Abstract
Periplasmic nitrate reductase (napFDAGHBC operon product) functions in anaerobic respiration. Transcription initiation from the Escherichia coli napF operon control region is activated by the Fnr protein in response to anaerobiosis and by the NarQ-NarP two-component regulatory system in response to nitrate or nitrite. The binding sites for the Fnr and phospho-NarP proteins are centered at positions −64.5 and −44.5, respectively, with respect to the major transcription initiation point. The E. coli napF operon is a rare example of a class I Fnr-activated transcriptional control region, in which the Fnr protein binding site is located upstream of position −60. To broaden our understanding of napF operon transcriptional control, we studied the Haemophilus influenzae Rd napF operon control region, expressed as a napF-lacZ operon fusion in the surrogate host E. coli. Mutational analysis demonstrated that expression required binding sites for the Fnr and phospho-NarP proteins centered at positions −81.5 and −42.5, respectively. Transcription from the E. coli napF operon control region is activated by phospho-NarP but antagonized by the orthologous protein, phospho-NarL. By contrast, expression from the H. influenzae napF-lacZ operon fusion in E. coli was stimulated equally well by nitrate in both narP and narL null mutants, indicating that phospho-NarL and -NarP are equally effective regulators of this promoter. Overall, the H. influenzae napF operon control region provides a relatively simple model for studying synergistic transcription by the Fnr and phospho-NarP proteins acting from class I and class II locations, respectively.
Facultative aerobes such as Escherichia coli can respire with a variety of terminal electron acceptors, including oxygen, nitrate, dimethyl sulfoxide (DMSO), and fumarate. Synthesis of the corresponding respiratory enzymes is regulated in response to the preferred acceptors, oxygen and nitrate. During anaerobic growth, the Fnr protein (fumarate and nitrate reductases) activates transcription initiation at many operons, including the narGHJI and dmsABC operons encoding the respiratory enzymes cytochrome b-linked nitrate reductase and DMSO reductase, respectively (17, 21, 59).
The Fnr protein is homologous to the well-studied Crp protein (cyclic AMP receptor protein; also known as Cap, catabolite gene activator protein). The Crp and Fnr proteins bind as homodimers to DNA sites of dyad symmetry upstream of regulated promoters, from whence they stimulate transcription initiation. The Crp protein is activated upon binding its allosteric effector, cyclic AMP, whereas the Fnr protein is activated upon assembly of its oxygen-labile iron-sulfur cluster (23, 28).
Two types of simple Crp- and Fnr-dependent transcription control regions are defined (6, 8). Class I control regions have a Crp or Fnr binding site located 60 or more nucleotides (nt) upstream of the transcription initiation point (8, 65). From these distal locations, Crp and Fnr make specific contacts with the RNA polymerase α-subunit carboxyl-terminal domain (α-CTD). Class II control regions have a Crp or Fnr binding site located near position −40 with respect to the transcription initiation site, overlapping or replacing the −35 promoter element. From this proximal location, Crp and Fnr make specific contacts with both the α-CTD and the σ70 subunit of RNA polymerase. Several examples of class I and class II Crp-dependent promoters are known (8). However, expression from most known Fnr-dependent promoters, including those for the narG and dmsA operons, is activated through Fnr class II mechanisms (6, 31).
As defined formally, class I and II promoters are regulated by only a single activator protein, whereas promoters controlled by multiple activators are designated class III (8). Nevertheless, we refer here to multiply activated promoters as class I or class II in order to denote the locations of the respective regulatory protein binding sites.
Transcription initiation for a subset of Fnr-activated operons is further regulated by nitrate, which induces synthesis of enzymes for nitrate respiration and represses synthesis of enzymes for respiration of other anaerobic acceptors. Response to nitrate is mediated by the NarX sensor kinase, which controls phosphorylation of the NarL response regulator (57). Phospho-NarL binds to upstream sites in the narG operon control region to stimulate transcription activation in synergy with the Fnr protein (10, 61), and it also binds to operator sites in the dmsA operon control region to repress transcription (3).
The E. coli napFDAGHBC operon encoding cytochrome c-linked nitrate reductase (periplasmic nitrate reductase) contains an Fnr site centered at −64.5 nt with respect to the major transcription initiation point and therefore is a rare example of a class I Fnr-activated operon (9, 11). Expression of the napFE. coli (napFEc) operon is further induced by nitrate and nitrite, acting through the NarQ-NarP two-component regulatory system, a paralog of the NarX-NarL system (53). The phospho-NarP protein binds to a site centered at −44.5 nt with respect to the major transcription initiation point to activate transcription in synergy with the Fnr protein (11, 13). Thus, in this context, the phospho-NarP protein can be considered a class II activator.
The napFEc operon control region exhibits complexities that may limit its utility as a simple model for studying Fnr class I and phospho-NarP class II transcription activation. The phospho-NarL protein also binds to the site centered at −44.5 but fails to stimulate transcription activation. Thus, it competes for binding with, but antagonizes activation by, the phospho-NarP protein (11, 13). In addition, the major promoter for napFEc operon transcription overlaps a minor promoter of uncertain physiological significance (9, 13, 54). Finally, expression of the napFEc operon is also regulated by the molybdate-responsive ModE protein, which binds to a site centered at −134.5 with respect to the major transcription initiation point (41, 54).
We report here our analysis of the Haemophilus influenzae Rd napF control region, which we transplanted into E. coli as a Φ(napFHi-lacZ) monocopy operon fusion (where napFHi denotes napFH. influenzae). Results demonstrate that in E. coli at least, transcription from the napFHi promoter (i) is stimulated by the Fnr protein from a site centered at −81.5 nt upstream of the transcription initiation point, (ii) is further stimulated by either the phospho-NarP or phospho-NarL protein from a site centered at −42.5, and (iii) is not responsive to molybdate limitation or ModE protein control. Therefore, this control region provides a relatively simple example of a promoter that is controlled by an Fnr class I transcription activation mechanism, in synergy with phospho-NarP or phospho-NarL bound at a class II location.
