sequence on the template strand is 5′GTCAAG3′, while that on the wt is 5′TTCAAG3′ (the −32 position is underlined; also refer to Fig. 1B). Our use of the A264R substitution constitutes the inverse of the recent demonstration that an alanine substitution at R584 of σ70 (homologous to A264 of σ32) alters the specificity (8), leading to the recognition of a mutant −35 region with the sequence 5′TTTCAA3′ rather than of the canonical 5′TGTCAA3′ on the template strand.
Substitutions in region 2.4.
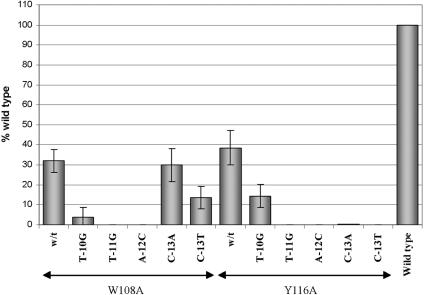
Seven residues in the stretch from A111 to N120 of region 2.4 of σ32 were replaced based on homology and structural considerations (red side chains in Fig. 5B). Three substitutions, A111Q, H114A, and E115A, were for residues homologous to those of T. aquaticus σA and E. coli σ70, which are known to protrude from the surface of the sigma factor (3, 19). In σ70, two of these (Q437 and T440, homologous to σ32 A111 and H114) are thought to participate in the recognition of the most-upstream T-A base pair of the −10 element (9). The A111Q substitution resulted in a core-binding defect (Table 1) and will not further be considered. For H114A, the expression of β-galactosidase was reduced by less than 10% compared to that of wt σ32. Somewhat larger effects were obtained for the E115A, Y116A, and R119A substitutions (with 65% or less of the wt activity). By analogy to the studies carried out with substitutions in region 4.2 of σ32 (see above), we also searched for possible substitutions in region 2.4 that would affect σ32 specificity. We tested the Y116A σ32 for (partial) rescue of promoters with the T-10G, T-11G, A-12C, C-13A and C-13T substitutions in the upstream region of the −10 element (see Fig. 7) as well as with C-14A, C-14T, C-15A, C-15T, C-16A, C-16T, A-18T, and A-18C in the downstream region. No evidence for such rescue was observed (Fig. 7 and data not shown). Recognition of the −10 regions by σ32 and σ70 residues appears to involve nonhomologous residues of the two sigma factors. The latter was not surprising in view of the striking sequence differences between the −10 regions utilized by the two sigma factors (TATAAT and GNCCCCATNT for σ70 and σ32, respectively).
FIG. 7.
Search for altered specificity mutations in region 2 of σ32 protein. BB1556 (DnaK−) cells were cotransformed with the pSAKT32 and pQF50KgroE plasmids bearing, respectively, the gene encoding wt σ32 or that with the indicated substitutions in region 2 and the wt groE promoter or that with the indicated substitutions in the −10 promoter element. β-Galactosidase activities were normalized to those of cells with wt RNAP and wt promoter as described in the text. All values are averages of five to six independent experiments, with the error bars representing the standard deviations.
Identification of a contact between a region 2.3/2.4 amino acid and a −10 base pair.
Residue W108 is at the boundary between regions 2.3 and 2.4 of σ32, occupying the same face of the helix as A111, H114, and E115 (Fig. 5B). Recognition of the −10 region of the σ32 promoter by W108 was demonstrated by the identification of a substitution that leads to a reduced specificity (Fig. 7). In agreement with our previous results for wt σ32 (35), the promoter mutation C-13A in the −10 region reduced activity to about 30% of that of the wt promoter. The combination of the C-13A promoter mutation and the W108A substitution in σ32 also showed an activity of 30% of that of wt, while just 10% would be expected if the two substitutions acted independently. These expectations may actually be overestimated, as shown with the Y116A mutant of σ32, where the combination of promoter and σ32 mutations leads to much lower observed activity levels. The observation that the W108A substitution in σ32 renders the RNA polymerase impervious to the presence of the C-13A promoter mutation (i.e., a reduced rather than an altered specificity) is consistent with the recognition of the −13 C-G base pair by the W108 residue. It is unclear why the C-13T promoter substitution is tolerated less well by the W108A σ32.
