Abstract
Nitrite reductase (NirK) of Nitrosomonas europaea confers tolerance to nitrite (NO2−). The nirK gene is clustered with three genes of unknown physiological function: ncgABC. At present, this organization is unique to nitrifying bacteria. Here we report that the ncgABC gene products facilitate NirK-dependent NO2− tolerance by reversing the negative physiological effect that is associated with the activity of NirK in their absence. We hypothesize that the ncg gene products are involved in the detoxification of nitric oxide that is produced by NirK.
Nitrosomonas europaea is a nitrifying bacterium that makes free energy available by the aerobic oxidation of ammonia (NH3) to NO2− (12). During nitrification, this organism expresses NirK and nitric oxide reductase, both enzymes that are classically associated with anaerobic respiration in denitrifying bacteria (1, 3). Inactivation of the nirK gene of N. europaea rendered the cells more sensitive to the toxic effects of NO2− produced during nitrification (1). Recently, we demonstrated that this bacterium expresses increasing levels of NirK in response to the accumulation of NO2− in its environment (2). This NirK-dependent tolerance to NO2− might constitute a defense mechanism that protects the cell against the toxic product of aerobic NH3 oxidation (2, 10).
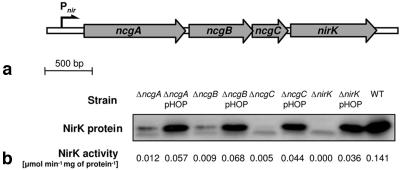
The nirK gene of N. europaea is clustered with three other genes (NE0927, NE0926, and NE0925) (1, 5). We have designated these nirK cluster genes ncgA, ncgB, and ncgC, respectively (Fig. 1a). Inspection of the preliminary genome sequence of the NO2−-oxidizing nitrifying bacterium Nitrobacter hamburgensis revealed the presence of a similar nirK gene cluster (U.S. Department of Energy Joint Genome Institute [http://www.jgi.doe.gov/]). Other bacterial nirK genes characterized thus far are transcribed either monocistronically or polycistronically in conjunction with nirV (8). The physiological role of the latter gene is not known; nirV of Rhodobacter sphaeroides is required neither for the synthesis of active NirK nor for wild-type growth (8). The ncg genes of N. europaea do not bear significant homology to nirV.
FIG. 1.
(a) nirK gene cluster of N. europaea. The small arrow indicates the nirK gene cluster promoter (Pnir) that was described previously (2). (b) Western blot detection of NirK protein and NirK activity in wild-type cells (WT) and in ncgA, ncgB, ncgC, and nirK mutant cells with and without the NirK expression vector pHOP. The second (lower) band is not related to NirK protein.
The close association of ncgABC and nirK, along with the correlation between NirK expression and the activity of a promoter located upstream of ncgA (1, 2), suggests that their gene products might also engage in functional interactions. To test this hypothesis, we constructed mutants of N. europaea in which the ncg genes were disrupted and determined the effects on (i) NirK expression and activity, (ii) cell growth, (iii) NO2− tolerance, and (iv) the physiological consequences of NirK expression.
nirK cluster genes of N. europaea.
ncgA encodes a periplasmic “blue” copper oxidase that has oxidase activity with a range of electron donors and a minor nitrite reductase activity with reduced N. europaea cytochrome c in vitro (1, 6). The N terminus of NcgA has some sequence similarity with the copper resistance protein (CopA) of Pseudomonas syringae (4). A full-length copA homologue is present at another location in the genome of N. europaea (5). ncgB and ncgC encode c-heme containing polypeptides that copurified upon isolation from the soluble protein fraction (11). The number of c-heme binding motifs (Cys-X-Y-Cys-His) present in NcgB and NcgC suggests that they are di- and monoheme c-type cytochromes, respectively. An N-terminal leader peptide was predicted for both proteins, suggesting that they reside in the periplasm, as has been established experimentally for NirK and NcgA (1, 9).
ncgABC are not required for synthesis of active NirK.
