Abstract
Group A streptococcus (GAS) is a leading cause of severe, invasive human infections, including necrotizing fasciitis and toxic shock syndrome. An important element of the mammalian innate defense system against invasive bacterial infections such as GAS is the production of antimicrobial peptides (AMPs) such as cathelicidins. In this study, we identify a specific GAS phenotype that confers resistance to host AMPs. Allelic replacement of the dltA gene encoding d-alanine-d-alanyl carrier protein ligase in an invasive serotype M1 GAS isolate led to loss of teichoic acid d-alanylation and an increase in net negative charge on the bacterial surface. Compared to the wild-type (WT) parent strain, the GAS ΔdltA mutant exhibited increased susceptibility to AMP and lysozyme killing and to acidic pH. While phagocytic uptake of WT and ΔdltA mutants by human neutrophils was equivalent, neutrophil-mediated killing of the ΔdltA strain was greatly accelerated. Furthermore, we observed the ΔdltA mutant to be diminished in its ability to adhere to and invade cultured human pharyngeal epithelial cells, a likely proximal step in the pathogenesis of invasive infection. Thus, teichoic acid d-alanylation may contribute in multiple ways to the propensity of invasive GAS to bypass mucosal defenses and produce systemic infection.
Group A streptococcus (GAS) is a leading cause of superficial bacterial infections such as pharyngitis (“strep throat”) and impetigo. In the last two decades, GAS has been increasingly associated with severe invasive diseases, including necrotizing fasciitis (NF) and toxic shock syndrome (TSS) (42, 46), revealing a propensity of the pathogen to bypass host mucosal defense barriers. One key component of mammalian innate immunity that contributes to defense against invasive bacterial infection is epithelial and leukocyte production of antimicrobial peptides (AMPs) such as cathelicidins and defensins (30, 34). The specific means by which invasive GAS isolates avoid AMP killing to produce serious infections are not well understood.
A number of bacterial pathogens exhibit intrinsic resistance to mammalian AMPs (33, 38). Because of the cationic nature of most AMPs, electrostatic attraction to the negatively charged bacterial cell envelope is hypothesized to represent a critical early event in the bactericidal process (11). Consequently, modifications to bacterial cell surface constituents to incorporate positively charged residues can decrease the affinity of AMPs to reach their cell wall target of action and afford the organism relative protection. Documented examples of this phenomenon include addition of l-lysine to phosphotidylglycerol (Staphylococcus aureus), modification of lipopolysaccharide with aminoarabinose (e.g., Salmonella enterica), or d-alanylation of lipoteichoic acid polymers (e.g., Listeria monocytogenes) (1, 17, 23).
A component of most gram-positive bacterial cell walls, lipoteichoic acid (LTA) is an amphiphilic polymer bound to the cytoplasmic membrane through its glycolipid anchor (14). LTA may be involved in processes such as the control of cell shape, autolytic enzyme activity, and maintenance of cation homeostasis (32). In GAS, LTA contributes to pharyngeal epithelial cell attachment (3, 44). Classical biochemical and enzymologic studies indicated that GAS could incorporate d-alanine into its membrane-bound LTA through a two-step process involving a d-alanine-activating enzyme and d-alanine-d-alanyl carrier protein ligase (5). Genome sequencing (12) now reveals that GAS harbors a genetic locus with homology to the four-gene d-alanyl-LTA (dltABCD) operons first described in the model gram-positive organisms Lactobacillus casei and Bacillus subtilis (18, 37).
In this study, we apply targeted mutagenesis to a serotype M1T1 isolate from a patient with NF and TSS to determine the potential contribution(s) of GAS teichoic acid d-alanylation to (i) cationic AMP resistance, (i) impairment of human neutrophil killing, and (i) interaction with human pharyngeal epithelial cells.
MATERIALS AND METHODS
Bacteria and growth conditions.
M1T1 GAS wild-type (WT) strain 5448 is an isolate from a patient with NF and TSS that is genetically representative of a globally disseminated clone associated with invasive GAS infections (21). GAS cells were grown in Todd-Hewitt broth (THB), pH 7.5, or on THB agar plates (THA). For antibiotic selection, 10 μg/ml erythromycin (Em) or 3 μg/ml chloramphenicol (Cm) was used. Escherichia coli strains were grown in Luria-Bertani broth (LB); antibiotic selection employed 500 μg/ml Em or 50 μg/ml Cm. For functional assays, unless otherwise noted, bacteria were grown to early exponential phase in THB, washed three times with pyrogen-free phosphate-buffered saline (PBS), resuspended in appropriate buffers, and adjusted to the desired concentrations using a spectrophotometric method confirmed by pour-plate colony counts.
Allelic replacement of the GAS dltA gene and complementation vector construction.
