Abstract
During evolution, pathogenic bacteria have developed complex interactions with their hosts. This has frequently involved the acquisition of virulence factors on pathogenicity islands, plasmids, transposons, or prophages, allowing them to colonize, survive, and replicate within the host. In contrast, Mycoplasma species, the smallest self-replicating organisms, have regressively evolved from gram-positive bacteria by reduction of the genome to a minimal size, with the consequence that they have economized their genetic resources. Hence, pathogenic Mycoplasma species lack typical primary virulence factors such as toxins, cytolysins, and invasins. Consequently, little is known how pathogenic Mycoplasma species cause host cell damage, inflammation, and disease. Here we identify a novel primary virulence determinant in Mycoplasma mycoides subsp. mycoides Small Colony (SC), which causes host cell injury. This virulence factor, released in significant amounts in the presence of glycerol in the growth medium, consists of toxic by-products such as H2O2 formed by l-α-glycerophosphate oxidase (GlpO), a membrane-located enzyme that is involved in the metabolism of glycerol. When embryonic calf nasal epithelial cells are infected with M. mycoides subsp. mycoides SC in the presence of physiological amounts of glycerol, H2O2 is released inside the cells prior to cell death. This process can be inhibited with monospecific anti-GlpO antibodies.
Mycoplasma species represent the smallest self-replicating organisms detected on earth. Their genomes range from the 580 kb of Mycoplasma genitalium (24) to the 1,358 kb of Mycoplasma penetrans (40) and serve as a blueprint for the design of synthetic living organisms (18). This leads to the drastic economizing of genetic resources and to an obligate parasite lifestyle. Pathogenic Mycoplasma species cause mainly atypical pneumonia, urogenital infections, and arthritis in humans and in animals (7, 8, 25). In contrast to other pathogenic bacteria where virulence is mostly determined by toxins, invasins, and cytolysins, pathogenic Mycoplasma species appear to have no such typical primary virulence factors, as revealed by the genomic sequence analysis of the eight species that have been completely sequenced (17, 24, 26, 28, 32, 35, 40, 48). Although diagnosis of mycoplasmal infections has improved significantly since the introduction of PCR methods, and antigenic variability has been studied in detail in several Mycoplasma species (20, 39), there is currently very little knowledge available on the molecular mechanisms and the effectors that allow pathogenic mycoplasmas to cause host cell damage, inflammation, and disease.
We have studied Mycoplasma mycoides subsp. mycoides Small Colony (SC), the etiological agent of contagious bovine pleuropneumoniae (CBPP), a severe infectious disease causing major losses of livestock, as a model to investigate the molecular basis of mycoplasmal virulence. M. mycoides subsp. mycoides SC is an extracellular pathogen with a genome size of 1,211 kb (48) that lives in close association with the host cells. The rationale for the use of this species as a model is the high virulence of this species as well as the fact that it is clearly established as the etiological agent of CBPP. Furthermore, this severe cattle disease is of extraordinary socioeconomic importance to livestock production in countries that currently suffer CBPP outbreaks (23). In addition, countries that are free of this epidemic are continuously threatened by reemerging infections.
Recently, we suggested that M. mycoides subsp. mycoides SC might use particular, highly efficient metabolic pathways and the release of toxic intermediates or side products as virulence determinants (46). This hypothesis is based on the observation that highly virulent strains of M. mycoides subsp. mycoides SC that cause CBPP in Africa possess a particularly active ATP-binding cassette (ABC) transport system for the assimilation of glycerol, which is metabolized to dihydroxyacetone-phosphate (DHAP) with the release of hydrogen peroxide (H2O2), while less virulent strains from recent European outbreaks lacked part of the glycerol uptake genes due to a deletion. These latter consequently produced less H2O2 in the presence of glycerol compared to the highly virulent African strains.
It has been proposed that H2O2 production of certain pathogenic Mycoplasma species causes lysis of erythrocytes, peroxidation of lipids of infected fibroblasts, and inhibition of ciliary movement in infected tracheal organs (34, 45). Using catalase-deficient mice, H2O2 production of Mycoplasma pulmonis was suggested as a mycoplasmal virulence factor (15). Thus, since M. mycoides subsp. mycoides SC, and mycoplasmas in general, are devoid of catalase and dismutase activities as determined by genome sequence analysis (48), targeted release of H2O2 and other accompanying reactive oxygen species (ROS) to host cells may be responsible for inflammation and tissue damage. The current study investigates the function of l-α-glycerophosphate oxidase (GlpO) and the impact of release of H2O2/ROS, resulting from glycerol metabolism of M. mycoides subsp. mycoides SC, on cytotoxicity toward host cells. For this purpose, we have developed an in vitro system using primary embryonic calf nasal epithelial cells (ECaNEp cells) to assess mycoplasma-induced cytotoxicity.
MATERIALS AND METHODS
Strains, cells, growth conditions, and DNA extraction.
M. mycoides subsp. mycoides SC strain Afadé, a highly virulent field strain isolated in 1968 at Farcha Laboratory, N′Djaména, Chad, was used for the virulence studies unless otherwise noted. This strain causes CBPP under natural and experimental conditions (2). A less virulent strain, M. mycoides subsp. mycoides SC strain L2, which lacks the active glycerol uptake system GtsABC (46), has been used where stated specifically. Furthermore, the type strain PG1 and 10 other strains from African and European outbreaks were also used in this study for genetic analyses. Mycoplasmal cultures were grown in mycoplasma broth medium to a density of 108 to 109 CFU/ml or on solid mycoplasma agar medium (Axcell Biotechnologies, St. Genis l'Argentière, France). Growth and handling of live M. mycoides subsp. mycoides SC were performed in a biological safety laboratory fulfilling the BL3 containment safety standards. DNA extraction was performed as previously described (19). For genetic manipulation and subcloning, Escherichia coli strains DH5α [F− φ80dlacZΔM15 Δ(lacZYA-argF) U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1] and BL21(DE3) [F′ dcm ompT hsdS(rB− mB−) gal λ(DE3)] were used. The pETHIS-1 expression vector (41) was used for expression of recombinant polyhistidine N- and C-terminal fusion proteins.