Cells of Nitrosomonas europaea ATCC 19718 and mutants thereof were cultured in liquid mineral medium containing 25 mM (NH4)2SO4 as a nitrogen and free-energy source at 30°C in shaken (175 rpm) batch cultures (150-ml culture in a 500-ml bottle with a loose cap) as described by Hyman and Arp (7). Mutants in which one of the ncg genes was disrupted were constructed by the insertion of a suicide vector, containing a transcriptional terminator, via homologous recombination and were confirmed by PCR as described previously (2). The expression level of NirK in cells that were in the early stationary growth phase was measured by Western blot analysis of NirK protein in lysates with polyclonal NirK antibodies as described in detail elsewhere (2). Disruption of ncgA, ncgB, and ncgC all resulted in a diminished expression of NirK (Fig. 1b). This was most likely due to polar effects and suggests that ncgABC and nirK are expressed as an operon. A residual amount of NirK was still present in each of these mutants. Measurement of the specific NirK activity in these protein preparations with hydroxylamine as an electron donor, performed as described previously (2), revealed that the residual NirK protein in these mutants was active. Moreover, introduction of the NirK expression vector pHOP, which contained nirK under the control of a constitutive promoter and is described elsewhere (1), in these ncg mutants resulted in an increase of the specific NirK activity that corresponded to that observed in the nirK mutant upon insertion of the same vector. Taken together, these observations show that ncgABC are not required for the synthesis of active NirK in N. europaea.
The beneficial physiological effect of NirK requires ncgABC.
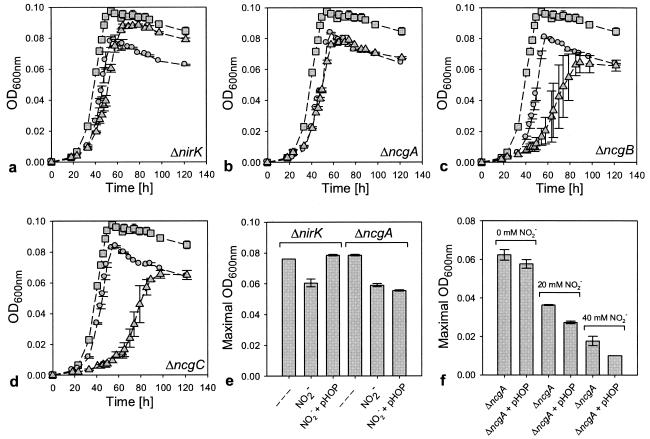
Physiologically, interruption of the ncg genes had negative effects on growth that were similar to those of the inactivation of nirK: both mutations caused cells to reach a lower maximal biomass concentration than wild-type cells (Fig. 2a through d). The negative consequences of the inactivation of nirK were partially compensated for by the introduction of the NirK expression vector, which increased the maximal biomass concentration that was reached by this mutant toward that of wild-type cells (Fig. 2a). To test whether the debilitated growth of the ncg mutants could also be reversed by increasing the level of NirK expression, we determined the growth characteristics of ncg mutant cells that harbored the NirK expression vector (Fig. 2b through d). In contrast to the nirK mutant, none of the ncg mutants reached higher maximal biomass concentrations as a result of the elevated NirK expression level (Fig. 2b through d). Strikingly, the expression of NirK in the ncgA mutant, and to a much larger extent in the ncgB and ncgC mutants, even decreased the maximal cell density that was reached. In addition, the growth rates of the ncgB and ncgC mutants were approximately halved by the introduction of the NirK expression vector.
FIG. 2.
Growth curves and maximal biomass concentrations of wild-type cells of N. europaea and of ncg and nirK mutants with and without the NirK expression vector pHOP. OD600nm, optical density at 600 nm. (a) Squares, wild-type cells; circles, nirK mutant cells; triangles, pHOP-harboring nirK mutant cells. (b) Squares, wild-type cells; circles, ncgA mutant cells; triangles, pHOP-harboring ncgA mutant cells. (c) Squares, wild-type cells; circles, ncgB mutant cells; triangles, pHOP-harboring ncgB mutant cells. (d) Squares, wild-type cells; circles, ncgC mutant cells; triangles, pHOP-harboring ncgC mutant cells. Error bars indicate the standard errors of the means (SEM) (n = 3). (e) Maximal cell densities reached by the nirK and ncgA mutants with and without pHOP and in the presence of 10 mM of NaNO2. Dashes indicate that cells did not contain pHOP and that incubation was carried out in the absence of added NaNO2. Error bars indicate the SEM (n = 2). (f) Maximal cell densities reached by ncgA mutant cells with and without pHOP in the presence of 0, 20, and 40 mM of NaNO2. Error bars indicate the SEM (n = 3).
Expression of NirK in the ncgA mutant decreases NO2− tolerance.