Precise, in-frame allelic replacement of the GAS dltA gene with the chloramphenicol acetyltransferase gene (cat) was performed using a modification of our established methods (20) and sequence information from the published GAS M1 genome (12). PCR and the primers dltA-upstream-F (5′-GCTCTAGAGCGCAAGAGGCAGCTGAATTAC-3′) and dltA-start-R (5′-CGGTGGTATATCCAGTGATTTTTTTCTCCATCAGTCAATCTMCCTAAAATTCATTAT-3′) were used to amplify an ∼500-bp sequence immediately upstream of dltA; ∼500 bp immediately downstream of dltA were amplified with the primers dltA-end-F (5′-TGAGTGGCAGGGCGGGGCGTAAGATATTAAAACTTTGATTAATGAGGTCAAT-3′) and dltA-downstream-R (5′-CGGGATCCCGATGGGGCCACTTGAGAAAGT-3′). The dltA-start-R and dltA-end-F primers were constructed with 25-bp 5′ extensions corresponding to the 5′ and 3′ ends of the cat gene, respectively. In a fusion PCR the upstream and downstream PCR products were then combined with a 650-bp amplicon of the complete cat gene (amplified from pACYC184) using primers dltA-upstream-F and dltA-downstream-R. The resultant amplicon, containing an in-frame substitution of dltA with cat, was subcloned into the temperature-sensitive vector pHY304, yielding targeting plasmid pΔdltA. Subsequent steps in transformation and mutagenesis were performed exactly as described previously (20). The precise in-frame, allelic replacement of dltA with cat in mutant 5448ΔdltA, from the ATG start codon to the native stop codon, was confirmed by PCR. For complementation analysis, dltA plus flanking DNA was PCR amplified from the WT 5448 chromosome using dltA-upstream-F and dltA-downstream-R; the PCR product was cloned directionally into the expression vector pDCerm (20), yielding plasmid pdltA.
d-alanine quantification of teichoic acids by high-performance liquid chromatography (HPLC).
Cells were grown overnight without agitation at 30°C in Hogg and Jago broth (19) containing 3% tryptone, 1% yeast extract, 0.2% beef extract, 0.5% lactose, 0.5% KH2PO4 supplemented with 0.4% sorbitol, and 1% glucose, final pH 6.5. Cells adjusted to an optical density at 578 nm (OD578) of 20 were heat inactivated (10 min, 99°C) and used for a mild alkaline hydrolysis carried out at 37°C for 1 h in 0.1 M NaOH. Supernatant was neutralized, three-fourths dried under vacuum, and used for precolumn derivatization with Marfey's reagent (1-fluoro-2,4-dinitrophenyl-5-l-alanine amide; Sigma) as described previously (22). Marfey's reagent reacts with the optical isomers of amino acids to form diastereomeric N-aryl derivatives that can be separated by HPLC. The separation of the amino acid derivates (detection at 340 nm) was accomplished using a C18 reversed-phase column (Hypersil ODS; column diameter = 3 mm, length = 125 mm; Bischoff Chromatography, Leonberg, Germany) on a Beckman Coulter HPLC system at 30°C with a flow rate of 1 ml per min by linear gradient elution from 0 to 50% acetonitrile in sodium acetate buffer (20 mM, pH 4) in 10 min followed by a 3-min isocratic elution at 50% acetonitrile in sodium acetate buffer (20 mM, pH 4). The d-Ala derivatives showed a linear relationship between the amount injected and the peak area in the range of 50 to 1,000 pmol.
Cytochrome c binding assay.
Bacteria were grown to early exponential phase, washed twice with morpholinepropanesulfonic acid (MOPS) buffer (20 mM, pH 7), adjusted to a final A600 of 7 in MOPS buffer plus 0.5 mg/ml cytochrome c (Sigma-Aldrich, St. Louis, MO), and incubated at room temperature. As a control MOPS buffer, 0.5 mg/ml cytochrome c was incubated under the same conditions without bacteria. After 10 min, bacteria were removed by centrifugation (21,000 × g, 3 min) and the cytochrome c content of the supernatants was quantified photometrically at 530 nm, the absorption maximum of the prosthetic group.
GAS phenotypic analyses.
The β-hemolytic phenotype reflecting streptolysin S (SLS) expression was determined by plating 10 μl of exponential phase GAS on THA plus 5% sheep's blood for a 24-h incubation at 37°C. M protein dot blot analysis on whole GAS cells was performed with antibodies to the N-terminal domain of M protein as described previously (21), with an isogenic M protein-deficient mutant (26) used as a control. The amount of mature cysteine protease SpeB secreted by each isolate was determined by dot blot developed with anti-SpeB antibodies, while the functional proteolytic activity of SpeB was measured using the EnzCheck protease assay kit (Molecular Probes) as described previously (21).
Autolysis assay.
The water-induced autolysis assay was performed as previously described (39), with some modifications. Briefly, cells grown to the mid-exponential phase in Hogg and Jago broth supplemented with 0.4% sorbitol and 1% glucose were harvested and washed twice with sodium phosphate buffer (10 mM, pH 7.0). Autolysis was induced by washing the cells with ice-cold double-distilled water. Cells were resuspended in sodium phosphate buffer (10 mM, pH 7.0) containing Triton X-100 (0.05%). The decrease of the absorbance at 600 nm (A600) was monitored at 30°C for 4 h, with the initial A600 set to be 100%. The assay was repeated three times with similar results.
pH sensitivity studies.
The pH sensitivities of WT and ΔdltA GAS were determined by comparing their ability to survive in sodium phosphate buffer (20 mM Na2HPO4/NaH2PO4 plus 1 mM MgCl2 plus 10 mM l-arginine) with pHs ranging from 4 to 7. The latter series of experiments was performed essentially as described previously (9). Briefly, the bacteria were grown to exponential phase and harvested by centrifugation, adjusted to 1 × 109 CFU/ml in phosphate buffer (pH 7.0), and then diluted 1:100 into phosphate buffer at the specified pH. Aliquots were removed at specified time points, and dilutions were plated on THA to enumerate surviving CFU.
Antimicrobial peptide, lysozyme, and hydrogen peroxide sensitivity.