ECaNEp cells were prepared from fetuses obtained from a local abattoir and were maintained in minimal essential medium (MEM)-Earle medium supplemented with 7% fetal calf serum and penicillin (100 IU/ml) in 24-well microtiter plates and used at a confluent density of 2 × 105 cells per well, at 37°C in a humidified 5% CO2 atmosphere (42). Fetal calf serum and cell culture media were purchased from Seromed (Biochrom, Munich, Germany). Cells were routinely screened for contamination by mycoplasmas using PCR or for contamination by bovine viral diarrhea virus using immunostaining.
PCR amplification, Southern blotting, and DNA sequence analysis.
A glpO-specific DNA probe was constructed by PCR in the presence of digoxigenin-11-dUTP (Roche Diagnostics, Rotkreuz, Switzerland) using the oligonucleotide primers glpO_EcoRI_N (5′-TCGAATTCAATGAAGCAAACAAAAGTTGATATTTG-3′) and glpO_NotI_C (5′-TTGCGGCCGCATTTCCATGGAAGAATAGCTTCTTC-3′) using standard PCR conditions (19). Extraction of genomic DNA from mycoplasmas and Southern blot analysis were performed as previously described (36).
DNA sequencing was performed with a DNA Sequenator AB 3100 genetic analyzer and a Taq DyeDeoxy Terminator cycle sequencing kit (Applied Biosystems, Norwalk, Conn.), with oligonucleotide primers glpO_EcoRI_N and glpO_NotI_C and by primer walking using glpO internal primers. The DNA and deduced amino acid sequences were analyzed with the PC/Gene program PROSITE (6). Sequence comparisons with sequences in the GenBank and EMBL databases were performed using BLAST (3). Analysis of proteins was carried out by using the programs Motif Scan (http://hits.isb-sib.ch/cgi-bin/hits_motifscan) and TMpred (http://www.ch.embnet.org/software/TMPRED_form.html), and “toppred” software (47).
Cloning, site-directed mutagenesis, and expression of recombinant GlpO.
The glpO gene of M. mycoides subsp. mycoides SC strain Afadé was first amplified with the primers glpO_EcoRI_N and glpO_NotI_C containing the restriction sites for EcoRI and NotI, respectively. Furthermore, primer glpO_NotI_C included a mutated TGG to encode tryptophan in E. coli (TGGTrp). The other two mycoplasma-specific TGATrp codons in the glpO gene were replaced with TGGTrp codons, using the overlap extension-PCR method (14) with the primer pairs (carrying the appropriate nucleotide substitutions) glpO_mut1L (5′-GAAGACTGGATCAAAGAAATGGA-3′) and glpO_mut1R (5′-TTTGATCCAGTCTTCATAACGTTT-3′) or glpO_mut2L (5′-GCTAATTGGCAACCAAAAGAAGA-3′) and glpO_mut2R (5′-TGGTTGCCAATTAGCCTTTTTATC-3′). The PCR product was cloned into pETHIS-1 by flanking EcoRI and NotI cleavage sites. The construct was analyzed by DNA sequencing and introduced into E. coli BL21(DE3) for expression and purification via Ni2+ chelation chromatography of the polyhistidine-tailed fusion protein as described previously for other proteins (41).
Sera, polyclonal antibodies, immunoglobulin purification, and Fab preparation.
Bovine sera from a controlled experimental infection with the African M. mycoides subsp. mycoides SC strain Afadé have been described in detail by Abdo and colleagues (2). Polyclonal monospecific serum directed against recombinant GlpO was obtained by subcutaneous immunization of rabbits with 160 μg of purified recombinant polyhistidine-tailed protein GlpO in 500 μl of phosphate-buffered saline (PBS) buffer, pH 8.0 (50 mM Na2HPO4 · NaH2PO4, pH 8.0, 140 mM NaCl), mixed with 500 μl of Adjuvant 10 (Gerbu Biotechnik GmbH, Gaiberg, Germany), followed by booster immunizations with 40 and 20 μg of protein at 2 and 4 weeks, respectively. The rabbits were bled 10 days after the last booster immunization. Antisera were prepared from the blood samples and stored at −20°C.
Immunoglobulin G (IgG) fractions from rabbit anti-GlpO serum and from preserum of the same rabbit were purified with the HiTrap Protein G kit (Amersham Pharmacia Biotech, Uppsala, Sweden) as directed by the manufacturer. Preparation of Fab fragments was performed with the ImmunoPure Fab Preparation kit (Pierce, Rockford, Ill.) according to the manufacturer's instructions. Fab fragments were dialyzed overnight against PBS buffer, pH 7.4, and then filter sterilized. Protein concentrations were determined using the method of Bradford (12). Monospecific rabbit serum directed against lipoprotein LppC has been previously described (37).
Immunoblot analysis, Triton X-114 partitioning, and growth inhibition test.
Total antigens from mycoplasmas were prepared as previously described (22). Immunoblotting was carried out with bovine serum at a dilution of 1:2,000 and rabbit monospecific serum anti-GlpO at a dilution of 1:1,000 (4).
M. mycoides subsp. mycoides SC total antigen from a stationary phase culture was separated into hydrophobic and hydrophilic fractions by the Triton X-114 partitioning method (11). Samples from the Triton X-114 detergent phase and the aqueous phase were analyzed by immunoblotting with the monospecific, polyclonal antibodies directed against GlpO and with the bovine serum directed against M. mycoides subsp. mycoides SC.
The growth inhibition tests were performed by spotting 5 and 10 μl of undiluted, decomplemented monospecific rabbit serum against GlpO, purified IgG, or Fab fragments of anti-GlpO IgG onto the mycoplasma-containing agar medium and incubating the plates at 37°C for 4 days. Serum against M. mycoides subsp. mycoides SC (2) was used as a positive control, and monospecific rabbit serum against LppQ (1) was used as a negative control. Observation of growth inhibition was carried out under a light microscope as previously described (33, 38).
Scanning electron microscopy (SEM) and immunogold labeling.