Batch cultures of N. europaea typically produce NO2− to a concentration of ≈20 mM at the onset of the stationary growth phase. To assess whether the reversed phenotypic consequences of NirK in the ncg mutants involved changes in the NO2− tolerance of these cells, nirK and ncgA mutants, both with and without the NirK expression vector, were cultured in the presence of added sodium nitrite (NaNO2) (Fig. 2e). The addition of 10 mM NaNO2 at the start of cultivation reduced the maximal cell densities that were reached by cultures of the nirK and ncgA mutants to the same extent. In nirK mutants, this negative effect was fully alleviated by the expression of NirK from the NirK expression vector. In contrast, the negative effect of NaNO2 on the ncgA mutant was not reduced by the introduction of this vector but rather appeared to be aggravated. To substantiate the latter observation, the effects of increasing concentrations of NaNO2 (i.e., 0, 20, and 40 mM) on the maximal cell density were examined (Fig. 2f). In the presence of 20 and 40 mM NaNO2, ncgA mutants that harbored the NirK expression vector reached a lower maximal density than those without this vector.
Conclusion.
We have characterized three novel nirK cluster genes that are required for the NirK-dependent tolerance to NO2− observed previously in N. europaea (1). Expression of NirK in mutants in which the ncg genes were disrupted reduced rather than increased the tolerance of the cells to NO2−. The data suggest that the ncgABC gene products interact with NirK, reversing a negative effect of the activity of NirK, which is otherwise essential for tolerance to NO2−. The polar effects observed in this study hamper inference of the roles of the individual ncg genes. At present, the most parsimonious hypothesis regarding the underlying mechanism is that the ncgABC gene products facilitate scavenging of the toxic NO produced by NirK.
Acknowledgments
This work was financially supported by The Netherlands Organization for Scientific Research (NWO).
We are grateful to Bas van Schooten and Willem N. M. Reijnders for excellent technical assistance.
REFERENCES
- 1.Beaumont, H. J. E., N. G. Hommes, L. A. Sayavedra-Soto, D. J. Arp, D. M. Arciero, A. B. Hooper, H. V. Westerhoff, and R. J. M. van Spanning. 2002. Nitrite reductase of Nitrosomonas europaea is not essential for production of gaseous nitrogen oxides and confers tolerance to nitrite. J. Bacteriol. 184:2557-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaumont, H. J. E., S. I. Lens, W. N. M. Reijnders, H. V. Westerhoff, and R. J. M. van Spanning. 2004. Expression of nitrite reductase in Nitrosomonas europaea involves NsrR, a novel nitrite-sensitive transcription repressor. Mol. Microbiol. 54:148-158. [DOI] [PubMed] [Google Scholar]
- 3.Beaumont, H. J. E., B. van Schooten, S. I. Lens, H. V. Westerhoff, and R. J. M. van Spanning. 2004. Nitrosomonas europaea expresses a nitric oxide reductase during nitrification. J. Bacteriol. 186:4417-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cha, J. S., and D. A. Cooksey. 1991. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 88:8915-8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chain, P., J. Lamerdin, F. Larimer, W. Regala, V. Lao, M. Land, L. Hauser, A. Hooper, M. Klotz, J. Norton, L. Sayavedra-Soto, D. Arciero, N. Hommes, M. Whittaker, and D. Arp. 2003. Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J. Bacteriol. 185:2759-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiSpirito, A. A., L. R. Taaffe, J. D. Lipscomb, and A. B. Hooper. 1985. A ‘blue’ copper oxidase from Nitrosomonas europaea. Biochim. Biophys. Acta 827:320-326. [Google Scholar]
- 7.Hyman, M. R., and D. J. Arp. 1992. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J. Biol. Chem. 267:1534-1545. [PubMed] [Google Scholar]
- 8.Jain, R., and J. P. Shapleigh. 2001. Characterization of nirV and a gene encoding a novel pseudoazurin in Rhodobacter sphaeroides 2.4.3. Microbiology 147:2505-2515. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 10.Poth, M., and D. D. Focht. 1985. 15N kinetic analysis of N2O production by Nitrosomonas europaea: an examination of nitrifier denitrification. Appl. Environ. Microbiol. 49:1134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittaker, M., D. Bergmann, D. Arciero, and A. B. Hooper. 2000. Electron transfer during the oxidation of ammonia by the chemolithotrophic bacterium Nitrosomonas europaea. Biochim. Biophys. Acta 1459:346-355. [DOI] [PubMed] [Google Scholar]
- 12.Wood, P. M. 1986. Nitrification as bacterial energy source, p. 39-62. In J. I. Prosser (ed.), Nitrification. Society for General Microbiology, IRL Press, Oxford, United Kingdom.