The murine cathelicidin mCRAMP was synthesized and purified (>99%) by the Louisiana State University Protein Facility. Polymyxin B and chicken egg white lysozyme were purchased from Sigma, and hydrogen peroxide was obtained from Fisher Scientific. In sterile 96-well microtiter plates, logarithmic-phase GAS cells were adjusted to 105 CFU/ml in 100 μl THB containing one of the following antimicrobial compounds in serial dilutions: mCRAMP (0 to 32 μM), polymyxin B (0 to 256 μg/ml), lysozyme (0 to 12 mg/ml), or hydrogen peroxide (0 to 32 mM). Plates were incubated for 24 h at 37°C. The MIC was defined as the lowest antimicrobial concentration yielding no detectable bacterial growth (A600 measurement), and minimal bactericidal concentration (MBC) was defined as the lowest antimicrobial concentration yielding no surviving bacteria when the sample was plated on THA.
Isolation of human neutrophils and autologous plasma.
Blood was drawn from healthy volunteers and heparinized. Autologous plasma was obtained by centrifuging a blood aliquot at 1,000 × g for 5 min. The supernatant was subsequently centrifuged at 3,000 × g for 5 min. The plasma was then stored on ice until use. Neutrophils were isolated by density gradient centrifugation using Polymorphprep solution (Axis Shield PoC AS, Oslo, Norway) according to the manufacturer's instructions. The neutrophil layer was washed with pyrogen-free PBS without Ca2+ and Mg2+ at 4°C, and contaminating erythrocytes were hypotonically lysed. Subsequently, neutrophils were washed twice and finally resuspended in RPMI 1640. The viability of cells exceeded 95% as assessed by trypan blue exclusion.
Neutrophil killing assays.
Experiments were performed as described previously (23), with minor modifications. Early-exponential-phase WT and ΔdltA mutant GAS cells were washed, resuspended, and adjusted to 5 × 106 CFU/ml in PBS. Subsequently, the bacteria were diluted 1:5 in 100% prewarmed plasma and incubated for 25 min at 37°C and then placed on ice until use. The preopsonized GAS cells were then diluted 1:4 into prewarmed RPMI 1640 containing 3.33 × 106 neutrophils/ml in 14-ml nonpyrogenic polypropylene round-bottom tubes (Becton Dickinson) to provide a final neutrophil:bacteria ratio of 10:1. As a control, preopsonized GAS were incubated under the same conditions without neutrophils. All tubes were shaken at 200 rpm at 37°C, and samples were drawn after 0, 7.5, 15, 30, and 60 min. Neutrophils were hypotonically lysed by the addition of distilled water, and the numbers of surviving bacteria were quantified by plating on THA plates and 24 h of incubation at 37°C.
Phagocytosis studies.
To compare uptake kinetics of WT GAS and the ΔdltA mutant by human neutrophils, bacteria were grown to early exponential phase and fluorescence tagged in THB plus 30 μM calcein AM (Molecular Probes, Eugene, OR) for 1 h at 37°C. Subsequently, the cells were washed four times in cold PBS and adjusted to 5 × 106 bacteria/ml in PBS using a Neubauer improved counting chamber for bacteria (depth, 0.02 mm; PolyOptik GmbH, Bad Blankenburg, Germany) under fluorescence microscopy. Bacteria were preopsonized and incubated with neutrophils as described above, and then samples were drawn and diluted into cold PBS to stop the phagocytosis reaction. Neutrophils were then separated from non-cell-associated bacteria by two differential centrifugation steps (180 × g, 10 min, 4°C) and placed on ice until analysis. Finally, ethidium bromide (EtBr) at a final concentration of 16 μg/ml was added to the samples and 200 neurophils/sample were examined for the presence of intracellular bacteria by fluorescence microscopy as described previously (10). EtBr causes extracellular calcein-labeled GAS to fluoresce red-orange, whereas phagocytosed, intracellular GAS remain green because live neutrophils do not take up the dye. This process allows distinction between intracellular and extracellular bacteria by simultaneous visualization of both populations. Dead neutrophils were excluded from analysis (always <6% and equivalent to control wells without bacteria).
Epithelial cell adherence and invasion.
GAS adherence and invasion assays with human HEp-2 epithelial cells were performed as previously described (44), with some modifications. Briefly, newly confluent monolayers of ∼2 × 105 Hep-2 cells in 24-well plates were washed thrice with RPMI 1640. Early-exponential-phase WT and ΔdltA GAS cells were grown and washed as above, adjusted to the desired concentrations in RPMI 1640, and added to the wells in a 300-μl volume. Multiplicities of infection (MOI) of 1:1 to 20:1 bacteria:cell were used. Plates were centrifuged at 400 × g for 5 min to place GAS cells on the monolayer surface and incubated for 1 h at 37°C under 5% CO2, and wells were washed thrice with RPMI 1640 to remove non-cell-associated bacteria. In adherence assays, the monolayers were subsequently lysed by the addition of H2O-0.025% Triton X-100, and the dilutions were plated on THA to enumerate total cell-associated CFU. For cellular invasion assays, 300 μl RPMI 1640 plus 100 μg/ml gentamicin (Gm) plus 5 μg/ml penicillin (Pen) was added to the washed infected monolayers to kill surface-associated but noninternalized bacteria. After incubation for 2 h at 37°C, the wells were washed thrice and Hep-2 cells were lysed for enumeration of intracellular bacteria as described above.
RESULTS
The dlt operon is responsible for the d-alanylation of teichoic acids in GAS and affects bacterial surface charges.
