Abstract
srfA is an operon required for the synthesis of surfactin and the development of genetic competence in Bacillus subtilis. We observed that the expression of srfA is downregulated upon treatment with H2O2. Thus, we examined the involvement of several oxidative stress-responsive transcription factors in srfA expression. Our DNA microarray analysis revealed that the H2O2 stress-responsive regulator PerR is required for srfA expression. This was confirmed by lacZ fusion analysis. A ComX feeding assay and epistatic analyses revealed that the role of PerR in srfA expression is independent of other known regulators of srfA expression, namely, comQXP, rapC, and spx. Gel mobility shift and footprint assays revealed that PerR binds directly to two tandemly arranged noncanonical PerR boxes located in the upstream promoter region of srfA. A transcriptional srfA-lacZ fusion lacking both PerR boxes showed diminished and PerR-independent expression, indicating that the PerR boxes we identified function as positive cis elements for srfA transcription.
The Bacillus subtilis srfA operon encodes the biosynthetic genes for surfactin, a biosurfactant that has been reported to be important for the swarming motility and fruiting body formation of natural isolates of B. subtilis (3, 7, 18-20). In addition, the srfA operon is involved in the development of genetic competence, since the srfA operon encodes another gene, comS, that is required for comK activation (9). The comK gene encodes the competence transcription factor required for the expression of late competence operons, including comE (42). srfA expression is dependent on two extracellular controlling systems, namely, ComQXP and RapC-CSF (competence and sporulation factor) (9, 22, 38). When the ComX pheromone is secreted into the medium, the increase in its concentration is sensed by the ComP receptor, which activates the cognate response regulator ComA by phosphorylation (22, 36), resulting in ComA-P. RapC inhibits the DNA binding of ComA-P, while the extracellular CSF peptide inhibits RapC after the CSF peptide is incorporated into the cytoplasm (6, 38). Thus, RapC and CSF constitute a positive regulatory device for ComA-P, which leads to the binding of ComA-P to the srfA promoter. Another regulatory molecule that affects ComA-P-mediated srfA transcription is Spx, which inhibits the interaction between ComA-P and RNA polymerase; when the ClpXP protease degrades Spx, activation of the ComA regulon results (29-30). In addition, the expression of the spx gene is induced by diamide stress (30).
B. subtilis has three Fur (ferric uptake regulator) homologues, one of which is PerR, a repressor of several members of the peroxide stress regulon, such as ahpCF, katA, zosA, and mrgA (24). The PerR protein has two divalent ion-binding sites, one for the zinc cation and the other for various regulatory cations. For example, Mn(II)-, Ni(II)-, or Fe(II)-bound PerR is active as a repressor (17). It has been shown that H2O2 inactivates the DNA-binding activity of PerR (17, 24).
srfA expression has been reported to decrease upon H2O2 treatment (see supplementary data in reference 25). We also observed a similar phenomenon. These hint at another mechanism that regulates the srfA operon, namely, one that is linked to the oxidative stress response. To characterize this mechanism, we tested the effect of disrupting transcription factor genes that regulate the expression of oxidative stress-responsive genes on the expression of srfA and other genes by using DNA microarray analysis. This analysis revealed that the disruption of perR blocks srfA expression. When we analyzed the mechanism by which PerR regulates srfA, we found that PerR binds directly to the srfA promoter region. Furthermore, srfA promoter deletion analysis using lacZ fusions confirmed that two tandemly oriented PerR-binding sites function as positive cis elements for srfA transcription.
MATERIALS AND METHODS
Bacterial strains and culture media.
All the B. subtilis strains used in this study are listed in Table 1. B. subtilis and Escherichia coli cells were grown in modified competence (MC) (21) medium and Luria-Bertani (LB) medium, respectively. The concentrations of antibiotics used have been described previously (33).
TABLE 1.
Bacillus subtilis strains used in this study
| Strain | Relevant phenotype and description | Reference or source |
|---|---|---|
| 168 | trpC2 | Laboratory stock |
| OAM240 | trpC2 perR::Cmr | This study |
| LAB358 | trpC2 pheA SPβ c2del2::Tn917::srfA-lacZ (Emr Cmr) | 27 |
| YBCPd | trpC2 ybcP::pMutInIII (EmrskfB-lacZ) | 43 |
| OAM186 | trpC2 SPβ c2del2::Tn917::srfA-lacZ (Emr Cmr) | This study |
| OSM102 | trpC2 SPβ c2del2::Tn917::srfA-lacZ (Emr Cmr) ΔcomQXPA (Emr) | 34 |
| OAM241 | trpC2 SPβ c2del2::Tn917::srfA-lacZ (Emr Cmr) perR::Cmr | This study |
| OAM245 | trpC2 SPβ c2del2::Tn917::srfA-lacZ (Emr Cmr) rapC::pMutInIII (EmrlacZ::Tcr) | This study |
| OAM246 | trpC2 SPβ c2del2::Tn917::srfA-lacZ (Emr Cmr) rapC::pMutInIII (EmrlacZ::Tcr) perR::Cmr | This study |
| RAPCd | trpC2 rapC::pMutInIII (EmrrapC-lacZ) | This study |
| OAM262 | trpC2 rapC::pMutInIII (EmrrapC-lacZ) perR::Cmr | This study |
| ORB3834 | trpC2 pheA1 spx::Kmr | 29 |
| OAM233 | trpC2 SPβ c2del2::Tn917::srfA-lacZ (Emr Cmr) spx::Kmr | This study |
| OAM247 | trpC2 SPβ c2del2::Tn917::srfA-lacZ (Emr Cmr) spx::KmrperR::Cmr | This study |
| 8G33 | trpC2 comK-lacZ (Kmr) | 42 |
| OAM242 | trpC2 comK-lacZ (Kmr) perR::Cmr | This study |
| OGM113 | trpC2 leuC7 comE-lacZ (Emr) | 32 |
| OAM243 | trpC2 comE-lacZ (Emr) | This study |
| OAM244 | trpC2 comE-lacZ (Emr) perR::Cmr | This study |
| OAM248 | trpC2 amyE::srfA1-lacZ (Cmr::Tcr) | This study |
| OAM249 | trpC2 amyE::srfA1-lacZ (Cmr::Tcr) perR::Cmr | This study |
| OAM250 | trpC2 amyE::srfA2-lacZ (Cmr::Tcr) | This study |
| OAM251 | trpC2 amyE::srfA2-lacZ (Cmr::Tcr) perR::Cmr | This study |
| OAM252 | trpC2 amyE::srfA3-lacZ (Cmr::Tcr) | This study |
| OAM253 | trpC2 amyE::srfA3-lacZ (Cmr::Tcr) perR::Cmr | This study |
Materials.