Substitutions in region 2.3/2.2.
A large body of data indicates that aromatic residues in region 2.3 of σ70 are important for the initiation of DNA melting that ultimately results in the strand separation of 12 to 14 base pairs of promoter DNA. Interestingly, region 2.3 of E. coli σ70 has seven aromatic residues, but the homologous region of σ32 has only three. To compare the strand separation processes orchestrated by the two different sigma factors, we have targeted for mutagenesis four residues in region 2.3 previously shown to be important for DNA melting in both E. coli σ70 and Bacillus subtilis σA (7, 15, 32). These are (for σ32/σ70) V99/Y425, F104/Y430, H107/W433, and W108/W434 (discussed above). Residues homologous to E. coli Y430 and W433 have also been demonstrated to be important for DNA melting in T. aquaticus σA (29). In both the E. coli σ70 (19) and the T. aquaticus σA structures (3), these residues are seen to occupy the same face on the surface of the sigma factor, poised to interact with promoter DNA, at or near the −10 region (Fig. 5B).
The F104A and F104H substitutions greatly reduced σ32 activity (Fig. 3), but no firm conclusions concerning the roles of these amino acid residues could be drawn, as the resulting mutants, surprisingly, were found to display notable core RNAP-binding defects (Fig. 4 and Table 1) not seen with the homologous σ70 substitutions. It is possible that this finding is an artifact of the coimmunoprecipitation assay for σ32-core RNAP interaction used here. We believe that this is unlikely, as not only the duplicates but also the values for the two different substitutions were in very good agreement (see Table 1). On the other hand, the F104W and F104Y substitutions both resulted in σ32 with over 75% of wt activity. Apparently, an aromatic group at position 104 is sufficient for retaining the structural integrity and activity of σ32. The H107A substitution in σ32 did not reduce activity in the β-galactosidase assay, while the σ32s with H107F and H107W substitutions had 83% and 62% of the wt activity, respectively, indicating that under our conditions the histidine at position 107 was not critical. Even so, this histidine is highly conserved in heat shock sigma factors. It is possible that the presence of a histidine at position 107 is crucial only under particular conditions of stress and/or that the H107 might be involved in the specific recognition of an as-yet-unknown subset of promoter sequences.
W108, the σ32 residue shown above to recognize the base pair at position −13, might additionally be involved in promoter DNA melting, although our data do not allow separate assessments of these two functions. However, the homologous tryptophans of E. coli σ70 (434) and B. subtilis σA (193) likely are involved in both promoter recognition and DNA melting (1, 15). σ32s bearing the V99A and V99Y substitutions had 125% and 110% of the wt activity, respectively, indicating that under our conditions V99 was not important to strand separation of promoter DNA in vivo. The Y425A substitution in σ70 (homologous to σ32 V99) reduces in vivo activity by over 50%, while the effect of substitution at residue W433 of σ70, which reduced activity by 75% (23), is also greater than that of the substitution at the homologous σ32 residue (H107). The in vivo effects of substitutions in region 2.3 of B. subtilis σA are also considerably greater than those of substitutions for the homologous amino acids in σ32 (24). Interestingly, the effects of peptide insertions in the region of σ32 immediately N terminal to residues V106 and H107 (21) were also found to be more deleterious than those observed here with substitutions for H107.
For σ70, two basic amino acids, one in region 2.3 (K418) and the other in region 2.2 (K414), had been shown to be important for open complex formation by the RNAP holoenzyme (32). Indeed, we found that of the homologous residues (K92 and R88, respectively), R92 especially was important to σ32 function: R92A had less than 30% of wt activity, and K88A had about 60% (Fig. 3). Thus, these basic residues that likely stabilize the holoenzyme-promoter DNA complex by interactions with DNA phosphates may play similar roles in functional promoter complex formation by holoenzymes containing σ70 and σ32. Alanine substitution for an additional basic amino acid (R91A) had a relatively small effect (74% of wt activity).