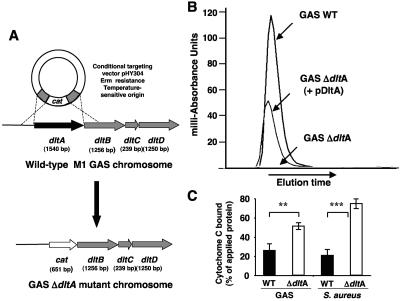
Inactivation of the dltA gene in GAS WT strain 5448 was achieved by precise, in-frame allelic replacement (Fig. 1A). d-alanine of teichoic acids was liberated from heat-inactivated cells by using a mild alkaline hydrolysis and quantified by HPLC analysis. Figure 1B shows an overlay of the d-alanine chromatogram peaks of WT GAS, the ΔdltA mutant, and the ΔdltA mutant with plasmid pdltA expressing the GAS dltA gene under the control of its own promoter. While the GAS ΔdltA mutant revealed no detectable amounts of d-alanine, we found 7.06 ± 1.31 nmol/ml d-alanine at an OD578 of 1 (means ± standard deviations of four independent derivatization reactions). Complementation of the GAS dltA mutant led to a considerable but not complete restoration of wild-type d-alanine levels (3.54 ± 0.27 nmol/ml d-alanine at an OD578 of 1; Fig. 1B). To determine whether the observed defect in LTA d-alanylation significantly altered the surface charge characteristics of GAS, WT and mutant strains were incubated with the cationic protein cytochrome c (Cyt c). The GAS ΔdltA mutant bound significantly more Cyt c than the WT parent strain (Fig. 1C), confirming a functional increase in net negative surface charge analogous to that observed in a previously characterized dltA deletion mutant of S. aureus (24) used as an assay control.
FIG. 1.
The dlt operon encodes teichoic acid d-alanylation in GAS and affects bacterial surface charge. (A) Scheme for allelic replacement of the dltA gene in a GAS chromosome. (B) HPLC analysis of the d-alanine content of teichoic acids in WT, ΔdltA, and ΔdltA plus pdltA GAS strains. (C) Binding of the cationic protein cytochrome c to WT and ΔdltA GAS strains compared to analogous mutations in S. aureus strain 113 (24). Data represent the mean values of protein bound by the bacterium ± standard deviations of four independent experiments (**, P < 0.005; ***, P < 0.0005; two-tailed t test).
The ΔdltA mutant is unaltered in expression of other GAS virulence factors.
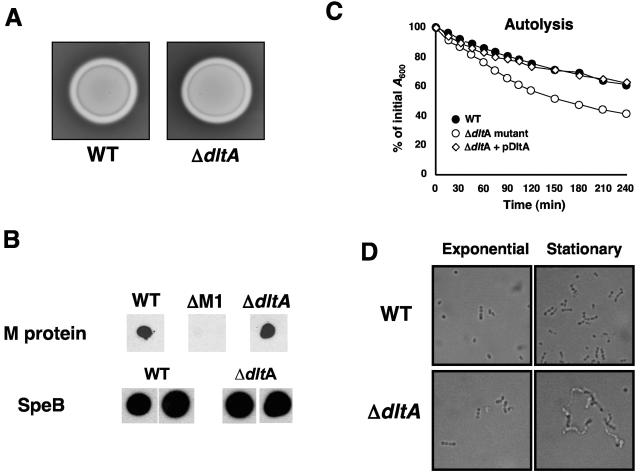
The GAS ΔdltA mutant was examined for potential pleiotrophic effects on the known GAS virulence factors SLS, M protein, and cysteine protease. The β-hemolytic phenotype of GAS on blood agar is produced by SLS, a cytotoxin that may impede neutrophil killing (8). WT and ΔdltA mutant GAS produced equivalent zones of β-hemolysis on blood agar media (Fig. 2A). The M protein has a well-established role in promoting GAS resistance to neutrophil killing (45), but no differences in M1 protein expression between the WT and the ΔdltA mutant were observed by immunoblot analysis (Fig. 2B). Finally, the GAS cysteine protease SpeB may complex to the bacterial surface and act to degrade cathelicidin antimicrobial peptides (35). WT and ΔdltA mutant GAS produced equivalent amounts of SpeB protein (Fig. 2B), and culture supernatants from each strain showed equivalent cysteine protease activity (data not shown). Two phenotypic differences were apparent between the WT and the ΔdltA mutant. As previously shown upon insertional disruption of the dlt operon in Bacillus subtilis (48), the GAS dltA mutant showed an increased rate of autolysis; complementation of the mutant with dltA on an expression plasmid returned the autolysin activity to WT levels (Fig. 2C). The GAS dltA mutant was also observed to form longer chains in stationary-phase culture compared to the WT strain (Fig. 2D). This phenotype was reminiscent of increased clumping of dltA-deficient Streptococcus agalactiae described in stationary-phase cultures (40).
FIG. 2.
Effect of the ΔdltA mutation on additional GAS phenotypes. (A) β-Hemolytic (streptolysin S) phenotype on sheep's blood agar. (B) M protein and cysteine proteinase SpeB expression by dot blot analysis. (C) Rate of water-induced autolysis activity as determined by decreasing A600. (D) Phase-contrast transmission microscopy of WT and ΔdltA mutant GAS in exponential and stationary growth phases. Note the long chains formed by the ΔdltA mutant in stationary phase.
The GAS ΔdltA mutant exhibits increased pH sensitivity.