Synthetic oligonucleotides were commercially prepared by Tsukuba Oligo Service (Ibaraki, Japan) and are shown in Table 2.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence |
|---|---|
| perR-R1 | 5′-GTAATGACTATAGAAATCGGAC-3′ |
| perR-R2 | 5′-GTTATCCGCTCACAATTCTGTGCAGCCATCCGTCATGC-3′ |
| perR-F1 | 5′-CGTCGTGACTGGGAAAACGCGTCTGCCAAGAGTGTTCG-3′ |
| perR-F2 | 5′-CCATCACTATTCCTCCAATGC-3′ |
| pUC1 | 5′-GTTTTCCCAGTCACGACG-3′ |
| pUC2 | 5′-GAATTGTGAGCGGATAAC-3′ |
| rapC-F | 5′-GCGCAAGCTTGTAATTCCTTCTTCAGCGGT-3′ |
| rapC-R | 5′-GCCGAGATCTCCTGGTTGCTGTCAATGTTT-3′ |
| perR-HisB | 5′-ATTGGATCCATGGCTGCACATGAACTAAA-3′ |
| perR-HisH | 5′-GCTAAGCTTTCAATGATTTTCTTTTTTCGAACAC-3′ |
| srfA-1 | 5′-biotin-GCGCGGTACACATAGTCATGTAAA-3′ |
| srfA-2 | 5′-TTATCTTTCTACCGTTCAGT-3′ |
| srfA-2a | 5′-biotin-TTATCTTTCTACCGTTCAGT-3′ |
| srfA-3 | 5′-TAGTGGAAATGATTGCGGCA-3′ |
| srfA-4 | 5′-biotin-GTTGTAAGACGCTCTTCGCA-3′ |
| srfA-4a | 5′-GTTGTAAGACGCTCTTCGCA-3′ |
| katA-1 | 5′-biotin-AGCTGTTACAACAAGGTTT-3′ |
| katA-2 | 5′-TTTGATTATCTCCAACCGGA-3′ |
| rapG-1 | 5′-biotin-ATCATCTCTCCTTCATATA-3′ |
| rapG-2 | 5′-CATGTTTCTTGATGGCAAGG-3′ |
| srfA-B1 | 5′-CATGGATCCAGTTTGGTTTAAAAATTTTT-3′ |
| srfA-B2 | 5′-CATGGATCCTGTAAATAATGTTTAGTGG-3′ |
| srfA-B3 | 5′-CATGGATCCATGATTGCGGCATCCCGC-3′ |
| srfA-H1 | 5′-CATAAGCTTCCGCTATTAAAGCAGGCT-3′ |
Construction of plasmids and strains.
A chloramphenicol-resistant perR disruptant was constructed by a PCR-based method without cloning of DNA into E. coli as described previously (31). For this, six primers were used, namely, perR-F1, perR-R1, perR-F2, perR-R2, pUC-1, and pUC-2. All the plasmids used in this study are listed in Table 3. To construct pHis-perR, a PCR product generated by using the oligonucleotide primer pairs perR-HisB and perR-HisH was treated with BamHI and HindIII and cloned into pQE8 (QIAGEN, Hilden, Germany) treated with the same restriction enzymes. This added the histidine tag to the N terminus of PerR. To construct pMutin-rapC, a PCR product amplified by using rapC-F and rapC-R was treated with HindIII and BglII and cloned between the HindIII and BamHI sites of pMutinIII (41). pMutin-rapC was transformed into B. subtilis 168, and the lacZ gene in the resultant strain was inactivated by transformation of placZ::Tc. To construct pIS-srfA1, pIS-srfA2, and pIS-srfA3, PCR products amplified by using srfA-B1 and srfA-H1, srfA-B2 and srfA-H1, and srfA-B3 and srfA-H1, respectively, were treated with BamHI and HindIII and cloned into pIS284 treated with the same enzymes. After linearization with PstI, each plasmid was transformed into B. subtilis 168, after which the chloramphenicol resistance markers of the resultant strains were replaced with tetracycline markers by transformation with plasmid ECE75. Sequencing of the cloned PCR fragments was performed with a 377 DNA sequencer (Perkin-Elmer) and a Dye Terminator cycle sequencing kit (Applied Biosystems).
TABLE 3.
Plasmids used in this study
| Plasmid | Description | Reference or source |
|---|---|---|
| pQE8 | pUC19-based Ampr plasmid | Qiagen |
| pHis-perR | pQE8 carrying perR | This study |
| pMutInIII | Ampr Emr | 41 |
| pIS284 | Ampr CmrlacZ | I. Smith |
| ECE75 | Ampr Cmr::Tcr | Bacillus Stock Center |
| placZ | AmprlacZ::Tcr | 35 |
| pIS-srfA-1 | pIS284 carrying the srfA promoter region (−170 to +134) | This study |
| pIS-srfA-2 | pIS284 carrying the srfA promoter region (−144 to +134) | This study |
| pIS-srfA-3 | pIS284 carrying the srfA promoter region (−122 to +134) | This study |
β-Galactosidase assays.
For β-galactosidase assays, samples were withdrawn at hourly intervals and the β-galactosidase activities were measured as described previously (33). Conditioned medium was prepared by removing the cells by centrifugation and filter sterilizing the supernatant. Cells were grown in MC medium to early log phase and divided into two equal volumes. To each half, either conditioned or fresh medium was added, and cultivation was continued, after which samples were withdrawn. In all assays using strains carrying the perR disruption, the strains were used immediately after construction to avoid the accumulation of suppressors.
Production and purification of His-tagged PerR.
The protein was produced in E. coli and purified as described previously (35). His-tagged PerR was produced as a soluble protein in E. coli, and thus, purification was performed by stepwise elution with imidazole from a Ni-affinity column. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the fractions, the purified protein was dialyzed against TEDG buffer (26).