(Studies with H. influenzae presented here were submitted by Catherine T. Yen in 1998 as part of an undergraduate thesis for the Cornell University Division of Biological Sciences Honors Program.)
MATERIALS AND METHODS
Strains and plasmids.
Strains and plasmids are listed in Table 1. Genetic crosses were performed by P1 kc-mediated generalized transduction (42). Null alleles of nar regulatory genes have been described previously (46). Standard methods were used for restriction endonuclease digestion, ligation, transformation, and PCR amplification of DNA (38).
TABLE 1.
E. coli K-12 strains
| Strain | Genotype | Reference or source |
|---|---|---|
| VJS676 | F− λ− prototroph Δ(argF-lacIZYA)U169 | 56 |
| Derivatives of strain VJS676 | ||
| VJS4797 | λΦ(napFEc-lacZ) Δ85 | 11 |
| VJS5101 | λΦ(napFEc-lacZ) Δ85 narL215::Tn10 | 11 |
| VJS5109 | λΦ(napFEc-lacZ) Δ85 narP253::Tn10d(Cm) | 11 |
| VJS5117 | λΦ(napFEc-lacZ) Δ85 narL215::Tn10 narP253::Tn10d(Cm) | 11 |
| VJS6621 | λΦ(napFHi-lacZ) Δ110 | This study |
| VJS6623 | λΦ(napFHi-lacZ) Δ110 narL215::Tn10 | This study |
| VJS6625 | λΦ(napFHi-lacZ) Δ110 narP253::Tn10d(Cm) | This study |
| VJS6627 | λΦ(napFHi-lacZ) Δ110 narL215::Tn10 narP253::Tn10d(Cm) | This study |
| VJS6906 | λΦ(napFHi-lacZ) Δ260 | This study |
| VJS6907 | λΦ(napFHi-lacZ) Δ260 (NarP/NarL site mutant) | This study |
| VJS6908 | λΦ(napFHi-lacZ) Δ260 (Fnr site mutant) | This study |
Culture media and conditions.
Defined, complex, and indicator media for genetic manipulations were used as described previously (38). Defined medium to grow E. coli cultures for enzyme assays and for RNA extraction was buffered with 3-{N-morpholino}propanesulfonic acid (MOPS) as previously described (56). The initial pH of this medium is set at 8.0 to ameliorate nitrite toxicity. Because the pKa of MOPS is 7.2, the buffering capacity of this medium continually increases as acidic fermentation products accumulate; at harvest, cultures typically had a pH value of about 7.5. Medium for culturing H. influenzae was full-strength heart infusion broth supplemented with NAD and hemin (1) and buffered with MOPS (pH 8.0). The respiratory oxidants NaNO3 (40 mM), NaNO2 (5 mM), DMSO (40 mM), and sodium fumarate (40 mM) were added as indicated.
Cultures were grown at 37°C to the mid-exponential phase. Culture densities were monitored with a Klett-Summerson photoelectric colorimeter (Klett Manufacturing Co., New York, N.Y.) equipped with a number 66 (red) filter. Anaerobic cultures for enzyme assays and for RNA extraction were grown in screw-cap tubes as described previously (32).
Enzyme assays.
β-Galactosidase activities were determined at room temperature (approximately 21°C) by following the hydrolysis of o-nitrophenyl-β-d-galactopyranoside in CHCl3-sodium dodecyl sulfate-permeabilized cells. Specific activities are expressed in arbitrary (Miller) units (22).
Nitrate reductase activities were determined at room temperature by following the production of nitrite in intact cells (56). Cells were suspended in 0.32 M potassium phosphate, pH 7.1, and stored on ice. Samples (0.8 ml) were mixed with 0.1 ml of 0.5 mg ml−1 benzyl viologen. Reactions were started by adding 0.1 ml of a mixture containing 4 mg ml−1 Na2S2O4, 4 mg ml−1 NaHCO3, and 0.5 M NaNO3. Reactions were terminated by vigorous vortex mixing (to oxidize the viologen), and 1 ml each of sulfanilic acid and N-1-napthylethylenediamine solutions was added. Specific activities are expressed in arbitrary units analogous to Miller units (56).
All cultures were assayed in duplicate, and reported values are averaged from at least two independent experiments.
Construction of napFHi control region alterations.
The source DNA for the napFHi control region was a plasmid pUC8 shotgun subclone, designated GHIEP28, isolated for the H. influenzae genome sequencing project (16) and purchased from the American Type Culture Collection (Manassas, Va.). Oligonucleotide-directed site-specific mutagenesis was used to introduce substitutions into the napFHi operon control region or its flanking sequences. Mutagenesis followed the ampicillin selection protocol (33). PCRs were performed with a high-fidelity thermostable DNA polymerase (Accuzyme; Bioline USA, Reno, Nev.).
Following each round of mutagenesis, the DNA sequence for the entire fragment was determined to eliminate isolates with spurious nucleotide substitutions. The control region cassettes were then recloned into the operon fusion vector pRS415 (51). The resulting Φ(napFHi-lacZ) operon fusions were crossed into bacteriophage λRS45 (51), and monocopy lysogens were identified by a whole-colony PCR test (45).
Transcript analysis.