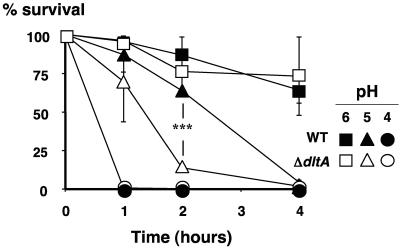
WT and ΔdltA mutant GAS cells grew equally well in THB media at the unadjusted pH of 7.5 to 7.8 (data not shown). Because an important host bactericidal mechanism involves maturation and acidification of the phagosome (27), we examined the sensitivity of WT and ΔdltA to decreasing pH by assessing their survival kinetics in 20 mM Na2HPO4/NaH2PO4, 1 mM MgCl2, 10 mM l-arginine buffered to a pH value of 7.0, 6.0, 5.0, or 4.0. Both WT and ΔdltA GAS showed ∼75% survival after 4 h at pH 6.0 but were killed rapidly within 1 h at pH 4.0 (Fig. 3). However, at pH 5.0, corresponding to the late stages of neutrophil phagolysosome maturation, the ΔdltA mutant exhibited a significantly accelerated rate of death compared to the WT parent strain (Fig. 3). These results suggest that d-alanylation of teichoic acids provides GAS relative protection against killing by acidification, a finding similar to that observed in S. agalactiae (40).
FIG. 3.
GAS ΔdltA mutant exhibits increased pH sensitivity. Exponential phase WT and ΔdltA mutant GAS were incubated up to 4 h in sodium phosphate buffer containing d-arginine buffered to the indicated pH. (***, P < 0.0005; two-tailed paired t test).
GAS teichoic acid d-alanylation contributes to antimicrobial peptide and lysozyme resistance.
Polycationic peptides with bactericidal activity can be produced by microbes in niche competition with other species or by higher organisms as part of their innate immune defense. We tested whether the increased negative surface charge of the GAS ΔdltA mutant was associated with enhanced susceptibility to cationic AMP killing. As shown in Table 1, the isogenic GAS ΔdltA mutant exhibited clearly decreased MICs and MBCs for the murine cathelicidin mCRAMP compared to the WT parent strain. Similarly, the ΔdltA mutant was more susceptible to the bacteria-derived cationic AMP polymyxin B. With both mCRAMP and polymyxin B, complementation of the ΔdltA mutant with an expression plasmid bearing a copy of the WT GAS dltA gene increased resistance toward WT levels. These results indicate that d-alanylation of teichoic acids is an important component of the intrinsic resistance of GAS to cationic AMP killing. The murein hydrolase lysozyme is produced by phagocytes to digest the cell wall peptidoglycan of engulfed bacteria. We found the GAS dltA mutant showed significantly enhanced susceptibility to lysozyme (MIC, 4 mg/ml; MBC, 8 mg/ml) compared to the WT strain (MIC and MBC, both >12 mg/ml). In contrast, the WT and dltA mutant GAS strains were equivalent in their susceptibility to hydrogen peroxide (MIC and MBC, both 4 mM), a key effector of neutrophil oxidative burst killing.
TABLE 1.
MICs and MBCs for antimicrobials used in this study
| GAS | mCRAMP
|
Polymyxin B
|
||
|---|---|---|---|---|
| MIC (μM) | MBC (μM) | MIC (μg/ml) | MBC (μg/ml) | |
| WT | 14.0 | 32.0 | 28.0 | 117.3 |
| ΔdltA mutant | 3.5 | 14.0 | 8.7 | 42.7 |
| ΔdltA + pDltA | 16.0 | 28.0 | 24.0 | 80.0 |
GAS teichoic acid d-alanylation decreases susceptibility to neutrophil killing.
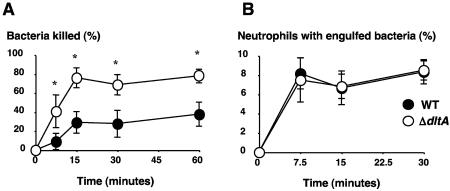
As vacuole acidification and production of AMPs and lysozyme are all components of effective neutrophil bactericidal activity, we hypothesized that GAS d-alanylation of teichoic acid could help the bacterium to survive neutrophil killing. Comparing the killing kinetics of preopsonized WT and ΔdltA GAS strains by purified human neutrophils, the mutant strain was eliminated more readily (Fig. 4A). As WT and ΔdltA strains were phagocytosed by neutrophils with similar efficiencies (Fig. 4B), the observed difference in killing kinetics likely represents an increased susceptibility of the ΔdltA bactericidal killing mechanisms, including acidification and cationic AMP production.
FIG. 4.
GAS LTA d-alanylation decreases susceptibility to neutrophil killing. (A) Phagocytosis and killing kinetics of WT and ΔdltA mutant GAS by human neutrophils at a bacteria:cell ratio of 1:10. The values represent the means and standard deviations of three independent experiments (*, P < 0.05, two-tailed t test). (B) Human neutrophil phagocytosis of fluorescence-tagged GAS. The percentage of neutrophils with intracellular bacteria is depicted (200 cells examined per sample); the graph shows mean values ± standard deviations in one representative study of three experiments with similar results.
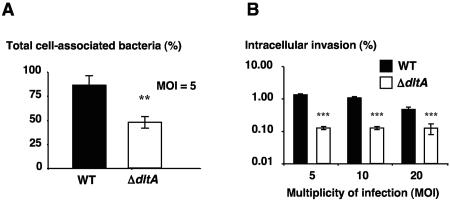
GAS teichoic acid d-alanylation promotes epithelial cell adherence and invasion.