For electron microscopy, M. mycoides subsp. mycoides SC cells were cultured at 37°C for 5 days in mycoplasma broth medium on gold or platinum-sputtered coverslips that had been precoated with poly-l-lysine. Cells were washed three times with PBS buffer, pH 7.4, at 37°C and fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. After being washed with PBS, the coverslips were blocked in PBS buffer supplemented with 0.2 M glycine and 1% bovine serum albumin for 15 min at room temperature, and thereafter they were incubated with IgG from rabbit serum anti-GlpO diluted 1:100 or 1:50 in PBS supplemented with 1% bovine serum albumin overnight at 4°C. Samples were then washed with PBS for 10 min and labeled with 15-nm colloidal gold-conjugated goat anti-rabbit IgG (British Biocell International, Cardiff, United Kingdom) diluted 1:50 in PBS for 90 min at room temperature. Coverslips were washed with 0.1 M cacodylate buffer, pH 7.4, and processed for SEM following standard protocols. Briefly, samples were osmicated in 1.33% OsO4 with 0.11% ruthenium red in 0.13 M cacodylate buffer, pH 7.4, for 15 min (43, 44), washed with 0.1 M cacodylate buffer, dehydrated through an ascending ethanol series, and dried by evaporation of hexamethyldisilazane (Sigma, Buchs, Switzerland) (13).
Secondary electron and corresponding back-scattered electron signals were examined in a high-resolution field emission scanning electron microscope DSM 982 Gemini (Zeiss, Oberkochen, Germany) at an accelerating voltage of 5 kV, a working distance of 8 mm, and a magnification of ×50,000 to ×100,000.
Control experiments included omission of primary antibody as well as the use of a rabbit anticalcitonine antibody (Anawa Biomedical Services and Products, Zurich, Switzerland) and of rabbit preimmune serum, respectively.
Quantification of H2O2 production and inhibition assay.
To measure H2O2 production, strains of M. mycoides subsp. mycoides SC were grown in mycoplasma culture medium for 3 days at 37°C to a density of approximately 5 × 108 CFU/ml. The culture was centrifuged at 8,000 × g for 10 min at 4°C, washed once in incubation medium (67.6 mM HEPES, pH 7.3, 140 mM NaCl, 7 mM MgCl2), resuspended in prewarmed incubation medium at 37°C at a density of 109 CFU/ml, portioned in aliquots of 1 ml, and incubated at 37°C for 1 h. To induce H2O2 production, glycerol was added to the mycoplasma suspensions at a final concentration of 100 μM, the physiological concentration in bovine serum. The production of H2O2 was measured with the peroxide test (Merck KgaA, Darmstadt, Germany) as described previously (46) at time zero and at 1, 2, 5, 10, 20, and 120 min after the addition of glycerol. In order to inhibit the l-α-glycerolphosphate oxidase GlpO, mycoplasmas were pretreated with purified Fab fragments of IgG directed against GlpO by incubation at concentrations of 0.26 μg/ml to 2.6 μg/ml, followed by two washes with PBS buffer. To assess the viability of M. mycoides subsp. mycoides SC cells after induction of H2O2 production, aliquots of the reaction assays were plated on mycoplasma agar plates at the end of the assay and grown at 37°C.
Assessment of cytotoxic activity.
ECaNEp cells (42) were grown in 24-well plates until confluence was reached. Prior to the assay, the medium was removed and replaced by 200 μl of MEM-Earle medium without supplements or by MEM-Earle medium supplemented with 100 μM glycerol. The ECaNEp cells were then infected at a multiplicity of infection (MOI) of 50 mycoplasmas per cell. To block the GlpO activity, M. mycoides subsp. mycoides SC cells were pretreated with anti-GlpO Fab fragments at 0.26 μg/ml. Viable ECaNEp cells were counted after fixation and staining with 0.75% crystal violet, 0.25% NaCl, 1.75% formaldehyde, and 50% ethanol and photographed under phase-contrast microscopy at various times after infection. Purified Fab fragments from polyclonal IgG directed against the membrane lipoprotein LppC of M. mycoides subsp. mycoides SC were used as a control since LppC is not correlated to the glycerol metabolism (37). In order to determine whether H2O2 produced during growth of M. mycoides subsp. mycoides SC prior to contact with ECaNEp cells would be deleterious to the bovine cells, the supernatant of cultures of M. mycoides subsp. mycoides SC grown in the presence of glycerol was filtered through a 0.22-μm-pore-size filter (Millipore, Bedford, Mass.) and added to ECaNEp cells. The ECaNEp cell viability was assessed by trypan blue exclusion.
Detection of oxidative stress caused by H2O2 and other ROS in ECaNEp cells.
The oxidation of 5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester (CM-H2DCFDA; Molecular Probes, Eugene, Oreg.) was used to assess oxidative stress by intracellular ROS in ECaNEp cells. This dye enters the cells and produces a fluorescent signal after intracellular oxidation by ROS. The same conditions used for the assessment of cytotoxic activity were employed with the following modifications. As controls, half of the wells containing ECaNEp cells were treated with 30 mM N-acetyl-l-cysteine to prevent oxidation by H2O2 and ROS. Then, the ECaNEp cells were incubated for 1 h with 10 μM CM-H2DCFDA and washed once with MEM-Earle medium. Cells were then infected at an MOI of 500 mycoplasmas per cell in the presence or absence of glycerol. To block the GlpO activity, M. mycoides subsp. mycoides SC cells were pretreated with anti-GlpO Fab fragments at a concentration of 0.26 μg/ml. As a control, cells were treated with H2O2 solutions ranging from 150 μM to 4.4 mM for 20 min. Intracellular H2O2 and ROS were monitored 20 min after infection with mycoplasmas by fluorescence microscopy using a Nikon Eclipse TE 300 microscope. Note that all steps involving CM-H2DCFDA, including handling of this chemical, were performed in the dark.
Nucleotide sequence accession numbers.
The EMBL/GenBank accession numbers for the nucleotide sequences of glpO from M. mycoides subsp. mycoides SC strains Afadé and L2 are AJ581566 and AJ581564, respectively. The sequences of the glpO genes from Mycoplasma sp. bovine group 7 strain PG50 and M. mycoides subsp. capri strain PG3 have been deposited under accession numbers AJ581565 and AJ581567, respectively.
RESULTS
Genetic and functional analysis of the glpO gene.