Analysis by rapid amplification of cDNA ends (5′-RACE) (47), also termed anchored PCR, used reagents purchased from Invitrogen Life Technologies (5′-RACE system, version 2.0; Invitrogen Life Technologies, Carlsbad, Calif.) and was performed essentially as described by the manufacturer's instructions. Oligonucleotide primers used were as follows: 5′-GGGTAACGCCAGGGTTTTCC (gene-specific primer 1 in lacZ), 5′-GTTTTCCCAGTCACGAC (gene-specific primer 2 in lacZ; M13 forward primer), 5′-GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG (abridged anchor primer), 5′-AAGCTTAGTGAATCCGTAATCATGGTCATAG (gene-specific primer 3 in lacZ), and 5′-GGCCACGCGTCGACTAGTAC (universal amplification primer).
RESULTS
H. influenzae is a facultative aerobe.
Haemophilus spp. are classified phylogenetically as members of the gamma subdivision of the proteobacteria and are very close relatives of the enterobacteria (14). H. influenzae is indigenous to the mucous membranes of the human upper respiratory tract, and many strains cause infections of the middle ear (otitis media) or respiratory tract (40). Although defined media have been developed (25), even complex media must be supplemented with NAD (factor V) and hemin (factor X).
H. influenzae Rd is known to use both oxygen and nitrate as respiratory oxidants (62). More-extensive analysis of the hemin-independent species H. parainfluenzae revealed that oxygen, nitrate, and fumarate serve as electron acceptors. Furthermore, in H. parainfluenzae, the amount and types of cytochromes are regulated in response to oxygen and nitrate availability, and nitrate reductase synthesis is induced by nitrate during anaerobic growth (52, 63). Thus, like their enterobacterial relatives, Haemophilus spp. regulate the synthesis of respiratory enzymes in response to growth conditions.
The H. influenzae Rd strain KW20 genome encodes very few transcriptional regulatory proteins (16). Nevertheless, it does encode the regulators that in E. coli are known to control anaerobic respiration: the Fnr and ModE proteins and the NarQ-NarP two-component system, described in the introduction, as well as the ArcB-ArcA two-component system, which controls citrate cycle enzyme synthesis in response to ubiquinone-dependent respiration (18). Together with the physiological studies summarized immediately above, this indicates that regulation of respiratory gene expression in H. influenzae Rd is likely very similar to that in E. coli.
As deduced from the respective DNA sequences, the H. influenzae Fnr protein (257 residues) shares 80% sequence identity over 236 residues with the E. coli Fnr protein (254 residues). The FnrHi protein (encoded by the fnr gene; locus tag HI1425) contains all four Cys residues shown to be essential for FnrEc function (20, 28), and the two protein sequences are identical in a 32-residue span that encompasses the helix-turn-helix DNA binding domain (29). The fnrHi gene complements an E. coli fnr null allele (19, 39).
As with E. coli, the H. influenzae narP (locus tag HI0726) and narQ (locus tag HI0267) genes are unlinked (16). The NarPHi protein (208 residues) shares 59% identity over 204 residues with the NarPEc protein (215 residues), and the NarQHi protein (567 residues) shares 38% identity over 204 residues with the NarQEc protein (566 residues). Identities along the multidomain NarQ sequences are localized in discrete patches corresponding to discrete functions (53). The narPHi gene is essential for nitrate induction of nitrate reductase synthesis (this study; see below), and the narQHi gene complements an E. coli narQ null allele (C. T. Yen and V. Stewart, unpublished data).
NarP-dependent nitrate respiration by H. influenzae.
We cultured H. influenzae Rd strain KW20 and its narP null derivative, strain MGH90 (22), as described in Materials and Methods. Both strains grew well with aeration, exhibiting exponential-phase doubling times of approximately 1 h and achieving relatively high culture densities of about 120 Klett units after 8 h of cultivation (data not shown). Both strains grew more slowly with DMSO or fumarate as the electron acceptor, with exponential-phase doubling times of approximately 3.5 to 4 h and culture densities of only about 50 Klett units. Cultures with no added electron acceptor exhibited very slow growth to a culture density of about 30 Klett units.
The narP+ strain grew relatively well with nitrate as the electron acceptor, exhibiting an exponential-phase doubling time of approximately 1 h and achieving a final culture density of about 60 Klett units. By contrast, the narP null strain grew very slowly with nitrate (doubling time of >8 h) and achieved a final culture density of only about 40 Klett units. Thus, the narP+ gene was specifically required only for nitrate respiration.
The H. influenzae Rd genome contains the napFDAGHBC operon encoding cytochrome c-linked nitrate reductase but does not contain a narGHJI operon for cytochrome b-linked nitrate reductase (44). We measured periplasmic nitrate reductase specific activity as described in Materials and Methods. The narP+ strain synthesized about 25 U of activity after anaerobic growth with fumarate as the electron acceptor and about 120 U after anaerobic growth with nitrate plus fumarate. The narP null strain by contrast synthesized only about 5 U irrespective of added nitrate. Enzyme activity was insensitive to azide (55), as expected for cytochrome c-linked nitrate reductase (44). Thus, the narP+ gene was required for nitrate induction of periplasmic nitrate reductase synthesis in H. influenzae.
napFHi transcription initiation point in E. coli.
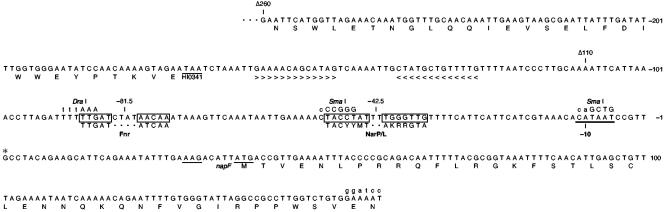
We constructed a Φ(napFHi-lacZ) operon fusion in the moderate-copy-number plasmid pRS415 as described in Materials and Methods. The insert encompasses a sequence from a native EcoRI site at position −260 with respect to the transcription initiation site, within the upstream conserved hypothetical gene designated HI0341, through a BamHI site, introduced via oligonucleotide-directed site-specific mutagenesis, within the napFHi coding region (Fig. 1). We introduced this plasmid into the wild-type E. coli strain VJS676, grew cultures anaerobically in the presence of nitrate, and used the method of rapid amplification of cDNA ends (47) to determine the 5′ end of the napFHi mRNA as described in Materials and Methods.