LTA has been shown to play a role in GAS epithelial cell attachment and uptake (3, 44). To assess the potential contribution of the d-alanyl modification of teichoic acids to these processes, we compared interactions of the WT and the ΔdltA mutant with cultured monolayers of human pharyngeal epithelial cell line Hep-2. After 1 h of incubation of GAS with the Hep-2 monolayer and vigorous washing, we found a marked decrease in the total number of cell-associated ΔdltA mutant bacteria compared to the WT parent strain (44.9% ± 3.7% reduction; P < 0.005) (Fig. 5A). Using antibiotic protection to quantify those bacteria which had invaded the intracellular compartment, an even more marked reduction of approximately 10-fold fewer internalized ΔdltA mutant cells than WT GAS cells were observed at each of three MOI tested (5, 10, 20) (Fig. 5B). As a control, no difference was observed in the susceptibility of the WT GAS and the mutant to the antibiotics used in the assay (gentamicin MIC, 6.25 μg/ml; gentamicin MBC, 12.5 μg/ml; penicillin MIC, 160 ng/ml; penicillin MBC, 320 ng/ml). Taken together, these studies reveal that d-alanylation of teichoic acids itself plays a role in facilitating GAS epithelial cell attachment and invasion.
FIG. 5.
GAS teichoic acid d-alanylation promotes Hep-2 human pharyngeal epithelial cell adherence and invasion. (A) Total cell-associated bacteria after 1 h of incubation. Samples were run in triplicate, and the mean values ± standard deviations of three independent experiments are shown (**, P < 0.005; two-tailed t test). (B) Intracellular bacteria quantified in an antibiotic protection assay after 1 h of incubation followed by 2 h of antibiotic treatment to kill extracellular bacteria. Samples were run in triplicate, and the mean values ± standard deviations of one representative experiment of three performed are shown (***, P < 0.005; two-tailed t test).
DISCUSSION
GAS is a major human pathogen increasingly associated with deep-seated invasive infections. The ability of GAS to produce invasive infection reflects the organism's ability to resist innate immune defenses and penetrate mucosal epithelial barriers. In this study, we demonstrate that the dlt operon of GAS functions to incorporate d-alanine into the teichoic acids expressed on the bacterial cell surface. This modification results in an increase in positive surface charge; increased resistance to AMP, lysozyme, and neutrophil killing; and greater adherence and invasion of pharyngeal epithelial cells. Thus, d-alanylation of teichoic acids represents a potential virulence phenotype that could contribute in multiple fashions to the pathogenesis of invasive GAS infection.
An emerging theme in AMP resistance among pathogenic bacteria is the modification of normally anionic cell surface constituents with cationic molecules; the net effect of these substitutions is to repel positively charged AMPs before they can reach the cytoplasmic membrane to disrupt its integrity. In a number of medically important gram-positive pathogens, d-alanylation of cell-wall-bound LTA appears to serve this function, including S. aureus, S. agalactiae, and L. monocytogenes (1, 7, 24, 41, 49). Our demonstration of dltA-encoded AMP resistance in the leading pathogen GAS further illustrates the potential broad significance of this bacterial adaptation.
Cathelicidin antimicrobial peptides contribute to innate defense against invasive bacterial infection, as recently demonstrated experimentally with GAS and Salmonella enterica (34, 43). dltA-mediated d-alanylation of GAS teichoic acids represents the first general mechanism for GAS cationic AMP resistance proven by targeted gene deletion. Studies with purified GAS proteins suggest GAS possesses additional virulence determinants that may contribute to AMP resistance. The SpeB cysteine protease of GAS can degrade human cathelicidin, a phenomenon reversed by the SpeB-specific protease inhibitor E64 (35, 45). Invasive serotype M1 strains (such as 5448, used in this study) also secrete a protein known as SIC that can interfere with host complement function (2). Recently, it was shown that purified SIC binds and inactivates the human cathelicidin and β-defensin (15). The AMP-repelling effects of teichoic acid d-alanylation and the proteolytic and binding effects of these GAS virulence factors may combine to pose a considerable challenge to host AMP-mediated innate immune defenses.
Once pathogenic bacteria such as GAS reach subepithelial tissues, neutrophils are recruited to the site of infection to limit further spread of the invading microorganisms. Human neutrophils employ vacuole acidification and multiple cationic substances, including cathelicidins, defensins, myeloperoxidase, lysozyme, and phospholipase A2, to kill engulfed microorganisms (16). We found that although the WT and ΔdltA mutant GAS strains were phagocytosed with equal efficiency, the mutant was much more rapidly killed by human neutrophils. We conclude the association of teichoic acid d-alanylation with GAS resistance to low pH, lysozyme, and cationic AMPs acts to promote the organism's survival within neutrophils, a phenotype recently appreciated to contribute to GAS virulence (28, 29). Additional studies have recently challenged the classical notion that the surface expression M protein acts to block GAS phagocytosis but rather indicate that the well-known GAS virulence factor functions to promote neutrophil intracellular survival (45). As d-alanylated LTA interacts to form complexes with M protein on the GAS surface (36), the combination of the two moieties in surface fibrillar extensions may work in concert to retard intracellular killing.
The amphiphilic molecule LTA has been known to participate in host cell attachment by certain gram-positive pathogens, including GAS (4, 50). In other streptococci, LTA-mediated host cell interactions have been shown to involve both hydrophobic interactions with the lipid portion of the polymer as well as more specific interactions due to the glycerol phosphate polymer (31). Recently, free LTA was found to specifically block intracellular invasion of Hep-2 cells by S. pyogenes without affecting overall cellular adherence (44). Our present discovery represents the first demonstration that the d-alanine modification of teichoic acid may itself contribute to the process of GAS adherence and invasion. We hypothesize that the incorporation of positive charges on the bacterial cell surface promotes interaction with the negatively charged host cell surface. An analogous situation may exist in Streptococcus pneumoniae, where removal of surface choline-binding proteins was associated with a net increase in negative charge on the bacterial surface and decreased adherence to eukaryotic cells (47).