The glpO gene from M. mycoides subsp. mycoides SC encodes a 387-amino-acid polypeptide, GlpO, with a predicted molecular mass of 42.7 kDa and a pI of 8.14. It contains three TGATrp codons. The protein has a putative FAD-binding site at amino acid positions 8 to 36 and appears to be surface exposed. TMpred identified two significant transmembrane regions, spanning amino acids 6 to 25 and 140 to 157. The glpO gene is followed by the genes glpK (putative glycerol phosphate kinase) and glpF (putative glycerol facilitator factor) in the highly virulent strain Afadé. The same gene arrangement is observed for the type strain PG1 (48). Southern blot analysis of genomic DNA with the gene probe for glpO showed the presence of glpO in M. mycoides subsp. mycoides SC strains Afadé, L2, and the type strain PG1 and in 10 other strains from African and European outbreaks that were analyzed (data not shown). Immunoblot analysis of total antigens with monospecific anti-GlpO IgG revealed a distinct 45-kDa protein band confirming the expression of the glpO gene in all strains of M. mycoides subsp. mycoides SC tested (data not shown). The amino acid sequence of GlpO from M. mycoides subsp. mycoides SC strain Afadé and strain L2 was found to be identical to that of the GlpO of the type strain PG1 (48) and showed similarity to the glycerol-3-phosphate dehydrogenases of various Mycoplasma species (Table 1).
TABLE 1.
Comparison of the protein sequences of GlpO and GtsABC from M. mycoides subsp. mycoides SC strain Afadé with sequences of the homologues from other mycoplasmas
| Mycoplasma species | Protein namea | EMBL/GenBank accession no. | Identity (%) | Similarity (%) | |||
|---|---|---|---|---|---|---|---|
| GlpO (CAE46342)
|
|||||||
| M. sp. bovine group 7 | GlpO | CAE46341 | 99 | 99 | |||
| M. mycoides subsp. capri | GlpO | CAE46343 | 97 | 99 | |||
| M. penetrans | GlpA | BAC44427 | 58 | 75 | |||
| M. gallisepticum | MGA_0646 | AAP56366 | 52 | 73 | |||
| M. mobile | GlpD | AAT27781 | 52 | 71 | |||
| M. pulmonis | GlpD | CAC13437 | 46 | 70 | |||
| M. hyopneumoniae | GlpD | AAV27992 | 44 | 64 | |||
| M. pneumoniae | GlpD | AAB95751 | 44 | 64 | |||
| M. genitalium | MG039 | AAC71255 | 40 | 62 | |||
| GtsA (AAG41804)
|
|||||||
| M. sp. bovine group 7 | GtsA | CAD12044 | 96 | 97 | |||
| M. hyopneumoniae | GtsA | AAV27549 | 42 | 62 | |||
| M. gallisepticum | MGA_1076 | AAP56596 | 39 | 59 | |||
| M. pulmonis | MYPU_4970 | CAC13670 | 33 | 52 | |||
| GtsB (AAG41805)
|
|||||||
| M. sp. bovine group 7 | GtsB | CAD12045 | 96 | 99 | |||
| M. hyopneumoniae | MHP392 | AAV27550 | 37 | 55 | |||
| M. pulmonis | MYPU_4980 | CAC13671 | 33 | 56 | |||
| M. gallisepticum | MGA_1077 | AAP56597 | 33 | 53 | |||
| GtsC (AAG41806)
|
|||||||
| M. sp. bovine group 7 | GtsC | CAD12046 | 89 | 96 | |||
| M. hyopneumoniae | MHP393 | AAV27551 | 37 | 57 | |||
| M. gallisepticum | MGA_1078 | AAP56598 | 36 | 51 | |||
| M. pulmonis | MYPU_4990 | CAC13672 | 31 | 55 | |||
Or open reading frame designation.
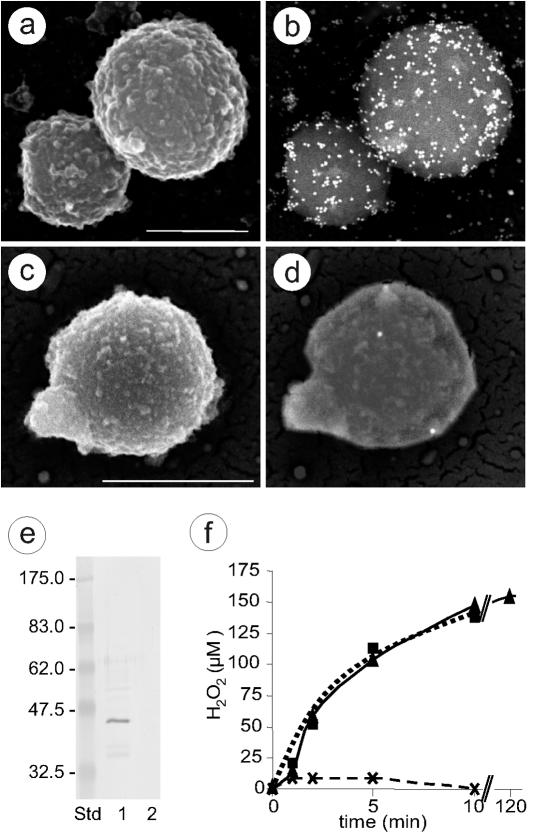
Genome sequence analysis predicts that mycoplasmas are devoid of catalase and dismutase activities (17, 24, 26, 28, 32, 35, 40, 48). Since cytoplasmic GlpO activity would induce intracellular H2O2 toxicity that in its turn would be deleterious to the mycoplasma itself, we hypothesized that the enzyme must be located at the surface membrane. Sequence analysis of GlpO or homologous enzymes from various pathogenic Mycoplasma species (listed in Table 1) using “toppred” software revealed transmembrane structures predicting domains of these enzymes to be surface located. Direct evidence for the presence of GlpO at the cell surface of M. mycoides subsp. mycoides SC was provided by SEM after immunogold labeling using IgG from monospecific rabbit anti-GlpO serum (Fig. 1a to d). To confirm the location of GlpO in the mycoplasmal membrane, total protein of M. mycoides subsp. mycoides SC was subjected to Triton X-114 phase partitioning. Anti-GlpO serum reacted strongly with a 45-kDa protein (GlpO) in the Triton X-114 phase, which was absent from the aqueous phase, indicating that GlpO is an integral membrane protein of M. mycoides subsp. mycoides SC (Fig. 1e). Immunoblot analysis of the Triton X-114 extract of M. mycoides subsp. mycoides SC with bovine serum from a cow that was experimentally infected with this strain (2) revealed the GlpO-specific band at 45 kDa that comigrated on the gel with GlpO (data not shown), showing that GlpO is involved in seroconversion during CBPP infection of cattle.
FIG. 1.