DNA microarray analysis.
For DNA microarray analysis, both the control strain 168 and its chloramphenicol-resistant derivative bearing the perR disruption were grown in LB liquid medium and harvested at an optical density of 0.4. The procedures used to isolate RNA and perform DNA microarray analysis have been described previously (34, 45).
Gel mobility shift and DNase I footprint assays.
For the gel shift assay, we employed a procedure using biotinylated DNA probes as described previously (35). The footprint assay was performed as follows. Probe DNAs were prepared by PCR using srfA-4 and srfA-2 or srfA-4a and srfA-2a as primers. The 60-μl reaction mixtures contained 100 ng DNA, 1 mg bovine serum albumin, 20 mM HEPES, pH 7.6, 1 mM EDTA, 10 mM (NH4)2SO4, 1 mM dithiothreitol, 0.2% Tween 20 (wt/vol), 30 mM KCl, an appropriate amount of the protein solution in TEDG buffer, and 4 units of DNase I (Roche, Indianapolis). The reaction mixture was left at room temperature for 5 min and then subjected to phenol extraction and subsequent ethanol precipitation after the addition of stop solution (0.1% SDS, 20 mM EDTA, 200 mM NaCl, 40 μg/ml tRNA). After the addition of a loading dye, the samples were applied onto a 6% polyacrylamide gel. After electrophoresis, the DNA was transferred to a positively charged nylon membrane. Biotinylated DNA was detected as described previously (35). A sequence ladder was generated by using a cycle sequencing kit (Toyobo Co.) employing the biotinylated srfA-2a or srfA-4 primer.
RESULTS
Expression of srfA is susceptible to H2O2 stress.
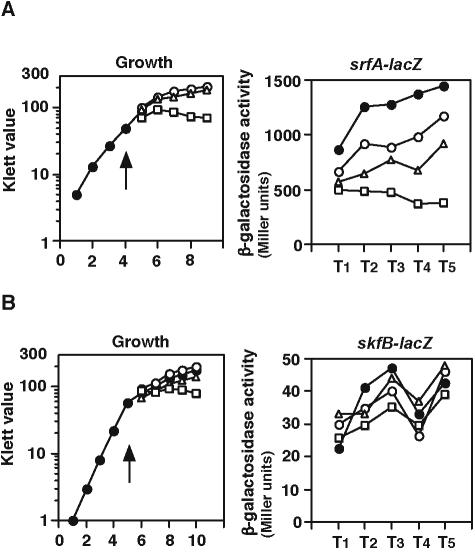
We observed that srfA expression was downregulated upon H2O2 treatment in a dose-dependent manner (Fig. 1A). It has been known that E. coli β-galactosidase is unstable under stress conditions, including heat shock (1); hence, it was possible that this decrease might not be due to an actual change in srfA expression. To rule out this, we chose skfB encoding a protein involved in the cell killing factor production and examined its expression in the same condition (43). The expression of the skfB-lacZ fusion was not influenced by the addition of H2O2 (Fig. 1B), supporting the observation with respect to srfA expression.
FIG. 1.
Decrease in srfA expression by H2O2 treatment. Cells were grown in modified competence medium, and their β-galactosidase activities were determined as described in Materials and Methods. Growth of the cells monitored with an Klett optical density meter and activities of β-galactosidase are shown in the left and right panels, respectively. The x axis represents the growth time in hours (left panels) and the duration of culture in hours relative to the end of vegetative growth (right panels). Several experiments were performed, and typical results are shown. Arrows in the left panels indicate the time when the indicated concentration of H2O2 was added. Closed circles, no addition of H2O2; open circles, 0.2 mM H2O2; open triangles, 1 mM H2O2; open squares, 5 mM H2O2. (A) and (B) show the experiments using srfA-lacZ (OAM186) and skfB-lacZ (YBCPd), respectively.
DNA microarray analysis of the effect of disrupting perR.
The observation that the expression of srfA is susceptible to H2O2 stress suggested that PerR, which is a repressor of several members of the peroxide stress regulon, may be involved in regulating srfA expression. To test this notion, we disrupted the perR gene in B. subtilis and tested the effect of this on the expression of srfA and other genes by DNA microarray analysis. Microarray analysis was performed twice using a glass plate on which pairs of the arrays were printed. In other words, four sets of hybridization data were obtained. Genes whose expression level in the perR disruptant differed from that in the parental strain by threefold in every data set were considered to be genes that could be regulated by PerR. We detected many such genes, since 88 genes were upregulated by >3-fold in the perR disruptant while 111 genes were downregulated by >3-fold (Hayashi et al. in list of experimental data available at Kegg expression database [http://www.genome.jp/kegg/expression/]). These included genes whose expression was not been previously shown to be regulated by PerR. We detected all of the known PerR-regulated genes, namely, ahpCF, hemAXCDBL, katA, zosA, fur, and mrgA, with the exception of perR itself, in addition to several of the function-unknown “y genes” detected by the DNA microarray analysis conducted by Helmann et al. (16). As shown in Table 4, transcription of the anaerobic and respiratory genes controlled by the ResE-ResD two-component system (e.g., the cydABC, nasCDEF, and resABCDE operons) was highly induced by the deletion of perR (28, 44). It is possible that the stress caused by the perR mutation may directly or indirectly activate resDE transcription, thereby upregulating the ResD regulon. Alternatively, these genes may be induced by the stress caused by the perR disruption, which acts independently of the ResD activity. Moreover, the expression of spx (yjbD) was induced in the perR disruptant.
TABLE 4.