In summary, GAS d-alanylation of teichoic acids is encoded by the dlt operon and contributes phenotypically to increased net positive surface charge, relative resistance to cationic AMPs, lysozyme, and low pH, greater survival against host neutrophil killing, and greater epithelial cell adherence and invasion. The pharyngeal epithelium represents the major reservoir for invasive GAS disease (13), and serotype M1 isolates associated with serious infection show an increased frequency of epithelial cell invasion in vitro (6, 25). Thus, incorporation of d-alanine into surface teichoic acids may contribute to GAS pathogenesis both through interference with innate immune clearance mechanisms and by facilitating penetration of host epithelial barriers. The biochemical modification encoded by the dlt operon may represent a novel therapeutic target for prevention of invasive GAS infection, working to render the organism susceptible to normal host mucosal barrier and immune functions.
Acknowledgments
This work was supported by NIH grants AR45676 (R.L.G.), AI48176 (R.L.G.), and AI048694 (V.N.), a VA Merit Award (R.L.G.), and an Edward J. Mallinckrodt, Jr., Scholar Award (to V.N.).
We thank Diana Schultz for help with the autolysis assay and Gabriele Hornig and Katja Klöpper for their expert technical assistance.
REFERENCES
- 1.Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. [DOI] [PubMed] [Google Scholar]
- 2.Akesson, P., A. G. Sjoholm, and L. Bjorck. 1996. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271:1081-1088. [DOI] [PubMed] [Google Scholar]
- 3.Beachey, E. H., and H. S. Courtney. 1989. Bacterial adherence of group A streptococci to mucosal surfaces. Respiration 55(Suppl. 1):33-40. [DOI] [PubMed] [Google Scholar]
- 4.Beachey, E. H., and H. S. Courtney. 1987. Bacterial adherence: the attachment of group A streptococci to mucosal surfaces. Rev. Infect. Dis. 9(Suppl. 5):S475-S481. [DOI] [PubMed] [Google Scholar]
- 5.Chevion, M., C. Panos, R. Linzer, and F. C. Neuhaus. 1974. Incorporation of d-alanine into the membrane of Streptococcus pyogenes and its stabilized L-form. J. Bacteriol. 120:1026-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary, P. P., D. LaPenta, R. Vessela, H. Lam, and D. Cue. 1998. A globally disseminated M1 subclone of group A streptococci differs from other subclones by 70 kilobases of prophage DNA and capacity for high-frequency intracellular invasion. Infect. Immun. 66:5592-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins, L. V., S. A. Kristian, C. Weidenmaier, M. Faigle, K. P. Van Kessel, J. A. Van Strijp, F. Gotz, B. Neumeister, and A. Peschel. 2002. Staphylococcus aureus strains lacking D-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 186:214-219. [DOI] [PubMed] [Google Scholar]
- 8.Datta, V., S. M. Myskowski, L. A. Kwinn, D. N. Chiem, N. Varki, R. G. Kansal, M. Kotb, and V. Nizet. 2005. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol. Microbiol. 56:681-695. [DOI] [PubMed] [Google Scholar]
- 9.Degnan, B. A., M. C. Fontaine, A. H. Doebereiner, J. J. Lee, P. Mastroeni, G. Dougan, J. A. Goodacre, and M. A. Kehoe. 2000. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect. Immun. 68:2441-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drevets, D. A., and P. A. Campbell. 1991. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J. Immunol. Methods 142:31-38. [DOI] [PubMed] [Google Scholar]
- 11.Epand, R. M., and H. J. Vogel. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11-28. [DOI] [PubMed] [Google Scholar]
- 12.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorentino, T. R., B. Beall, P. Mshar, and D. E. Bessen. 1997. A genetic-based evaluation of the principal tissue reservoir for group A streptococci isolated from normally sterile sites. J. Infect. Dis. 176:177-182. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, W. 1988. Physiology of lipoteichoic acids in bacteria. Adv. Microb. Physiol. 29:233-302. [DOI] [PubMed] [Google Scholar]
- 15.Frick, I. M., P. Akesson, M. Rasmussen, A. Schmidtchen, and L. Bjorck. 2003. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J. Biol. Chem. 278:16561-16566. [DOI] [PubMed] [Google Scholar]
- 16.Ganz, T. 2001. Fatal attraction evaded. How pathogenic bacteria resist cationic polypeptides. J. Exp. Med. 193:F31-F34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunn, J. S., S. S. Ryan, J. C. Van Velkinburgh, R. K. Ernst, and S. I. Miller. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaton, M. P., and F. C. Neuhaus. 1992. Biosynthesis of d-alanyl-lipoteichoic acid: cloning, nucleotide sequence, and expression of the Lactobacillus casei gene for the d-alanine-activating enzyme. J. Bacteriol. 174:4707-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogg, M. C. D., and G. R. Jago. 1970. Extraction of the 260 nm absorbing material from group N streptococci as a method for estimating cell growth. J. Dairy Res. 37:199-202. [Google Scholar]
- 20.Jeng, A., V. Sakota, Z. Li, V. Datta, B. Beall, and V. Nizet. 2003. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J. Bacteriol. 185:1208-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kansal, R. G., A. McGeer, D. E. Low, A. Norrby-Teglund, and M. Kotb. 2000. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect. Immun. 68:6362-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochhar, S., and P. Christen. 1989. Amino acid analysis by high-performance liquid chromatography after derivatization with 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide. Anal. Biochem. 178:17-21. [DOI] [PubMed] [Google Scholar]
- 23.Kristian, S. A., M. Dürr, J. A. Van Strijp, B. Neumeister, and A. Peschel. 2003. MprF-mediated lysinylation of phospholipids in Staphylococcus aureus leads to protection against oxygen-independent neutrophil killing. Infect. Immun. 71:546-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristian, S. A., X. Lauth, V. Nizet, F. Goetz, B. Neumeister, A. Peschel, and R. Landmann. 2003. Alanylation of teichoic acids protects Staphylococcus aureus against Toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J. Infect. Dis. 188:414-423. [DOI] [PubMed] [Google Scholar]
- 25.LaPenta, D., C. Rubens, E. Chi, and P. P. Cleary. 1994. Group A streptococci efficiently invade human respiratory epithelial cells. Proc. Natl. Acad. Sci. USA 91:12115-12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauth, X., C. W. McNamara, S. Myskowski, E. Igwe, B. Beall, P. Ghosh, R. L. Gallo, and V. Nizet. 2004. A new virulence role for group A streptococcal M1 protein is protection against cathelicidin antimicrobial peptides. Abstr. Gen. Meet. Am. Soc. Microbiol. 2004, abstr. E-84, p. 160, 2004.