Location and activity of l-α-glycerophosphate oxidase. (a to d) SEM photographs showing immunogold labeling of M. mycoides subsp. mycoides SC strain Afadé incubated with IgG from anti-GlpO (a and b) and preimmune serum (c and d). Secondary electron micrographs show the cell surface (a and c), and back-scattered electron micrographs reveal 15-nm colloidal gold-conjugated secondary antibody (b and d). Scale bar, 500 nm. (e) Immunoblot analysis of Triton X-114-fractionated total antigens of M. mycoides subsp. mycoides SC strain Afadé with GlpO antiserum. Lane 1, detergent phase, 20 μg of proteins; lane 2, aqueous phase, 20 μg of proteins; Std, molecular mass standard. (f) Hydrogen peroxide production of M. mycoides subsp. mycoides SC strain Afadé after the addition of 100 μM glycerol. The data shown are the mean values of five independent measurements. Standard deviations of the individual measurements were below 5% of the mean values. Triangles and solid line, untreated M. mycoides subsp. mycoides SC; crosses and dashed line, M. mycoides subsp. mycoides SC pretreated with Fab fragments from anti-GlpO IgG; squares and dotted line, M. mycoides subsp. mycoides SC pretreated with Fab fragments from anti-LppC.
In order to confirm the function of GlpO as an l-α-glycerophosphate oxidase, we measured the production of H2O2 after the addition of glycerol to an axenic culture of M. mycoides subsp. mycoides SC in the presence or absence of Fab fragments of monospecific anti-GlpO IgG. As shown in Fig. 1f, the addition of 100 μM glycerol to the culture at mid-exponential growth phase instantly resulted in the release of H2O2 into the growth medium, reaching 150 μM after 10 min, a concentration that was retained up to 2 h (Fig. 1f). The release of H2O2 could be blocked specifically when the mycoplasma suspensions were pretreated with monospecific polyclonal anti-GlpO antibodies or Fab fragments of anti-GlpO IgG at a minimal concentration of 0.26 μg/ml but not by Fab fragments of anti-LppC IgG used as a control antibody (Fig. 1f). LppC is a surface lipoprotein of M. mycoides subsp. mycoides SC that is not related to the glycerol metabolism. The addition of catalase to the culture of M. mycoides subsp. mycoides SC after supplementation with glycerol reduced the H2O2 levels in the medium to a concentration below 1 μM, which is the detection level of the assay. The generation time of M. mycoides subsp. mycoides SC was 3.3 h without or with the addition of anti-GlpO antibodies or anti-GlpO Fab fragments. Furthermore, anti-GlpO antibodies had no effect in the serum-drop growth inhibition tests (data not shown). An axenic culture that was grown in the presence of 100 μM glycerol for 2 h and that produced 150 μM H2O2 showed a generation time of 3.2 h, while the control culture to which no glycerol was added had a generation time of 3.3 h. Hence, the viability of mycoplasmas was not affected by the glycerol-induced production of H2O2.
Cytotoxicity of M. mycoides subsp. mycoides SC toward bovine epithelial cells.
To assess whether the production of H2O2 and related ROS, resulting from glycerol metabolism, contributes to the virulence of M. mycoides subsp. mycoides SC, we analyzed the cytotoxicity toward ECaNEp cells under various conditions (Fig. 2). As shown in Fig. 2b, infection of ECaNEp cells with M. mycoides subsp. mycoides SC strain Afadé at an MOI of 50 mycoplasmas per cell resulted in only a weak cytotoxic effect 1 h postinfection in the absence of glycerol in the culture medium. However, when the infection of the ECaNEp cells with the mycoplasma was made in the presence of physiological concentrations of glycerol, most ECaNEp cells detached from the surface and subsequently underwent complete lysis (Fig. 2c). This cytotoxic effect could be blocked when the mycoplasmas were pretreated with anti-GlpO Fab fragments at a concentration of 0.26 μg/ml, which concomitantly block H2O2 production. Under these conditions, the ECaNEp cells showed no morphological changes 1 h postinfection (Fig. 2d). The cytotoxicity of M. mycoides subsp. mycoides SC grown in glycerol-containing medium was not inhibited by anti-LppC Fab fragments that were used as a control (Fig. 2e). Only a weak cytotoxic effect was observed when ECaNEp cells were infected, in the presence of glycerol, with a less virulent strain (L2) that lacks genes for the active glycerol transporter GtsABC and produces only low amounts of H2O2 (46) (Fig. 2f). In control experiments, the addition of glycerol, anti-GlpO Fab or anti-LppC Fab alone or in combination did not affect the ECaNEp cells (data not shown). The kinetics of cytotoxicity experiments (Fig. 2g) show that induction of cytotoxicity occurs rapidly after the addition of glycerol to ECaNEp cells infected with M. mycoides subsp. mycoides SC, reaching 75% mortality after 2 h. At a higher MOI (500 mycoplasmas per cell), full mortality of the cells was reached at 30 min after the addition of glycerol. Pretreatment of mycoplasmas with anti-GlpO Fab fully prevented cell mortality over the entire observation period (Fig. 2g). In the absence of added glycerol, infection of ECaNEp cells with M. mycoides subsp. mycoides SC shows only a weak cytotoxic effect (Fig. 2g), which could also be inhibited by anti-GlpO Fab and might therefore be due to residual amounts of glycerol in the cell or culture medium. Interestingly, filtered supernatant of M. mycoides subsp. mycoides SC cultures grown in the presence of glycerol, containing approximately 150 μM H2O2, or the addition of 150 μM H2O2 to the cell cultures had no visible cytotoxic effect on ECaNEp cells after a 1-h exposure. Cytotoxicity induced by the addition of exogenous H2O2 to ECaNEp cell cultures was first reached at a concentration of 4.4 mM, with most of the cells dying after 1 h of exposure. This concentration is 30 times higher than that measured in growth medium of M. mycoides subsp. mycoides SC in presence of glycerol.
FIG. 2.