Genes regulated by PerRa
| Gene | Function | Transcriptional ratio (perR/WT)b | Known regulator (reference) |
|---|---|---|---|
| ahpCF | Alkyl hydroperoxide reductase | 23.6, 16.4 | PerR (24) |
| alsD | Alpha-acetolactate decarboxylase | 14.7 | ResD (44) |
| alsS | Alpha-acetolactate synthase | 16.6 | ResD (44) |
| ctaA | Cytochrome caa3 oxidase | 6.5 | ResD (44) |
| cydABC | Cytochrome bd ubiquinol oxidase | 239.6, 195.0, 101.8 | ResD (44) |
| fnr | Transcriptional regulator of anaerobic genes | 9.2 | ResD (44) |
| fur | Iron uptake repressor | 5.1 | PerR (24) |
| glpD | Glycerol-3-phosphate dehydrogenase | 7.7 | ResD (44) |
| hemEHY | Heme synthesis | 7.2, 6.1, 4.5 | ResD (44) |
| hemAXCDBL | Heme synthesis | 21.9, 12.8, 9.1, 9.4, 6.6, 4.5 | PerR (24) |
| hmp | Flavohemoglobin | 32.6 | ResD (44) |
| katA | Catalase | 32.9 | PerR (24) |
| lctEP | l-lactate dehydrogenase and permease | 104.5, 40.1 | ResD (44) |
| mrgA | DNA-binding stress protein | 24.1 | PerR (24) |
| nasCDEF | Assimilatory nitrate reductase | 4.7, 21.3, 7.4, 4.6 | ResD (44) |
| resABC | Cytochrome c biogenesis protein | 3.1, 3.7, 3.5 | ResD (44) |
| resDE | Two-component system | 3.2, 3.2 | ResD (44) |
| spx | Inhibitor of interaction between ComA and RNAP | 9.8 | SigM (39) |
| zosA | Zinc-transporting ATPase, | 41.7 | PerR (24) |
| hag | Flagellin | 0.17 | SigD (15) |
| srfAA, AB, AC, AD | Surfactin synthetase | 0.27, 0.29, 0.041, 0.26 | ComA (9) |
| mcpB | Methyl-accepting chemotaxis protein | 0.29 | SigD (15) |
Genes that constitute an operon are shown in a single line. Ratios of increases or decreases in gene expression are shown according to gene order.
Averages of the four data sets. WT, wild type.
Notably, regarding the genes that were downregulated by perR disruption, these include the srfA operon and chemotaxis-related genes. This suggests that PerR positively regulates the expression of these genes.
srfA expression is decreased in perR disruptant cells.
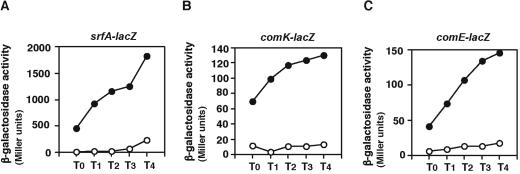
We further characterized the positive regulation of srfA expression by PerR. First, we confirmed that the disruption of perR downregulated srfA in competence medium (Fig. 2A). Since the effects of the perR mutation on expression of genes in the cell are highly pleiotropic, a control experiment was needed to demonstrate a specific effect of the perR mutation on srfA expression. We examined the expression of rapC-lacZ in the perR cells and did not observe any fluctuation of rapC-lacZ expression by the perR mutation (data not shown). Thus, it was concluded that srfA is indeed downregulated by the perR mutation. Any decrease in srfA expression would be expected to reduce the expression of comK and the late com operons and reduce the transformation efficiency of the bacterium. Indeed, the expression of both comK and the late com operon comE was almost abolished by the disruption of perR (Fig. 2B and C). Moreover, the perR disruptant exhibited a low efficiency of transformation (Table 5), which is consistent with the decrease in comK expression. Notably, this phenotype was highly unstable, probably because of the occurrence of a suppressor mutation distinct from that restoring the slow-growth phenotype (see Discussion).
FIG. 2.
Expression of srfA-lacZ, comK-lacZ, and comE-lacZ in perR disruptant cells. Cells were grown in modified competence medium, and their β-galactosidase activities were determined as described in Materials and Methods. The x axis represents the duration of culture in hours relative to the end of vegetative growth (T0). Several experiments were performed, and typical results are shown. (A) Closed circles, wild-type OAM186; open circles, perR disruptant OAM241. (B) Closed circles, wild-type 8G33; open circles, perR disruptant OAM242. (C) Closed circles, wild-type OAM243; open circles, perR disruptant OAM244.
TABLE 5.
Transformation efficiency of perR cells
| Strain | Relevant genotype | No. of viable cells (cells/ml) | No. of transformants (cells/ml) | Transformation efficiency (%)a | Ratio (%) |
|---|---|---|---|---|---|
| 168 | 2.43 × 108 | 6.5 × 103 | 0.26 × 10−2 | 100 | |
| OAM240 | perR | 0.30 × 108 | 0.05 × 103 | 0.016 × 10−2 | 6.1 |
Total DNA containing a kanamycin resistance marker was added to the cell culture at T2. After the transformation, cells were subjected to serial dilutions. Each fraction was plated onto an LB agar plate with or without kanamycin to count numbers of transformed cells and viable cells, respectively.
The ComQXP system is not involved in the reduction of srfA expression by the disruption of perR.
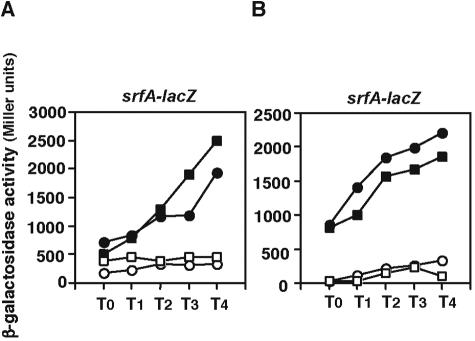
The ComQXP quorum-sensing system is a major regulator of srfA expression. Previously, it has been shown that adding conditioned medium prepared from a wild-type cell culture in stationary phase to wild-type cells in early logarithmic phase immediately induces srfA expression (22). To test the possibility that the perR disruptant cells may produce less ComX pheromone, this feeding assay was performed. As expected, the conditioned medium from wild-type cells induced early srfA expression by wild-type cells, whereas that from comXΔ cells did not (Fig. 3). However, the addition of the conditioned medium from perR disruptant cells induced levels of srfA expression by wild-type cells similar to those with the medium conditioned by wild-type cells. Thus, similar levels of ComX are present in the culture supernatants of the perR disruptant and wild-type cells.
FIG. 3.