FIG. 1.
napFHi control region sequence. Numbering is with respect to the transcription initiation point, indicated with an asterisk. The −10 promoter element is indicated with a thick underline, and translation initiation (Shine-Dalgarno element and initiation codon) and termination sequences are indicated with thin underlines. Sequences for binding the Fnr and phospho-NarP or -NarL proteins are boxed; consensus sequences are shown below boxes. Restriction endonuclease sites introduced to inactivate cis-acting regulatory sequences are indicated, with uppercase lettering denoting nucleotide changes from the wild type. The downstream BamHI restriction site introduced for constructing Φ(napFHi-lacZ) operon fusions is indicated in lowercase. Nucleotides in the inverted repeat forming the likely intrinsic terminator downstream of the HI0341 coding region are indicated with arrowheads.
Results (not shown) identified the G residue denoted by the asterisk in Fig. 1 as position +1. We conclude that this G is the transcription initiation point in E. coli. In contrast to the napFEc control region, which contains two distinct initiation points (54), we found no evidence for additional transcription initiation points for the napFHi control region.
Regulated expression of the napFHi control region in E. coli.
We isolated λ specialized transducing bacteriophage for two different Φ(napFHi-lacZ) operon fusion constructs and made monocopy lysogens, as described in Materials and Methods. The first construct, denoted Φ(napFHi-lacZ) Δ260, is described above (Fig. 1). The second construct, denoted Φ(napFHi-lacZ) Δ110, contains a sequence from the same downstream BamHI site to an EcoRI site (66) overlapping position −110 (Fig. 1). Thus, the two constructs differ only in the extent of the upstream sequence present.
We cultured the lysogenic strains to the mid-exponential phase with oxygen, nitrate, nitrite, or no added electron acceptor and measured β-galactosidase activity as described in Materials and Methods. Results are shown in Table 2. Expression levels from both the Φ(napFHi-lacZ) Δ260 construct and the Φ(napFHi-lacZ) Δ110 construct were qualitatively similar. Aerated cultures synthesized negligible levels of β-galactosidase, whereas anaerobic cultures synthesized readily measured amounts, demonstrating that Φ(napFHi-lacZ) expression was induced by anaerobiosis. In anaerobic cultures, added nitrate and nitrite resulted in further increases in β-galactosidase synthesis. Thus, the napFHi control region in E. coli exhibited transcription activation by anaerobiosis and further activation by nitrate or nitrite.
TABLE 2.
Effects of oxygen, nitrate, and nitrite on expression from Φ(napFHi-lacZ) constructs
| Strain | Fusionb | Sitec | LacZ sp acta
|
Activation by:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| +O2 | −O2 | +NO3−−O2 | +NO2−−O2 | O2 | NO3− | NO2− | |||
| VJS6621 | Φ(napFHi-lacZ) Δ110 | 3 | 150 | 1,280 | 420 | 25 | 8.5 | 2.8 | |
| VJS6906 | Φ(napFHi-lacZ) Δ260 | <1 | 16 | 390 | 50 | >16 | 24 | 3.1 | |
| VJS6907 | Φ(napFHi-lacZ) Δ260 | NarP/NarL | <1 | 10 | 7 | 5 | >10 | 1 | 1 |
| VJS6908 | Φ(napFHi-lacZ) Δ260 | Fnr | <1 | <1 | 2 | <1 | |||
Strains were cultured to the mid-exponential phase in MOPS medium (defined medium with glucose) with the terminal electron acceptor as indicated.
Location of upstream endpoint in construct.
Mutant regulatory protein binding site in control region (see Fig. 1).
The overall level of β-galactosidase synthesized was greater in strains carrying the Φ(napFHi-lacZ) Δ110 construct than in those with the Φ(napFHi-lacZ) Δ260 construct (Table 2). A probable intrinsic transcription terminator (43) lies between the end of the upstream HI0341 gene and the Δ110 deletion endpoint (Fig. 1). It is likely that the lower overall levels of β-galactosidase synthesis in strains carrying the Φ(napFHi-lacZ) Δ260 construct result from this terminator restricting low-level readthrough transcription from the upstream bacteriophage λ sequence (51).
cis-acting sites regulate expression of the napFHi control region in E. coli.
The consensus sequence for Fnr binding contains inverted repeats of the pentamer TTGAT separated by 4 nt (48). The consensus sequence for phospho-NarP and phospho-NarL binding contains inverted repeats of the heptamer TACYYMT (where Y represents C or T and M represents A or C) separated by 2 nt (12). Sequence inspection of the napFHi control region reveals likely sites for binding the Fnr and phospho-NarP proteins centered at positions −81.5 and −42.5, respectively (Fig. 1). A likely −10 promoter element for σ70-RNA polymerase recognition (consensus TATAAT) (24) is positioned appropriately with respect to the transcription initiation point.
To evaluate the in vivo roles for these sites, we used oligonucleotide-directed site-specific mutagenesis to introduce multiple nucleotide substitutions. These substitutions (which simultaneously introduced new restriction endonuclease sites) changed 3 nt in the upstream Fnr half-site, 5 nt in the upstream phospho-NarP half-site, and 4 nt in the −10 promoter element (Fig. 1).