Substitutions of amino acids possibly involved in contacting the α subunit.
Some promoters recognized by σ70-containing holoenzymes (e.g., rRNA promoters) have an A+T-rich element upstream of the −35 region (the UP element) that is recognized by one or both of the α subunits of RNAP (26). It is thought that the α subunits interacting with the UP element are able to activate the DNA-melting function of the σ70 subunit through protein-protein interactions. While some σ32 promoters, including the groE promoter, have A+T regions upstream of the −35 element, we have not been able to demonstrate a favorable effect of the presence of such regions on the activity of σ32-driven transcription (Y. Wang and P. L. deHaseth, data not shown). To further investigate this issue, we targeted three residues (N273, K277, and E283) homologous to σ70 residues K593, K597, and R603, respectively, which had been demonstrated to interact with residues of the α subunit of RNAP under some conditions (28). Two of the substitutions were shown to have modest effects on RNAP function: σ32 bearing the N273A and K277A substitutions displayed 67% and 56% of wt activity, respectively, and σ32 containing the E283A substitution was no worse than wt. While the groE test promoter used for these studies did not include the promoter's A+T-rich upstream region, it has been convincingly demonstrated that nonspecific interactions between the α subunits and DNA will also lead to the stimulation of open complex formation (5, 27, 31). The relatively small effects of substitutions in the putative region of σ32 that would interact with the α subunit may be due to the fact that with the promoters used here, the proper positioning of the α subunits for interaction with the σ subunit had to be accomplished through such nonspecific interactions only.
Conclusions.
The results of the mutagenesis study presented here demonstrate that the recognition of DNA sequences in the −35 region involves similar amino acid residues in regions 4.2 of E. coli σ32 and σ70, confirming a previously proposed model. There are clear differences between the amino acid sequences of the two proteins in region 2.3, likely reflecting differences in function. Three amino acids of σ32 in region 2.3 homologous to conserved and functionally important amino acids in σ70 were demonstrated to be only marginally important in determining activity. Pronounced differences are apparent between σ32 and σ70 in region 2.4 and between the −10 promoter regions recognized by RNAP containing the two sigma factors. Correspondingly notable differences have been observed in the effects of substituting homologous amino acids in region 2.4 on the activities of σ32 and σ70. These results were strengthened by the demonstration of a specific contact between the RNAP and the promoter DNA in both the −10 and −35 regions through suppression of promoter DNA mutations by substitutions in σ32.
Acknowledgments
This work was supported by NIH grant GM 31808 to P.L.D.
We thank W. Mayer and B. Bukau as well as C. Gross and C. Chan for generous gifts of anti-σ32 serum, and we thank Lisa Schroeder for critically reading the manuscript.