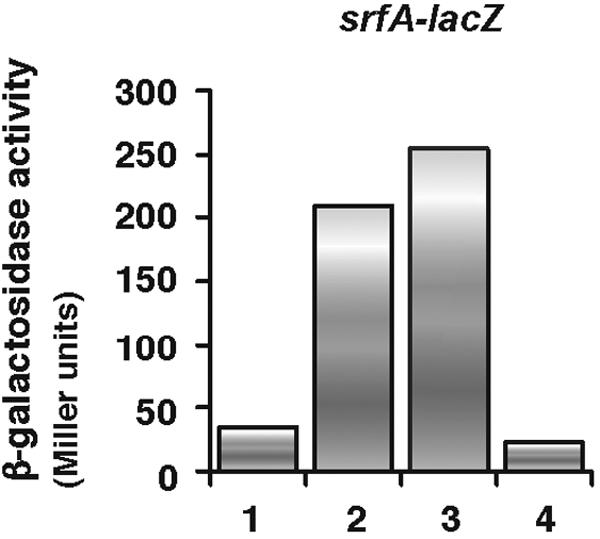
Amount of ComX in PerR disruptant cells. Various conditioned media were added to wild-type cell cultures carrying the srfA-lacZ fusion (OAM186) grown in modified competence medium at the early logarithmic phase. The conditioned media were prepared as described in Materials and Methods from the wild-type strain OAM186 (lane 2), the perR disruptant OAM241 (lane 3), and the comXΔ mutant OSM102 (lane 4). The effect of adding unconditioned modified competence medium is shown in lane 1. The β-galactosidase activities shown were those measured half an hour after the conditioned medium was added.
We also speculated on whether the decreased expression of srfA in perR disruptant cells was due to the impaired ability of the ComP receptor to bind ComX. However, this was not the case, since the transcription of pel, rapC, and rapF, which belong to the ComA regulon and require the functional ComP receptor for their expression (34), was not affected by the disruption of perR, as shown by our microarray data and the lacZ fusion analysis for rapC. These observations together suggest that the ComQXP regulatory system is functional in perR disruptant cells.
The RapC-CSF and ClpXP-Spx systems are also not involved in reduction of srfA expression by disruption of perR.
As described in the introduction, the expression of srfA is regulated by several distinct pathways. One involves RapC, an inhibitory molecule in the RapC-CSF system that directly interacts with ComA-P, and another is Spx, which destabilizes the interaction of ComA-P with RNA polymerase. Thus, both RapC and Spx are negative regulators of ComA-P (6, 29). To test whether the RapC pathway is involved in the decreased srfA expression induced by the perR disruption, an epistatic analysis of the perR mutation in the rapC mutant was performed. As shown in Fig. 4A, srfA expression was still decreased by the perR disruption in the rapC mutant, indicating that PerR does not affect srfA expression through the RapC-CSF system.
FIG. 4.
Effect of the perR disruption on the expression of srfA-lacZ in rapC and spx mutant cells. The cells were grown in modified competence medium, and their β-galactosidase activities were determined as described in Materials and Methods. The numbers on the x axis represent the duration of culture in hours relative to the end of the vegetative growth (T0). Several experiments were performed, and typical results are shown. (A) Closed circles, wild-type strain OAM186; closed squares, rapC disruptant OAM245; open circles, perR disruptant OAM241; open squares, rapC perR double disruptant OAM246. (B) Closed circles, wild-type strain OAM186; closed squares, spx disruptant OAM233; open circles, perR disruptant OAM241; open squares, spx perR double disruptant OAM247.
Since the expression of spx was induced in the perR disruptant, we speculated that higher levels of Spx may decrease the expression of srfA. If so, the introduction of the spx mutation into perR disruptant cells could suppress the effect of the perR disruption on srfA expression. To test this, we examined srfA expression in an spx perR double mutant. However, the negative effect of perR disruption on srfA expression was still observed, indicating that Spx has no role in repression of srfA in the perR cells (Fig. 4B). The result is consistent with the fact that the repressor activity of Spx has been observed only in the clpP or clpX mutant (30). Thus, we concluded that PerR positively regulates srfA expression independently of the RapC-CSF and ClpXP-Spx regulatory systems.
PerR binds to the srfA promoter.
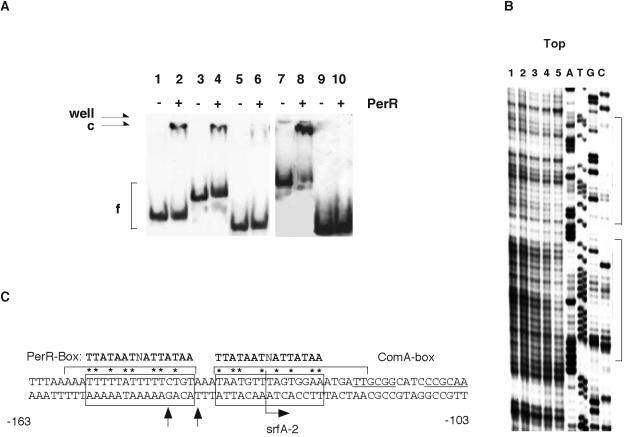
The above observations led us to hypothesize that PerR may directly regulate srfA expression by binding to the srfA promoter. To examine this, we produced a His-tagged PerR protein in E. coli and purified it from the cleared lysate of the cells. We used gel mobility shift analysis to first test whether this PerR protein could bind to the katA promoter and found it could do so in the absence of manganese (Fig. 5A, lane 2); moreover, the addition of manganese did not change the DNA-binding activity of the protein (data not shown). We speculated that the apparent manganese insensitivity of this PerR protein may be because it has already bound a metal ion to activate the protein on its second metal-binding site during its production and purification. An activated PerR protein in the production or purification process has been observed previously (17). Next, we tested whether PerR can bind directly to the srfA promoter region. As shown in Fig. 5A (lane 4), the addition of PerR to the reaction mixture containing the srfA promoter resulted in the formation of a DNA-protein complex. In contrast, PerR failed to form DNA-protein complexes with promoters of genes that PerR is known not to regulate, e.g., rapG (Fig. 5A, lane 6). To localize the PerR-bound region in the srfA promoter, we performed a gel shift assay using a srfA probe bearing a deletion. While PerR bound to the −237 to +10 region, it did not to the −130 to +10 region, indicating that the −237 to −130 region contains a PerR-bound sequence(s) (right panel in Fig. 5A). These results show clearly that PerR binds specifically to the srfA promoter.
FIG. 5.