Plasmid-borne Φ(napFHi-lacZ) Δ260 constructs carrying the Fnr and phospho-NarP site substitutions were converted to specialized transducing phage and used to form monocopy lysogens as described above. We cultured these strains and measured β-galactosidase activity in the same experiments described above for the wild-type versions (Table 2). Expression from the construct carrying the Fnr site alterations remained at the low level characteristic of aerobic cultures irrespective of culture conditions. Expression from the construct carrying the phospho-NarP site alterations exhibited essentially wild-type induction by anaerobiosis, but further induction by nitrate or nitrite was abolished. These results demonstrate that the sequence motifs identified by visual inspection are indeed the authentic binding sites for regulation by the Fnr and phospho-NarP proteins.
The plasmid-borne Φ(napFHi-lacZ) Δ260 construct carrying the −10 promoter element substitutions failed to direct synthesis of measurable levels of β-galactosidase activity (data not shown), demonstrating that the −10 sequence identified by visual inspection is essential for napFHi expression in E. coli. We did not convert this construct into a specialized transducing phage.
NarP and NarL proteins regulate expression of the napFHi control region in E. coli.
We next determined the effects of null alleles in the narP and narL genes, encoding the nitrate-responsive regulators, on expression of the napFHi control region in E. coli. To provide controls, we included analogous strains carrying the Φ(napFEc-lacZ) Δ85 fusion, which lacks the upstream ModE binding site (11, 41).
Patterns of expression from the Φ(napFEc-lacZ) fusion (Table 3) were essentially as described previously (11). In the narP+ narL+ strain, β-galactosidase synthesis was induced about 10-fold and 20-fold during growth with added nitrate and nitrite, respectively. Induction was increased in the narL null strain but essentially eliminated in the narP null strain. These patterns of expression have been interpreted as revealing phospho-NarP activation and phospho-NarL-dependent antagonism of expression from the napFEc control region (11).
TABLE 3.
Effects of narL and narP null alleles on on expression from Φ(napFHi-lacZ) and Φ(napFEc-lacZ) constructs
| Strain | Fusion | Genotype
|
LacZ sp acta
|
Activation by:
|
||||
|---|---|---|---|---|---|---|---|---|
| narL | narP | −NO3−−O2 | +NO3−−O2 | +NO2−−O2 | NO3− | NO2− | ||
| VJS4797 | Φ(napFEc-lacZ) Δ85 | + | + | 160 | 1,760 | 3,040 | 11 | 19 |
| VJS5101 | Φ(napFEc-lacZ) Δ85 | − | + | 220 | 8,940 | 7,010 | 40 | 32 |
| VJS5109 | Φ(napFEc-lacZ) Δ85 | + | − | 89 | 140 | 130 | 1.6 | 1.5 |
| VJS5117 | Φ(napFEc-lacZ) Δ85 | − | − | 91 | 88 | 84 | 1.0 | 0.9 |
| VJS6621 | Φ(napFHi-lacZ) Δ110 | + | + | 200 | 2,080 | 770 | 10 | 3.8 |
| VJS6623 | Φ(napFHi-lacZ) Δ110 | − | + | 200 | 1,660 | 1,180 | 8.3 | 5.9 |
| VJS6625 | Φ(napFHi-lacZ) Δ110 | + | − | 190 | 1,640 | 350 | 8.6 | 1.8 |
| VJS6627 | Φ(napFHi-lacZ) Δ110 | − | − | 180 | 180 | 150 | 1.0 | 0.8 |
Strains were cultured to the mid-exponential phase in MOPS medium (defined medium with glucose).
Patterns of expression from the Φ(napFHi-lacZ) fusion (Table 3) were strikingly different. In the narP+ narL+ strain, β-galactosidase synthesis was induced about 10-fold and 4-fold by growth with added nitrate and nitrite, respectively (see also Table 2). Induction by nitrate was unaffected by introduction of either the narL null or the narP null allele. However, the level of induction by nitrite was increased in the narL null strain and decreased in the narP null strain. As observed also with the Φ(napFEc-lacZ) fusion, expression in the narP narL double null strain remained at the anaerobic level irrespective of added nitrate or nitrite. These results indicate that phospho-NarP and phospho-NarL are equally effective activators of expression from the napFHi control region in nitrate-grown cultures. The different expression levels in nitrite-grown cultures in the narL versus narP null strain likely reflect differences in nitrite signaling by the cognate sensors, NarX and NarQ (53).
ModE protein does not regulate expression from the napFHi control region in E. coli.
Finally, we determined the effect of a null allele in the modE gene, encoding the molybdate-responsive regulator (49). Expression from the full-length napFEc control region requires both the modE+ gene and the cis-acting ModE protein binding site centered 70 nt upstream of the center of the Fnr binding site (41, 54). H. influenzae Rd contains the modE+ gene as well as several operons whose expression is likely regulated by the ModE protein (58). However, neither computer analysis (58) nor visual inspection (Fig. 1) has identified a candidate ModE protein binding site in the napFHi control region.
We examined Φ(napFHi-lacZ) expression in E. coli in response to molybdate and modE genotype as previously described (54). As controls, we included strains carrying the Φ(napFEc-lacZ) Δ146 and Φ(napFEc-lacZ) Δ123 fusions, which, respectively, retain and lack the upstream ModE protein binding site (54). Expression from both the Φ(napFHi-lacZ) Δ260 fusion and the Φ(napFHi-lacZ) Δ110 fusion was indifferent to molybdate and modE genotype (data not shown). We conclude that expression from the napFHi control region is not regulated by the ModE protein in E. coli and is likely not regulated by the ModE protein in H. influenzae.