REFERENCES
- 1.Aiyar, S. E., Y.-L. Juang, J. D. Helmann, and P. L. deHaseth. 1994. Mutations in sigma factor that affect the temperature dependence of transcription from a promoter, but not from a mismatch bubble in double-stranded DNA. Biochemistry 33:11501-11507. [DOI] [PubMed] [Google Scholar]
- 2.Bukau, B., and G. C. Walker. 1990. Mutations altering heat shock specific subunit of RNA polymerase suppress major cellular defects of E. coli mutants lacking DnaK chaperone. EMBO J. 9:4027-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell, E. A., O. Muzzin, M. Chlenov, J. L. Sun, C. A. Olson, O. Weinman, M. L. Trester-Zedlitz, and S. A. Darst. 2002. Structure of the bacterial RNA polymerase promoter specificity s subunit. Mol. Cell 9:527-539. [DOI] [PubMed] [Google Scholar]
- 4.Cowing, D. W., J. C. A. Bardwell, E. A. Craig, C. Woolford, R. W. Hendrix, and C. A. Gross. 1985. Consensus sequence for Escherichia coli heat shock gene promoters. Proc. Natl. Acad. Sci. USA 82:2679-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, C. A., M. W. Capp, M. T. Record, Jr., and R. M. Saecker. 2005. The effects of upstream DNA on open complex formation by Escherichia coli RNA polymerase. Proc. Natl. Acad. Sci. USA 102:285-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson, J. W., V. Vaughn, W. A. Walter, F. C. Neidhardt, and C. A. Gross. 1987. Regulation of the promoters and transcripts of rpoH, the Escherichia coli heat shock regulatory gene. Genes Dev. 1:419-432. [DOI] [PubMed] [Google Scholar]
- 7.Fenton, M. S., S. J. Lee, and J. D. Gralla. 2000. Escherichia coli promoter opening and −10 recognition: mutational analysis of σ70. EMBO J. 19:1130-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregory, B. D., B. E. Nickels, S. A. Darst, and A. Hochschild. 2005. An altered-specificity DNA-binding mutant of Escherichia coli σ70 facilitates the analysis of σ70 in vivo. Mol. Microbiol. 56:1208-1219. [DOI] [PubMed] [Google Scholar]
- 9.Gross, C., C. Chan, A. Dombroski, T. Gruber, M. Sharp, J. Tupy, and B. A. Young. 1998. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harbor Symp. Quant. Biol. 63:141-155. [DOI] [PubMed] [Google Scholar]
- 10.Gross, C. A. 1996. Function and regulation of the heat shock proteins, p. 1382-1399. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 11.Grossman, A. D., J. W. Erickson, and C. A. Gross. 1984. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell 38:383-390. [DOI] [PubMed] [Google Scholar]
- 12.Horikoshi, M., T. Yura, S. Tsuchimoto, Y. Fukumori, and M. Kanemore. 2004. Conserved region 2.1 of Escherichia coli heat shock transcription factor σ32 is required for modulating both metabolic stability and transcriptional activity. J. Bacteriol. 186:7474-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo, D. M., N. Ng, and R. Calendar. 1997. A σ32 mutant with a single amino acid change in the highly conserved region 2.2 exhibits reduced core RNA polymerase affinity. Proc. Natl. Acad. Sci. USA 94:4907-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joo, D. M., A. Nolte, R. Calendar, Y. N. Zhou, and D. J. Jin. 1998. Multiple regions on the Escherichia coli heat shock transcription factor σ32 determine core RNA polymerase binding specificity. J. Bacteriol. 180:1095-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juang, Y.-L., and J. D. Helmann. 1994. A promoter melting region in the primary sigma factor of Bacillus subtilis: identification of functionally important aromatic amino acids. J. Mol. Biol. 235:1470-1488. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, A., B. Grimes, M. Logan, S. Wedgwood, H. Williamson, and R. S. Hayward. 1995. A hybrid sigma subunit directs RNA polymerase to a hybrid promoter in Escherichia coli. J. Mol. Biol. 246:563-571. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Landick, R., V. Vaughn, E. T. Lau, R. A. VanBogelen, J. W. Erickson, and F. C. Neidhardt. 1984. Nucleotide sequence of the heat shock regulatory gene of E. coli suggests its protein product may be a transcription factor. Cell 38:175-182. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra, A., E. Severinova, and S. A. Darst. 1996. Crystal structure of a σ70 subunit fragment from E. coli RNA polymerase. Cell 87:127-136. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 21.Narberhaus, F., and S. Balsinger. 2003. Structure-function studies of Escherichia coli RpoH (σ32) by in vitro linker insertion mutagenesis. J. Bacteriol. 185:2731-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paek, K.-H., and G. C. Walker. 1987. Escherichia coli dnaK null mutants are inviable at high temperature. J. Bacteriol. 169:283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panaghie, G., S. E. Aiyar, K. L. Bobb, R. S. Hayward, and P. L. deHaseth. 2000. Aromatic amino acids in region 2.3 of Escherichia coli sigma 70 participate collectively in the formation of an RNA polymerase-promoter open complex. J. Mol. Biol. 299:1217-1230. [DOI] [PubMed] [Google Scholar]
- 24.Rong, J. C., and J. D. Helmann. 1994. Genetic and physiological studies of Bacillus subtilis sigma A mutants defective in promoter melting. J. Bacteriol. 176:5218-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen, R., and E. Z. Ron. 2002. Proteome analysis in the study of the bacterial heat-shock response. Mass Spectrom. Rev. 21:244-265. [DOI] [PubMed] [Google Scholar]
- 26.Ross, W., K. K. Gosink, J. Salomon, K. Igarashi, C. Zou, A. Ishihama, K. Severinov, and R. L. Gourse. 1993. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science 262:1407-1413. [DOI] [PubMed] [Google Scholar]
- 27.Ross, W., and R. L. Gourse. 2005. Sequence-independent upstream DNA-aCTD interactions strongly stimulate Escherichia coli RNA polymerase-lacUV5 promoter association. Proc. Natl. Acad. Sci. USA 102:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross, W., D. A. Schneider, B. J. Paul, A. Mertens, and R. L. Gourse. 2003. An intersubunit contact stimulating transcription initiation by E. coli RNA polymerase: interaction of the C-terminal domain and sigma region 4. Genes Dev. 17:1293-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroeder, L. A., and P. L. deHaseth. 2005. Mechanistic differences in promoter DNA melting by Thermus aquaticus and Escherichia coli RNA polymerases. J. Biol. Chem. 280:17422-17429. [DOI] [PubMed] [Google Scholar]
- 30.Siegele, D. A., J. C. Hu, W. A. Walter, and C. A. Gross. 1989. Altered promoter recognition by mutant forms of the σ70 subunit of Escherichia coli RNA polymerase. J. Mol. Biol. 206:591-603. [DOI] [PubMed] [Google Scholar]
- 31.Tang, Y., K. Murakami, A. Ishihama, and P. L. deHaseth. 1996. Upstream interactions at the lambda pRM promoter are sequence nonspecific and activate the promoter to a lesser extent than an introduced UP element of an rRNA promoter. J. Bacteriol. 178:6945-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomsic, M., L. Tsujikawa, G. Panaghie, Y. Wang, J. Azok, and P. L. deHaseth. 2001. Different roles for basic and aromatic amino acids in conserved region 2 of Escherichia coli σ70 in the nucleation and maintenance of the single-stranded DNA bubble in open RNA polymerase-promoter complexes. J. Biol. Chem. 276:31891-31896. [DOI] [PubMed] [Google Scholar]
- 33.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waldburger, C., T. Gardella, R. Wong, and M. M. Susskind. 1990. Changes in conserved region 2 of Escherichia coli sigma 70 affecting promoter recognition. J. Mol. Biol. 215:267-276. [DOI] [PubMed] [Google Scholar]
- 35.Wang, Y., and P. L. deHaseth. 2003. Sigma 32-dependent promoter activity in vivo: sequence determinants of the groE promoter. J. Bacteriol. 185:5800-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yura, T., H. Nagai, and H. Mori. 1993. Regulation of the heat-shock response in bacteria. Annu. Rev. Biochem. 47:321-350. [DOI] [PubMed] [Google Scholar]
- 37.Yura, T., T. Tobe, K. Ito, and T. Osawa. 1984. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc. Natl. Acad. Sci. USA 81:6803-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao, K., M. Liu, and R. R. Burgess. 2005. The global transcriptional response of Escherichia coli to induced σ32 protein involves σ32 regulon activation followed by inactivation and degradation of σ32 in vivo. J. Biol. Chem. 280:17758-17768. [DOI] [PubMed] [Google Scholar]
- 39.Zuber, P., J. Healy, L. Carter III, S. Cutting, C. P. Moran, and R. Losick. 1989. Mutation changing the specificity of an RNA polymerase sigma factor. J. Mol. Biol. 206:605-614. [DOI] [PubMed] [Google Scholar]