Gel mobility shift and footprint assays of the srfA promoter using PerR. The gel shift assay was performed as described in Materials and Methods. A 6% native polyacrylamide gel was used. (A) The katA and srfA probe DNAs span positions −131 to +65 and −237 to +10 relative to the transcription start site, respectively. The rapG probe spans the 180-bp-long promoter region of rapG. These probes were prepared by PCR using the katA-1 and katA-2, srfA-1 and srfA-2, and rapG-1 and rapG-2 primers. The srfA-2 probe DNA spans position −130 to +10 relative to the transcription start site and was amplified by PCR using srfA-3 and srfA-2a. c and f indicate the protein-DNA complex and free probe, respectively. “well” means the start point of the electrophoresis. Reactions contained poly(dI-dC) (0.1 μg/25 μl). The 2 nM probes were incubated with 200 nM His-tagged PerR. − and +, reactions without and with PerR, respectively. Left panel, lanes 1 and 2, katA; lanes 3 and 4, srfA; lanes 5 and 6, rapG. Right panel, lanes 7 and 8, srfA; lanes 9 and 10, srfA-2. (B) The srfA promoter prepared by PCR using srfA-4 and srfA-2 (40 nM) was incubated in separate reactions with the increased amount of His-tagged PerR and subjected to DNase I cleavage. Brackets along the gel indicate the protected regions. Lane 1 shows no PerR, while lanes 2, 3, 4, and 5 show the effect of 0.1 μM, 0.2 μM, 0.4 μM, and 0.6 μM of PerR, respectively. (C) The nucleotide sequence of the srfA promoter region is shown. Brackets over the nucleotide sequence and arrowheads indicate the protected regions and nucleotides, respectively. The numbers at either side of the nucleotide sequence show the nucleotide positions relative to the transcription start site. The PerR and ComA boxes are boxed and underlined, respectively. The bent arrow indicates the 5′ terminus of the srfA-2 probe. The asterisks show the nucleotides matching the consensus sequence of the PerR box (10).
To further characterize the sequence(s) bound by PerR in the srfA promoter, we performed DNase I footprinting analysis. On the top strand, protection was observed in the regions spanning −158 to −141 and −137 to −117 relative to the transcription start site (Fig. 5B). As shown in Fig. 5C, each protected region contains a sequence that weakly resembles the consensus sequence for PerR recognition, i.e., the −155 to −141 and −137 to −123 regions (8 matches of 14 bases). Both sequences are located immediately upstream from the ComA box in the srfA promoter (−118 to −103). With respect to the bottom strand, although the region that is protected from DNase I cleavage was not clearly observed due to the intrinsic insensitivity to DNase I of this region, the intensities of signals from −140T and −144G were nevertheless clearly weakened (data not shown).
Confirmation that the PerR boxes in the srfA promoter region act as positive cis elements.
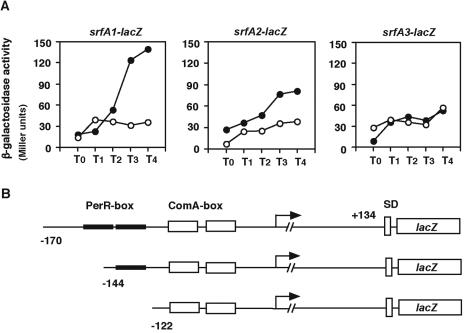
To confirm that the two PerR boxes in the srfA promoter region that we identified are positive cis elements, strains carrying transcriptional lacZ fusions using three different promoter regions of srfA at the amyE locus were constructed. The perR disruption was introduced into each strain, and the β-galactosidase activities of the strains were examined. The expression of srfA1-lacZ (−170 to +134) was decreased by the introduction of the perR disruption (Fig. 6A). This was expected, because this fusion carries both of the PerR boxes and the ComA boxes. The expression of srfA2-lacZ (−144 to +134), which carries only one of the PerR boxes, was decreased to about 65% of that of srfA1-lacZ. This decrease can be ascribed to the disruption of the upstream PerR box. The introduction of the perR disruption further decreased the expression of srfA2-lacZ, indicating that the downstream PerR box functions for the expression of the fusion. The expression of srfA3-lacZ (−122 to +134), which lacks both PerR boxes, decreased to levels similar to that of srfA1-lacZ on the perR disruption background. Thus, the srfA upstream region that lacks both PerR boxes cannot fully induce the expression of the lacZ fusion. Moreover, the expression of srfA3-lacZ was similar in wild-type and perR disruptant cells, indicating the PerR-independent expression of the fusion. This reinforced the functionality of the PerR boxes that we identified. Therefore, we concluded that these PerR boxes indeed function as positive cis elements for the expression of srfA.
FIG. 6.
Confirmation that the Per boxes function as cis elements in srfA expression. (A) Various cells were grown in modified competence medium, and sampling was initiated at late logarithmic phase. The β-galactosidase activities from the srfA-lacZ fusions were determined as described in Materials and Methods. The numbers on the x axis represent the duration of culture in hours relative to the end of vegetative growth (T0). The closed and open symbols indicate the wild-type and perR disruptant strains, respectively. Several experiments were performed, and typical results are shown. (B) Schematic representation of the srfA-lacZ fusions. The closed and open boxes indicate the PerR (−153 to −141 and −137 to −123) and ComA (−118 to −103 and −74 to −59) boxes, respectively. The 5′ and 3′ ends of the fusions are indicated as nucleotide positions relative to the transcription start point. The bent arrows show the promoter sequence of srfA. SD indicates the Shine-Dalgarno sequence of lacZ.
DISCUSSION
Here we showed for the first time that the B. subtilis molecule PerR, which represses members of the H2O2 stress regulon, can selectively activate gene expression by direct DNA binding. This is supported by a recent report showing that Fur (PerR is a B. subtilis homologue of Fur) functions as an activator by directly binding to several promoters in Neisseria meningitidis (8). In addition, the Streptococcus pyogenes PerR homologue positively regulates csp, a cold-shock protein gene, probably through DNA binding to the upstream region of csp (4). Moreover, the Borrelia oxidative stress response regulator BosR, which has 51% similarity to PerR, positively regulates napA encoding an oxidative-stress-related Dps/Dpr homologue by DNA binding to the promoter (2). In contrast, although several genes are known to be positively regulated by Fur in E. coli (40), the mechanism for this positive regulation has been elucidated as being indirect, at least with respect to genes involved in iron metabolism (23). Thus, it is not clear at this point whether all the genes that were positively regulated by PerR in B. subtilis, as determined by our microarray analysis, are direct targets of PerR binding or are downregulated only as indirect effects of the perR mutation.