Cytotoxicity of M. mycoides subsp. mycoides SC to ECaNEp cells (a to f) and cell viability assay (g). ECaNEp cells (a; control); ECaNEp 1 h after infection with M. mycoides subsp. mycoides SC strain Afadé (b); ECaNEp 1 h after infection with strain Afadé in the presence of 100 μM glycerol (c); ECaNEp 1 h after infection in the presence of glycerol (100 μM) with strain Afadé pretreated with Fab fragments from anti-GlpO IgG (d); ECaNEp 1 h after infection in the presence of glycerol (100 μM) with strain Afadé pretreated with Fab fragments from anti-LppC IgG (e); ECaNEp cells infected with M. mycoides subsp. mycoides SC strain L2 (which lacks an active glycerol uptake system) in the presence of 100 μM glycerol (f). The MOI in all experiments was 50 mycoplasmas per cell. (g) Viable ECaNEp cells at different times following infection with M. mycoides subsp. mycoides SC strain Afadé at an MOI of 50 mycoplasmas per cell. Triangles and thick solid line, infection with strain Afadé in the presence of 100 μM glycerol; crosses and dotted line, infection in the presence of 100 μM glycerol with strain Afadé pretreated with anti-GlpO Fab fragments (0.26 μg/ml); squares and dashed line, strain Afadé infection without glycerol; diamonds and thin solid line, ECaNEP cells alone (control).
To monitor oxidative stress in the ECaNEp cells caused by intracellular H2O2 and other ROS after infection with M. mycoides subsp. mycoides SC or addition of glycerol, we pretreated ECaNEp cells with CM-H2DCFDA and detected intracellular oxidation of this compound by fluorescence microscopy (Fig. 3). The cleavage of the ester groups by oxidation of CM-H2DCFDA results in the formation of dichlorofluorescein derivatives in the cells, which are highly fluorescent, while nonoxidized CM-H2DCFDA is nonfluorescent. As shown in Fig. 3c and d, infection of ECaNEp cells with M. mycoides subsp. mycoides SC resulted in a strong induction of fluorescence in ECaNEp cells 20 min after the addition of glycerol, reflecting the presence of intracellular H2O2 or ROS. In contrast, no fluorescence was detected in infected ECaNEp cells without the addition of glycerol (Fig. 3a and b). Intracellular oxidation of CM-H2DCFDA did not occur when the mycoplasmas were treated with anti-GlpO antibodies prior to the infection of ECaNEp cells (Fig. 3e and f). Furthermore, intracellular oxidation of CM-H2DCFDA was blocked by treating the ECaNEp cells with the antioxidant agent, N-acetyl-l-cysteine, prior to the addition of glycerol to the culture medium (Fig. 3g and h). Intracellular oxidation of ECaNEp cells did not occur upon the addition of exogenous H2O2 at 150 μM, a concentration corresponding to that released by M. mycoides subsp. mycoides SC 10 min after the addition of glycerol. As was the case for the induction of cytotoxicity, to achieve oxidation of intracellular CM-H2DCFDA in ECaNEp cells, concentrations of 4.4 mM exogenous H2O2 were necessary (Fig. 3i and j).
FIG. 3.
Detection of intracellular H2O2 and ROS in ECaNEp cells infected with M. mycoides subsp. mycoides SC strain Afadé at an MOI of 500 mycoplasmas per cell or treated with H2O2. Phase-contrast micrographs (a, c, e, g, and i) and fluorescence micrographs (b, d, f, h, and j) of ECaNEp cells as follows: 20 min after infection with M. mycoides subsp. mycoides SC strain Afadé in the absence of glycerol (a and b); infected with strain Afadé in medium supplemented with glycerol (c and d); infected in medium supplemented with glycerol with strain Afadé pretreated with Fab fragments from anti-GlpO IgG (e and f); pretreated with N-acetyl-l-cysteine and then infected with strain Afadé in medium with glycerol (g and h); incubated for 20 min in medium supplemented with 4.4 mM H2O2 (i and j).
DISCUSSION
In the present study, GlpO of M. mycoides subsp. mycoides SC was identified as a membrane protein that plays a central role in cytotoxicity toward ECaNEp cells. In the presence of physiological concentrations of glycerol, M. mycoides subsp. mycoides SC at a density of 109 CFU/ml releases relatively large amounts of H2O2, up to 150 μM into the culture medium. This amount of H2O2 is approximately 20-fold more than that produced by mycoplasmas grown in presence of glucose (31), which was reported to stimulate H2O2 production by just 50% (15). When ECaNEp cells were exposed to M. mycoides subsp. mycoides SC in the presence of physiological concentrations of glycerol, H2O2 was first detected in their cytosol, and, subsequently, cell death occurred. We noticed a marked discrepancy between the H2O2 concentration released by the mycoplasmas into the medium and the concentration required to trigger CM-H2DCFDA oxidation and cell death. Thus, filtered mycoplasmal growth medium containing 150 μM H2O2 or the addition of 150 μM exogenous H2O2 to ECaNEp cells did not lead to detectable CM-H2DCFDA oxidation in the cytosol, nor did it lead to cell death within 1 h. This indicates that close contact between mycoplasmas and host cells is necessary to successfully target the toxic compounds to the host cells. In this context, it is worth noting that M. mycoides subsp. mycoides SC—and most other pathogenic mycoplasmas for that matter—tightly attach to their host cells but do not penetrate. Moreover, active uptake of glycerol is necessary to obtain the glycerol-induced cytotoxic effect on ECaNEp cells. The European strain L2 of M. mycoides subsp. mycoides SC, which is devoid of the GtsABC transporter required for active uptake of glycerol and which produces 10 times less H2O2 at a significantly lower rate (46), has only a weak cytotoxic effect on ECaNEp cells under identical experimental conditions. The residual uptake of glycerol by strain L2 is thought to occur by means of a less efficient transport pathway mediated by the putative glycerol facilitator factor GlpF.
Based on our results, we propose the following model for triggering cellular damage to eukaryotic cells by M. mycoides subsp. mycoides SC (Fig. 4): glycerol present in the interstitial fluid is incorporated actively via the highly active ABC glycerol transporter (GtsA, GtsB, and GtsC) (46) and is subsequently phosphorylated into glycerol-3-phosphate. This, in turn, is oxidized in the presence of O2 by GlpO into DHAP, which enters in the glycolytic pathway, and produces one molecule of H2O2. Facilitated by the intimate contact of the mycoplasma with the host cell membrane, H2O2 and accompanying ROS enter the host cell. Inside the host cells, H2O2 and ROS act as powerful mediators of cell injury and inducers of inflammatory processes. They are expected to damage the host either by directly impairing tissue cells or inducing host gene expression, e.g., proinflammatory genes via activation of NF-κB (5) or via the Fenton reaction (21). Interestingly, mycoplasmas have previously been shown to induce a respiratory burst in phagocytes (30), suggesting that host-generated ROS might further contribute to tissue damage.
FIG. 4.