DISCUSSION
Surrogate genetics exploits a well-characterized host to study gene function from a related but experimentally less-tractable species (37). The close phylogenetic affiliation between E. coli and H. influenzae (14) makes the former a suitable surrogate for the latter. Thus, E. coli has been used as a host for studying aspects of H. influenzae gene function, including regulation by the Fnr and ArcB proteins (19, 30, 39). Results on control of Φ(napFHi-lacZ) expression in E. coli presented here therefore may provide a close approximation for control of napFHi operon expression in H. influenzae. Nevertheless, our primary motivation for studying Φ(napFHi-lacZ) expression in E. coli was to develop a relatively simple native model system for studying Fnr class I and phospho-NarP class II transcription activation mechanisms.
Results with E. coli suggest that the phospho-NarP protein directly activates transcription from the Fnr class I napF promoter (11, 13) and that the phospho-NarL protein directly activates transcription from the Fnr class II narG and fdnG operon promoters (34, 35). By contrast, the phospho-NarL and -NarP proteins act indirectly to stimulate transcription initiation from the Fnr class II nirB and nrfA promoters (7, 67). For discussion, we adopt the hypothesis that phospho-NarP and -NarL directly activate transcription from the napFHi promoter.
Transcription activation from Nar-regulated promoters is synergistic: the magnitude of expression is much greater when both the Fnr regulator and the NarL or NarP regulator are active than when either is inactive (for examples, see Tables 2 and 3). One model to explain synergistic transcription activation is that the two activators make contact simultaneously with distinct components of RNA polymerase (2, 26). This distinct-contact model can explain results from studies with the Fnr class II narG operon promoter, which we summarize here before considering how analogous models might apply to transcription activation at the Fnr class I napF operon promoters.
Transcription activation by phospho-NarL in synergy with Fnr (class II).
The Fnr protein interacts both with region 4 of the σ70 subunit and with the α-CTD subunit to stimulate narG operon transcription initiation from a class II site (position −42.5) (4, 31, 32, 36). These deduced interactions are supported by analogy to the well-studied homolog Crp (6). Fully induced narG operon transcription additionally requires the phospho-NarL protein, acting from upstream (class I) positions (57). The hypothesis that the Fnr and phospho-NarL proteins make distinct contacts with RNA polymerase is supported by two observations on expression of the narG operon control region.
The first observation concerns positive control missense substitutions in the fnr and rpoD genes that significantly decrease narG operon transcription initiation. For most of these mutants, addition of nitrate to generate phospho-NarL protein results in near-wild-type levels of expression (31, 36). This indicates that the second transcription activator (phospho-NarL) can overcome blocks to action by the first (Fnr).
The second observation concerns the narG operon promoter −10 region (5′-TACCTT), which is a relatively poor match to the consensus. Alteration to a consensus −10 sequence (5′-TATAAT) bypasses the need for Fnr activation (60), much as the UV5 alteration in the lacZ operon promoter bypasses the need for Crp activation (50). Remarkably, transcription initiation from this mutant narG promoter is stimulated by the phospho-NarL protein even in an fnr null mutant (60). This demonstrates that, given the proper promoter structure, the phospho-NarL protein can stimulate transcription initiation independently of the Fnr protein.
Class I transcription activation by Fnr.
Class I control regions have a Crp or Fnr binding site located upstream of the promoter, and studies with model control regions reveal optimal spacing of −61.5, −71.5, −82.5, or −92.5 nt from the transcription initiation point to the center of the dyad (8, 65). To date, the only well-characterized Fnr class I control regions have been synthetic constructs based on the melR promoter (32, 64, 65) and the native napFEc control region (9, 11, 13, 54). Here, we present evidence that the napFHi control region Fnr protein binding site is centered at position −81.5 (Fig. 1; Table 2) and therefore represents an additional characterized example of a native class I Fnr-activated promoter. Other recently described class I Fnr-activated promoters are those for the ydjX gene (27) and also for the hcp-hcr operon, transcription of which requires phospho-NarP or -NarL for full expression (15).
Transcription activation by phospho-NarP or -NarL in synergy with Fnr (class I).
Large-scale analysis of positive control mutants has not yet been applied to analysis of the napF operon control regions. Our working hypothesis is that activation of transcription from these promoters also involves distinct contacts to RNA polymerase. Phospho-NarP or -NarL, bound at a class II position (Fig. 1), is in position to make contact both with region 4 of the σ70 subunit and with the α-CTD, whereas the Fnr protein, bound at a class I position, is in position to make contact with the α-CTD (5).
Transcription from both the napFEc and the napFHi operon control regions is activated synergistically by the Fnr and phospho-NarP proteins. However, transcription from the native napFEc control region is antagonized rather than activated by the phospho-NarL protein, leading to the notion that phospho-NarL is not an effective transcription activator from a proximal (class II) binding site (11, 13). Recently, however, we found that transcription initiation from a mutant version of the napFEc control region, lacking the minor promoter P2, is stimulated by the phospho-NarL protein (54). Here, we present evidence that phospho-NarL and -NarP proteins, bound at a class II position in the wild-type napFHi control region, stimulated transcription initiation equally well in nitrate-grown cultures (Table 3). Therefore, given the appropriate promoter context, the phospho-NarL protein can stimulate transcription initiation from a class II position. Presumably, the structure of the wild-type napFEc control region prevents contacts between RNA polymerase and phospho-NarL but not between RNA polymerase and phospho-NarP. This would imply that the two Nar response regulators make different contacts to RNA polymerase.
Similar transcription control regions in proteobacteria.