Tandemly arranged PerR boxes were detected in the promoters of B. subtilis ahpC, hemA, and zosA, as well as in the srfA promoter (11, 17). In the gel shift assay using the katA or srfA probe shown in Fig. 5A, bands showing similar mobility were observed. This might be inconsistent with the fact that the katA and srfA probes carry one and two PerR boxes, respectively. In the footprint assay, His-tagged PerR only weakly protected the PerR box regions from DNase I cleavage. This suggests that PerR binds with a weak affinity to the PerR boxes in the srfA promoter. Thus, it seems that in the gel shift assay with the srfA promoter, a DNA-protein complex containing only one PerR dimer might be generated.
In the srfA promoter, the two PerR boxes are located in the promoter-distal region, whereas these boxes are located at the promoter-proximal region of genes known to be repressed by PerR (10, 17). This promoter-distal location of the PerR box in the srfA promoter is consistent with the fact that the positive and negative cis-acting sites of Fur are located in promoter-distal and -proximal regions, respectively (8). The mechanism by which PerR activates srfA remains unclear. The proximal location of the PerR boxes relative to the ComA box in the srfA promoter suggests that PerR may interact with ComA and that this is needed to induce the full activation of srfA expression by ComA. Alternatively, PerR may activate srfA expression independently of ComA.
The profile of the PerR regulon that our DNA microarray analyses revealed differs to some extent from the profile identified by Helmann et al. (16). These disparities may be due to the different media and harvesting times used (14). The perR mutant is known to grow slowly and to tend to accumulate suppressors that lead to rapid growth, like that seen with wild-type cells (5). It should be noted that the cell culture from which the RNA fraction used for the microarray analysis was obtained retained the slow-growth phenotype (data not shown).
Spo0A and DegU are response regulators that govern the initiation of sporulation and exoenzyme production, respectively (12, 21). The expression of srfA is known to be influenced by Spo0A-P and DegU-P, since disruption of spo0A or introduction of the degU32 mutation (which renders DegU-P resistant to dephosphorylation) decreases srfA expression (13, 21). The perR mutation would not affect the function of Spo0A-P, since in perR disruptant cells, the initiation of sporulation appears to be unimpaired (5; unpublished results). Moreover, we found by a plate assay that the perR disruption does not influence exoprotease production (unpublished results), which suggests that DegU-P is unlikely to be hyperactivated in perR disruptant cells, unlike when the DegU32 mutant protein is expressed. Consequently, it is unlikely that PerR affects srfA expression by influencing either regulator. Notably, CodY has also been known to repress srfA expression under an amino acid-rich condition (37). However, we used MC medium containing Casamino Acids and found srfA expression was fully induced. This indicates that CodY does not participate in the regulation of srfA expression under our experimental conditions.
In summary, we have shown here that B. subtilis PerR activates the expression of srfA by direct promoter DNA binding. It is known that H2O2 stress inactivates the DNA-binding activity of PerR (17, 24). Since PerR is required for activation of srfA, it seems that H2O2 stress inactivates PerR, thereby inhibiting srfA expression, as shown in Fig. 1A. Thus, PerR is involved in the regulatory system for expression of srfA, checking oxidative stress conditions in the cells.
Acknowledgments
We thank P. Zuber and M. M. Nakano for ORB3834.
This work was supported by the Research and Study Program of Tokai University Educational System General Research Organization to M.O. and by a Grant-in-aid for Scientific Research on Priority Areas (C) “Genome Biology” to N.O.
REFERENCES
- 1.Benson, A. K., and W. G. Haldenwang. 1993. The sigma B-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J. Bacteriol. 175:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boylan, J. A., J. E. Posey, and F. C. Gherardini. 2003. Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc. Natl. Acad. Sci. USA 100:11684-11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branda, S. S., J. E. Gonzalez-Pastor, S. Ben-Yehuda, R. Losick, and R. Kolter. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98:11621-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenot, A., K. Y. King, and M. G. Caparon. 2005. The PerR regulon in peroxide resistance and virulence of Strteptococcus pyogenes. Mol. Microbiol. 55:221-234. [DOI] [PubMed] [Google Scholar]
- 5.Casillas-Martinez, L., A. Driks, B. Setlow, and P. Setlow. 2000. Lack of a significant role for the PerR regulator in Bacillus subtilis spore resistance. FEMS Microbiol. Lett. 188:203-208. [DOI] [PubMed] [Google Scholar]
- 6.Core, L., and M. Perego. 2003. TPR-mediated interaction of RapC with ComA inhibits response regulator-DNA binding for competence development in Bacillus subtilis. Mol. Microbiol. 49:1509-1522. [DOI] [PubMed] [Google Scholar]
- 7.Cosmina, P., F. Rodriguez, F. de Ferra, G. Grandi, M. Perego, G. Venema, and D. van Sinderen. 1993. Sequence and analysis of the genetic locus responsible for surfactin synthesis in Bacillus subtilis. Mol. Microbiol. 8:821-831. [DOI] [PubMed] [Google Scholar]
- 8.Delany, I., R. Rappuoli, and V. Scarlato. 2004. Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis. Mol. Microbiol. 52:1081-1090. [DOI] [PubMed] [Google Scholar]
- 9.Dubnau, D., and C. M. Lovett, Jr. 2002. Transformation and recombination, p. 453-472. In A. L. Sonenshine, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 10.Fuangthong, M., and J. D. Helmann. 2003. Recognition of DNA by three ferric uptake regulator (Fur) homologs in Bacillus subtilis. J. Bacteriol. 185:6348-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaballa, A., and J. D. Helmann. 2002. A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Mol. Microbiol. 45:997-1005. [DOI] [PubMed] [Google Scholar]
- 12.Grossman, A. D. 1995. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu. Rev. Genet. 29:477-508. [DOI] [PubMed] [Google Scholar]
- 13.Hahn, J., and D. Dubnau. 1991. Growth stage signal transduction and the requirements for srfA induction in development of competence. J. Bacteriol. 173:7275-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatfield, G. W., S. P. Hung, and P. Baldi. 2003. Differential analysis of DNA microarray gene expression data. Mol. Microbiol. 47:871-877. [DOI] [PubMed] [Google Scholar]
- 15.Helmann, J. D., and C. P. Moran, Jr. 2002. RNA polymerases and sigma factors, p. 289-312. In A. L. Sonenshine, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 16.Helmann, J. D., M. F. Wu, A. Gaballa, P. A. Kobel, M. M. Morshedi, P. Fawcett, and C. Paddon. 2003. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbig, A. F., and J. D. Helmann. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41:849-859. [DOI] [PubMed] [Google Scholar]
- 18.Kearns, D. B., and R. Losick. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49:581-590. [DOI] [PubMed] [Google Scholar]
- 19.Kearns, D. B., F. Chu, R. Rudner, and R. Losick. 2004. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol. Microbiol. 52:357-369. [DOI] [PubMed] [Google Scholar]
- 20.Kinsinger, R. F., M. C. Shirk, and R. Fall. 2003. Rapid surface motility in Bacillus subtilis is dependent on extracellular surfactin and potassium ion. J. Bacteriol. 185:5627-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunst, F., T. Msadek, and G. Rapoport. 1994. Signal transduction network controlling degradative enzyme synthesis and competence in Bacillus subtilis, p. 1-20. In P. J. Piggot, C. P. Moran, Jr., and P. Youngman (ed.), Regulation of bacterial differentiation. American Society for Microbiology, Washington, D.C.