Model for triggering host cell inflammation by M. mycoides subsp. mycoides SC. GtsABC: active glycerol transport and phosphorylation system; GlpF: glycerol facilitator factor; GlpK: glycerol kinase; G3P: glycerol-3-phosphate. Thick arrows indicate the main virulence pathway of M. mycoides subsp. mycoides SC.
H2O2 and ROS can also activate NF-κB and, by doing so, induce the expression of a range of immune and proinflammatory genes in the eukaryotic host (16, 27, 29), a mechanism that might be of particular importance in respiratory tract infections caused by mycoplasmas. Infection with Mycoplasma species has also been linked to the development of AIDS, and it has been proposed that it could act as a cofactor of HIV, contributing to a faster progression of the disease (9). Since there are NF-κB binding sites in the human immunodeficiency virus long terminal repeat (10), H2O2 produced by mycoplasmas can be expected to promote transcription of the viral genome.
In summary, this study describes a novel mechanism of pathogenicity, involving a pathway consisting of active glycerol uptake and metabolism, which may apply to mycoplasmal pathogens in general. This is underlined by the presence of homologues of glpO and of the gtsABC genes involved in glycerol uptake in other Mycoplasma species, as revealed by analysis of genomic sequences (Table 1). Our observations provide new clues concerning the evolution of microbial pathogenicity. Moreover, surface-exposed metabolic enzymes that contribute to pathogenicity may also represent promising vaccine targets to control mycoplasmal diseases.
Acknowledgments
We are grateful to F. Thiaucourt, CIRAD-EMVT, Montpellier, France, for the gift of strains, to A. Galina, Universidade Federal do Rio de Janeiro, Brazil, for advice on fluorescence experiments, to D. Belin, University of Geneva, Switzerland, for fruitful discussions, to L. Nebel, University of Bern, Switzerland, for the cultivation of bovine cells, and to S. Burr, University of Bern, for editorial help. We thank I. Roditi for constructive criticisms.
This study was supported by Swiss Ministry of Education and Science grant no. BBW99.0849 as part of the EU 5th Framework, program INCO, contract no. ICA4-CT-2000-30015.
In recognition for his pioneering study of metabolic pathways of Mycoplasma species, this work is dedicated to Roger Miles, Kings College, London, United Kingdom, who died in 2002.
REFERENCES
- 1.Abdo, E.-M., J. Nicolet, and J. Frey. 2000. Antigenic and genetic characterization of lipoprotein LppQ from Mycoplasma mycoides subsp. mycoides SC. Clin. Diagn. Lab. Immunol. 7:588-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdo, E.-M., J. Nicolet, R. Miserez, R. Gonçalves, J. Regalla, C. Griot, A. Bensaide, M. Krampe, and J. Frey. 1998. Humoral and bronchial immune responses in cattle experimentally infected with Mycoplasma mycoides subsp. mycoides small colony type. Vet. Microbiol. 59:109-122. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1999. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 5.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 6.Bairoch, A., P. Bucher, and K. Hofmann. 1995. The PROSITE database, its status in 1995. Nucleic Acids Res. 24:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baseman, J. B., and J. G. Tully. 1997. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg. Infect. Dis. 3:21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanchard, A., and G. F. Browning (ed.). 2005. Mycoplasmas: molecular biology, pathogenicity and strategies for control. Horizon Bioscience, Wymondham, U.K.
- 9.Blanchard, A., L. Montagnier, and M. L. Gougeon. 1997. Influence of microbial infections on the progression of HIV disease. Trends Microbiol. 5:326-331. [DOI] [PubMed] [Google Scholar]
- 10.Bohnlein, E., J. W. Lowenthal, M. Siekevitz, D. W. Ballard, B. R. Franza, and W. C. Greene. 1988. The same inducible nuclear protein regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell 53:827-836. [DOI] [PubMed] [Google Scholar]
- 11.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. [PubMed] [Google Scholar]
- 12.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 13.Braet, F., R. De Zanger, and E. Wisse. 1997. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J. Microsc. 186:84-87. [DOI] [PubMed] [Google Scholar]
- 14.Braman, J., C. Papworth, and A. Greener. 1996. Site-directed mutagenesis using double-stranded plasmid DNA templates. Methods Mol. Biol. 57:31-44. [DOI] [PubMed] [Google Scholar]
- 15.Brennan, P. C., and R. N. Feinstein. 1969. Relationship of hydrogen peroxide production by Mycoplasma pulmonis to virulence for catalase-deficient mice. J. Bacteriol. 98:1036-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chabot, F., J. A. Mitchell, J. M. Gutteridge, and T. W. Evans. 1998. Reactive oxygen species in acute lung injury. Eur. Respir. J. 11:745-757. [PubMed] [Google Scholar]
- 17.Chambaud, I., R. Heilig, S. Ferris, V. Barbe, D. Samson, F. Galisson, I. Moszer, K. Dybvig, H. Wróblewski, A. Viari, E. P. C. Rocha, and A. Blanchard. 2001. The complete genome sequence of the murine respiratory pathogen Mycoplasma pulmonis. Nucleic Acids Res. 29:2145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Check, E. 2002. Venter aims for maximum impact with minimal genome. Nature 420:350. [DOI] [PubMed] [Google Scholar]
- 19.Cheng, X., J. Nicolet, F. Poumarat, J. Regalla, F. Thiaucourt, and J. Frey. 1995. Insertion element IS1296 in Mycoplasma mycoides subsp. mycoides small colony identifies a European clonal line distinct from African and Australian strains. Microbiology 141:3221-3228. [DOI] [PubMed] [Google Scholar]
- 20.Citti, C., and R. Rosengarten. 1997. Mycoplasma genetic variation and its implication for pathogenesis. Wien. Klin. Wochenschr. 109:562-568. [PubMed] [Google Scholar]
- 21.Crichton, R. R., S. Wilmet, R. Legssyer, and R. J. Ward. 2002. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J. Inorg. Biochem. 91:9-18. [DOI] [PubMed] [Google Scholar]