- 27.Lee, W. L., R. E. Harrision, and S. Grinstein. 2003. Phagocytosis by neutrophils. Microbes Infect. 5:1299-1306. [DOI] [PubMed] [Google Scholar]
- 28.Medina, E., O. Goldmann, A. W. Toppel, and G. S. Chhatwal. 2003. Survival of Streptococcus pyogenes within host phagocytic cells: a pathogenic mechanism for persistence and systemic invasion. J. Infect. Dis. 187:597-603. [DOI] [PubMed] [Google Scholar]
- 29.Medina, E., M. Rohde, and G. S. Chhatwal. 2003. Intracellular survival of Streptococcus pyogenes in polymorphonuclear cells results in increased bacterial virulence. Infect. Immun. 71:5376-5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moser, C., D. J. Weiner, E. Lysenko, R. Bals, J. N. Weiser, and J. M. Wilson. 2002. β-Defensin 1 contributes to pulmonary innate immunity in mice. Infect. Immun. 70:3068-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nealon, T. J., and S. J. Mattingly. 1984. Role of cellular lipoteichoic acids in mediating adherence of serotype III strains of group B streptococci to human embryonic, fetal, and adult epithelial cells. Infect. Immun. 43:523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nizet, V. 2006. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr. Issues Mol. Biol. 8:11-26. [PubMed] [Google Scholar]
- 34.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 35.Nyberg, P., M. Rasmussen, and L. Bjorck. 2004. α2-Macroglobulin-proteinase complexes protect Streptococcus pyogenes from killing by the antimicrobial peptide LL-37. J. Biol. Chem. 279:52820-52823. [DOI] [PubMed] [Google Scholar]
- 36.Ofek, I., W. A. Simpson, and E. H. Beachey. 1982. Formation of molecular complexes between a structurally defined M protein and acylated or deacylated lipoteichoic acid of Streptococcus pyogenes. J. Bacteriol. 149:426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perego, M., P. Glaser, A. Minutello, M. A. Strauch, K. Leopold, and W. Fischer. 1995. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J. Biol. Chem. 270:15598-15606. [DOI] [PubMed] [Google Scholar]
- 38.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 39.Peschel, A., C. Vuong, M. Otto, and F. Gotz. 2000. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob. Agents Chemother. 44:2845-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poyart, C., M. C. Lamy, C. Boumaila, F. Fiedler, and P. Trieu-Cuot. 2001. Regulation of d-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 183:6324-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M. C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in D-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol. Microbiol. 49:1615-1625. [DOI] [PubMed] [Google Scholar]
- 42.Proft, T., S. Sriskandan, L. Yang, and J. D. Fraser. 2003. Superantigens and streptococcal toxic shock syndrome. Emerg. Infect. Dis. 9:1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberger, C. M., R. L. Gallo, and B. B. Finlay. 2004. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. USA 101:2422-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sela, S., M. J. Marouni, R. Perry, and A. Barzilai. 2000. Effect of lipoteichoic acid on the uptake of Streptococcus pyogenes by HEp-2 cells. FEMS Microbiol. Lett. 193:187-193. [DOI] [PubMed] [Google Scholar]
- 45.Staali, L., M. Morgelin, L. Bjorck, and H. Tapper. 2003. Streptococcus pyogenes expressing M and M-like surface proteins are phagocytosed but survive inside human neutrophils. Cell Microbiol. 5:253-265. [DOI] [PubMed] [Google Scholar]
- 46.Stevens, D. L. 2000. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu. Rev. Med. 51:271-288. [DOI] [PubMed] [Google Scholar]
- 47.Swiatlo, E., F. R. Champlin, S. C. Holman, W. W. Wilson, and J. M. Watt. 2002. Contribution of choline-binding proteins to cell surface properties of Streptococcus pneumoniae. Infect. Immun. 70:412-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wecke, J., M. Perego, and W. Fischer. 1996. D-alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb. Drug Resist. 2:123-129. [DOI] [PubMed] [Google Scholar]
- 49.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243-245. [DOI] [PubMed] [Google Scholar]
- 50.Wicken, A. J., and K. W. Knox. 1975. Lipoteichoic acids: a new class of bacterial antigen. Science 187:1161-1167. [DOI] [PubMed] [Google Scholar]