- 22.Magnuson, R., J. Solomon, and A. D. Grossman. 1994. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell 77:207-216. [DOI] [PubMed] [Google Scholar]
- 23.Masse, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mongkolsuk, S., and J. D. Helmann. 2002. Regulation of inducible peroxide stress responses. Mol. Microbiol. 45:9-15. [DOI] [PubMed] [Google Scholar]
- 25.Mostertz, J., C. Scharf, M. Hecker, and G. Homuth. 2004. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology 150:497-512. [DOI] [PubMed] [Google Scholar]
- 26.Mukai, K., M. Kawata, and T. Tanaka. 1990. Isolation and phosphorylation of the Bacillus subtilis degS and degU gene products. J. Biol. Chem. 265:20000-20006. [PubMed] [Google Scholar]
- 27.Nakano, M. M., L. Xia, and P. Zuber. 1991. Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis. J. Bacteriol. 1732:5487-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano, M. M., and P. Zuber. 2002. Anaerobiosis, p. 393-404. In A. L. Sonenshine, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 29.Nakano, S., M. M. Nakano, Y. Zhang, M. Leelakriangsak, and P. Zuber. 2003. A regulatory protein that interferes with activator-stimulated transcription in bacteria. Proc. Natl. Acad. Sci. USA 100:4233-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano, S., E. Kuster-Schock, A. D. Grossman, and P. Zuber. 2003. Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 100:13603-13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanamiya, H., G Akanuma, Y. Natori, R. Murayama, S. Kosono, T. Kudo, K. Kobayashi, N. Ogasawara, S. M. Park, K. Ochi, and F. Kawamura. 2004. Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosome. Mol. Microbiol. 52:273-283. [DOI] [PubMed] [Google Scholar]
- 32.Ogura, M., and T. Tanaka. 2000. Bacillus subtilis comZ (yjzA) negatively affects expression of comG but not comK. J. Bacteriol. 182:4992-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogura, M., Y. Ohshiro, S. Hirao, and T. Tanaka. 1997. A new Bacillus subtilis gene, med, encodes a positive regulator of comK. J. Bacteriol. 179:6244-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogura, M., H. Yamaguchi, K. Yoshida, Y. Fujita, and T. Tanaka. 2001. DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res. 29:3804-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogura, M. K. Shimane, K. Asai, N. Ogasawara, and T. Tanaka. 2003. Binding of response regulator DegU to the aprE promoter is inhibited by RapG, which is counteracted by extracellular PhrG in Bacillus subtilis. Mol. Microbiol. 49:1685-1697. [DOI] [PubMed] [Google Scholar]
- 36.Piazza, F., P. Tortosa, and D. Dubnau. 1999. Mutational analysis and membrane topology of ComP, a quorum-sensing histidine kinase of Bacillus subtilis controlling competence development. J. Bacteriol. 181:4540-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serror, P., and A. L. Sonenshein. 1996. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J. Bacteriol. 178:5910-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon, J. M., B. A. Lazazzera, and A. D. Grossman. 1996. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 10:2014-2024. [DOI] [PubMed] [Google Scholar]
- 39.Thackray, P. D., and A. Moir. 2003. SigM, an extracytoplasmic function sigma factor of Bacillus subtilis, is activated in response to cell wall antibiotics, ethanol, heat, acid, and superoxide stress. J. Bacteriol. 185:3491-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Touati, D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1-6. [DOI] [PubMed] [Google Scholar]
- 41.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 42.van Sinderen, D., A. ten Berge, B. J. Hayama, L. Hamoen, and G. Venema. 1994. Molecular cloning and sequence of comK, a gene required for genetic competence in Bacillus subtilis. Mol. Microbiol. 11:695-703. [DOI] [PubMed] [Google Scholar]
- 43.Westers, H., P. G. Braun, L. Westers, H. Antelmann, M. Hecker, J. D. Jongbloed, H. Yoshikawa, T. Tanaka, J. M. van Dijl, and W. J. Quax. 2005. Genes involved in SkfA killing factor production protect a Bacillus subtilis lipase against proteolysis. Appl. Environ. Microbiol. 71:1899-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye, R. W., W. Tao, L. Bedzyk, T. Young, M. Chen, and L. Li. 2000. Global gene expression profiles of Bacillus subtilis grown under anaerobic conditions. J. Bacteriol. 182:4458-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida, K.-I., K. Kobayashi, Y. Miwa, C.-M. Kang, M. Matsunaga, H. Yamaguchi, S. Tojo, M. Yamamoto, R. Nishi, N. Ogasawara, T. Nakayama, and Y. Fujita. 2001. Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucleic Acids Res. 29:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]