- 22.Fleury, B., D. Bergonier, X. Berthelot, Y. Schlatter, J. Frey, and E. M. Vilei. 2001. Characterization and analysis of a stable serotype-associated membrane protein (P30) of Mycoplasma agalactiae. J. Clin. Microbiol. 39:2814-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Agriculture Organization of the United Nations. 2003. Contagious bovine pleuropneumonia. EMPRES Transboundary Anim. Dis. Bull. 24:2-7. [Google Scholar]
- 24.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, et al. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 25.Frey, J. 2002. Mycoplasmas of animals, p. 73-90. In S. Razin and R. Herrmann (ed.), Molecular biology and pathogenicity of mycoplasmas. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 26.Himmelreich, R., H. Hilbert, H. Plagens, E. Pirkl, B. C. Li, and R. Herrmann. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 24:4420-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Israel, A. 2000. The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol. 10:129-133. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe, J. D., N. Stange-Thomann, C. Smith, D. DeCaprio, S. Fisher, J. Butler, S. Calvo, T. Elkins, M. G. FitzGerald, N. Hafez, C. D. Kodira, J. Major, S. Wang, J. Wilkinson, R. Nicol, C. Nusbaum, B. Birren, H. C. Berg, and G. M. Church. 2004. The complete genome and proteome of Mycoplasma mobile. Genome Res. 14:1447-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karin, M., and M. Delhase. 2000. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin. Immunol. 12:85-98. [DOI] [PubMed] [Google Scholar]
- 30.Koppel, P., E. Peterhans, G. Bertoni, R. Keist, P. Groscurth, R. Wyler, and R. Keller. 1984. Induction of chemiluminescence during interaction of tumoricidal effector cell populations and tumor cells is dependent on the presence of mycoplasma. J. Immunol. 132:2021-2029. [PubMed] [Google Scholar]
- 31.Miles, R. J., R. R. Taylor, and H. Varsani. 1991. Oxygen uptake and H2O2 production by fermentative Mycoplasma spp. J. Med. Microbiol. 34:219-223. [DOI] [PubMed] [Google Scholar]
- 32.Minion, F. C., E. J. Lefkowitz, M. L. Madsen, B. J. Cleary, S. M. Swartzell, and G. G. Mahairas. 2004. The genome sequence of Mycoplasma hyopneumoniae strain 232, the agent of swine mycoplasmosis. J. Bacteriol. 186:7123-7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moller, B. R. 1979. A modification of the mycoplasma serum-drop growth inhibition test. J. Appl. Bacteriol. 47:97-104. [DOI] [PubMed] [Google Scholar]
- 34.Niang, M., R. F. Rosenbusch, M. C. Debey, Y. Niyo, J. J. Andrews, and M. L. Kaeberle. 1998. Field isolates of Mycoplasma ovipneumoniae exhibit distinct cytopathic effects in ovine tracheal organ cultures. Zentralbl. Veterinamed. A 45:29-40. [DOI] [PubMed] [Google Scholar]
- 35.Papazisi, L., T. S. Gorton, G. Kutish, P. F. Markham, G. F. Browning, D. K. Nguyen, S. Swartzell, A. Madan, G. Mahairas, and S. J. Geary. 2003. The complete genome sequence of the avian pathogen Mycoplasma gallisepticum strain R(low). Microbiology 149:2307-2316. [DOI] [PubMed] [Google Scholar]
- 36.Pilo, P., B. Fleury, M. Marenda, J. Frey, and E. M. Vilei. 2003. Prevalence and distribution of the insertion element ISMag1 in Mycoplasma agalactiae. Vet. Microbiol. 92:37-48. [DOI] [PubMed] [Google Scholar]
- 37.Pilo, P., S. Martig, J. Frey, and E. M. Vilei. 2003. Antigenic and genetic characterisation of lipoprotein LppC from Mycoplasma mycoides subsp. mycoides SC. Vet. Res. 34:761-775. [DOI] [PubMed] [Google Scholar]
- 38.Poveda, J. B., and R. Nicholas. 1998. Serological identification of mycoplasmas by growth and metabolic inhibition tests. Methods Mol. Biol. 104:105-111. [DOI] [PubMed] [Google Scholar]
- 39.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki, Y., J. Ishikawa, A. Yamashita, K. Oshima, T. Kenri, K. Furuya, C. Yoshino, A. Horino, T. Shiba, T. Sasaki, and M. Hattori. 2002. The complete genomic sequence of Mycoplasma penetrans, an intracellular bacterial pathogen in humans. Nucleic Acids Res. 30:5293-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaller, A., R. Kuhn, P. Kuhnert, J. Nicolet, T. J. Anderson, J. I. MacInnes, R. P. A. M. Segers, and J. Frey. 1999. Characterization of apxIVA, a new RTX determinant of Actinobacillus pleuropneumoniae. Microbiology 145:2105-2116. [DOI] [PubMed] [Google Scholar]
- 42.Schweizer, M., and E. Peterhans. 1999. Oxidative stress in cells infected with bovine viral diarrhoea virus: a crucial step in the induction of apoptosis. J. Gen. Virol. 80:1147-1155. [DOI] [PubMed] [Google Scholar]
- 43.Stoffel, M. H., A. Busato, and A. E. Friess. 2002. Density and distribution of anionic sites on boar ejaculated and epididymal spermatozoa. Histochem. Cell Biol. 117:441-445. [DOI] [PubMed] [Google Scholar]
- 44.Stoffel, M. H., and A. E. Friess. 2002. Demonstration and cytochemical analysis of anionic sites on ejaculated boar spermatozoa: a scanning electron microscopy study using cationised colloidal gold. Histochem. Cell Biol. 117:61-67. [DOI] [PubMed] [Google Scholar]
- 45.Tryon, V. V., and J. B. Baseman. 1992. Pathogenic determinants and mechanisms, p. 457-471. In J. Maniloff, R. N. McElhaney, L. R. Finch, and J. B. Baseman (ed.), Mycoplasmas: molecular biology and pathogenesis. American Society for Microbiology, Washington, D.C.
- 46.Vilei, E. M., and J. Frey. 2001. Genetic and biochemical characterization of glycerol uptake in Mycoplasma mycoides subsp. mycoides SC: Its impact on H2O2 production and virulence. Clin. Diagn. Lab. Immunol. 8:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Heijne, G. 1992. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487-494. [DOI] [PubMed] [Google Scholar]
- 48.Westberg, J., A. Persson, A. Holmberg, A. Goesmann, J. Lundeberg, K. E. Johansson, B. Pettersson, and M. Uhlen.2004. The genome sequence of Mycoplasma mycoides subsp. mycoides SC type strain PG1T, the causative agent of contagious bovine pleuropneumonia (CBPP). Genome Res. 14:221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]