The distances between the centers of the Fnr and phospho-NarP binding sites for the napFEc and napFHi control regions are 20 and 39 nt, respectively. This suggests that the DNA helical phase is important for their synergistic transcription activation functions. We therefore used the National Center for Biotechnology Information World Wide Web portals to search for additional examples of potential upstream Fnr and downstream phospho-NarP binding sites with center-to-center spacing in increments of approximately 10 nt (results not shown). Several such cases were apparent in the regions upstream of the napF operon in genome sequences from members of the families Pasteurellaceae and Vibrionaceae, as well as in the regions upstream of the ccmA operon, encoding cytochrome c maturation functions, from members of the family Pasteurellaceae. These and a few additional examples all have center-to-center spacing near 30 or 40 nt. Each of these genomes contains genes for the NarQ-NarP (but not the NarX-NarL) two-component system (53). Therefore, the architecture of the napFHi control region may be broadly representative of those subject to synergistic activation by the Fnr and phospho-NarP proteins in a range of species classified in the gamma subdivision of the proteobacteria.
Acknowledgments
We are grateful to Michelle L. Giwnn for providing cultures of the H. influenzae strains KW20 and MGH90, to Vinh Pham for constructing the phospho-NarP site mutant, and to Li-Ling Chen for determining transcriptional response to molybdate.
This study was supported by Public Health Service grant GM36877 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Barcak, G. J., M. S. Chandler, R. J. Redfield, and J.-F. Tomb. 1991. Genetic systems in Haemophilus influenzae. Methods Enzymol. 204:321-342. [DOI] [PubMed] [Google Scholar]
- 2.Barnard, A., A. Wolfe, and S. Busby. 2004. Regulation at complex bacterial promoters: how bacteria use different promoter organizations to produce different regulatory outcomes. Curr. Opin. Microbiol. 7:102-108. [DOI] [PubMed] [Google Scholar]
- 3.Bearson, S. M., J. A. Albrecht, and R. P. Gunsalus. 2002. Oxygen and nitrate-dependent regulation of dmsABC operon expression in Escherichia coli: sites for Fnr and NarL protein interactions. BMC Microbiol. 2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, A., and S. Busby. 1994. Location and orientation of an activating region in the Escherichia coli transcription factor, FNR. Mol. Microbiol. 11:383-390. [DOI] [PubMed] [Google Scholar]
- 5.Belyaeva, T. A., V. A. Rhodius, C. L. Webster, and S. J. Busby. 1998. Transcription activation at promoters carrying tandem DNA sites for the Escherichia coli cyclic AMP receptor protein: organisation of the RNA polymerase alpha subunits. J. Mol. Biol. 277:789-804. [DOI] [PubMed] [Google Scholar]
- 6.Browning, D., D. Lee, J. Green, and S. Busby. 2002. Secrets of bacterial transcription initiation taught by the Escherichia coli FNR protein. Symp. Soc. Gen. Microbiol. 61:127-142. [Google Scholar]
- 7.Browning, D. F., and S. J. Busby. 2004. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2:57-65. [DOI] [PubMed] [Google Scholar]
- 8.Busby, S., and R. H. Ebright. 1999. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293:199-213. [DOI] [PubMed] [Google Scholar]
- 9.Choe, M., and W. S. Reznikoff. 1993. Identification of the regulatory sequence of anaerobically expressed locus aeg-46.5. J. Bacteriol. 175:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darwin, A. J., J. Li, and V. Stewart. 1996. Analysis of nitrate regulatory protein NarL-binding sites in the fdnG and narG operon control regions of Escherichia coli K-12. Mol. Microbiol. 20:621-632. [DOI] [PubMed] [Google Scholar]
- 11.Darwin, A. J., and V. Stewart. 1995. Nitrate and nitrite regulation of the Fnr-dependent aeg-46.5 promoter of Escherichia coli K-12 is mediated by competition between homologous response regulators (NarL and NarP) for a common DNA-binding site. J. Mol. Biol. 251:15-29. [DOI] [PubMed] [Google Scholar]
- 12.Darwin, A. J., K. L. Tyson, S. J. W. Busby, and V. Stewart. 1997. Differential regulation by the homologous response regulators NarL and NarP of Escherichia coli K-12 depends on DNA binding site arrangement. Mol. Microbiol. 25:583-595. [DOI] [PubMed] [Google Scholar]
- 13.Darwin, A. J., E. C. Ziegelhoffer, P. J. Kiley, and V. Stewart. 1998. Fnr, NarP, and NarL regulation of Escherichia coli K-12 napF (periplasmic nitrate reductase) operon transcription in vitro. J. Bacteriol. 180:4192-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Rosa, R., and B. Labedan. 1998. The evolutionary relationships between the two bacteria Escherichia coli and Haemophilus influenzae and their putative last common ancestor. Mol. Biol. Evol. 15:17-27. [DOI] [PubMed] [Google Scholar]
- 15.Filenko, N. A., D. F. Browning, and J. A. Cole. 2005. Transcriptional regulation of a hybrid cluster (prismane) protein. Biochem. Soc. Trans. 33:195-197. [DOI] [PubMed] [Google Scholar]
- 16.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J.-F. Tomb, B. A. Dougherty, J. M. Merrick, K. McKenney, G. Sutton, W. FitzHugh, C. Fields, J. D. Gocayne, J. Scott, R. Shirley, L.-I. Liu, A. Glodek, J. M. Kelley, J. F. Weidman, C. A. Phillips, T. Spriggs, E. Hedblom, M. D. Cotton, T. R. Utterback, M. C. Hanna, D. T. Nguyen, D. M. Saudek, R. C. Brandon, L. D. Fine, J. L. Fritchman, J. L. Fuhrmann, N. S. M. Geoghagen, C. L. Gnehm, L. A. McDonald, K. V. Small, C. M. Fraser, H. O. Smith, and J. C. Venter. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 17.Gennis, R. B., and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 18.Georgellis, D., O. Kwon, and E. C. C. Lin. 2001. Quinones as the redox signal for the Arc two-component system of bacteria. Science 292:2314-2316. [DOI] [PubMed] [Google Scholar]
- 19.Georgellis, D., O. Kwon, E. C. C. Lin, S. M. Wong, and B. J. Akerley. 2001. Redox signal transduction by the ArcB sensor kinase of Haemophilus influenzae lacking the PAS domain. J. Bacteriol. 183:7206-7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green, J., C. Scott, and J. R. Guest. 2001. Functional versatility in the CRP-FNR superfamily of transcription factors: FNR and FLP. Adv. Microb. Physiol. 44:1-34. [DOI] [PubMed] [Google Scholar]
- 21.Guest, J. R., J. Green, A. S. Irvine, and S. Spiro. 1996. The FNR modulon and FNR-regulated gene expression, p. 317-342. In E. C. C. Lin and A. S. Lynch (ed.), Regulation of gene expression in Escherichia coli. RG Landes Co., Georgetown, Tex.
- 22.Gwinn, M. L., D. Yi, O. Smith, and J.-F. Tomb. 1996. Role of the two-component signal transduction and the phosphoenolpyruvate:carbohydrate phosphotransferase systems in competence development of Haemophilus influenzae Rd. J. Bacteriol. 178:6366-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harman, J. G. 2001. Allosteric regulation of the cAMP receptor protein. Biochim. Biophys. Acta 1547:1-17. [DOI] [PubMed] [Google Scholar]
- 24.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herriott, R. M., E. Y. Meyer, M. Vogt, and M. Modan. 1970. Defined medium for growth of Haemophilus influenzae. J. Bacteriol. 101:513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochschild, A., and J. K. Joung. 1997. Synergistic activation of transcription in E. coli, p. 101-114. In F. Eckstein and D. M. J. Lilley (ed.), Nucleic acids and molecular biology, vol. 11. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 27.Kang, Y., K. D. Weber, Y. Qiu, P. J. Kiley, and F. R. Blattner. 2005. Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function. J. Bacteriol. 187:1135-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiley, P. J., and H. Beinert. 1998. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol. Rev. 22:341-352. [DOI] [PubMed] [Google Scholar]
- 29.Körner, H., H. J. Sofia, and W. G. Zumft. 2003. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 27:559-592. [DOI] [PubMed] [Google Scholar]
- 30.Kroll, J. S., P. R. Langford, J. R. Saah, and B. M. Loynds. 1993. Molecular and genetic characterization of superoxide dismutase in Haemophilus influenzae type b. Mol. Microbiol. 10:839-848. [DOI] [PubMed] [Google Scholar]
- 31.Lamberg, K. E., and P. J. Kiley. 2000. FNR-dependent activation of the class II dmsA and narG promoters of Escherichia coli requires FNR-activating regions 1 and 3. Mol. Microbiol. 38:817-827. [DOI] [PubMed] [Google Scholar]
- 32.Lee, D. J., H. J. Wing, N. J. Savery, and S. J. W. Busby. 2000. Analysis of interactions between activating region 1 of Escherichia coli FNR protein and the C-terminal domain of the RNA polymerase α subunit: use of alanine scanning and suppression genetics. Mol. Microbiol. 37:1032-1040. [DOI] [PubMed] [Google Scholar]
- 33.Lewis, M. K., and D. V. Thompson. 1990. Efficient site directed in vitro mutagenesis using ampicillin selection. Nucleic Acids Res. 18:3439-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, J., and V. Stewart. 1992. Localization of upstream sequence elements required for nitrate and anaerobic induction of fdn (formate dehydrogenase-N) operon expression in Escherichia coli K-12. J. Bacteriol. 174:4935-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, S.-F., and J. A. DeMoss. 1988. Location of sequences in the nar promoter of Escherichia coli required for regulation by Fnr and NarL. J. Biol. Chem. 263:13700-13705. [PubMed] [Google Scholar]
- 36.Lonetto, M. A., V. Rhodius, K. Lamberg, P. Kiley, S. Busby, and C. Gross. 1998. Identification of a contact site for different transcription activators in region 4 of the Escherichia coli RNA polymerase σ70 subunit. J. Mol. Biol. 284:1353-1365. [DOI] [PubMed] [Google Scholar]
- 37.Maloy, S., and T. Zahrt. 2000. Surrogate genetics: the use of bacterial hybrids as a genetic tool. Methods 20:73-79. [DOI] [PubMed] [Google Scholar]
- 38.Maloy, S. R., V. J. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogeneic bacteria: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Manukhov, I. V., Y. V. Bertsova, D. Y. Trofimov, A. V. Bogachev, and V. P. Skulachev. 2000. Analysis of HI0220 protein from Haemophilus influenzae, a novel structural and functional analog of ArcB protein from Escherichia coli. Biochemistry (Moscow) 65:1321-1326. [PubMed] [Google Scholar]
- 40.Marrs, C. F., G. P. Krasan, K. W. McCrea, D. L. Clemans, and J. R. Gilsdorf. 2001. Haemophilus influenzae: human specific bacteria. Front. Biosci. 6:E41-E60. [DOI] [PubMed] [Google Scholar]
- 41.McNicholas, P. M., and R. P. Gunsalus. 2002. The molybdate-responsive Escherichia coli ModE transcriptional regulator coordinates periplasmic nitrate reductase (napFDAGHBC) operon expression with nitrate and molybdate availability. J. Bacteriol. 184:3253-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 43.Mooney, R. A., I. Artsimovitch, and R. Landick. 1998. Information processing by RNA polymerase: recognition of regulatory signals during RNA chain elongation. J. Bacteriol. 180:3265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potter, L., H. Angove, D. Richardson, and J. Cole. 2001. Nitrate reduction in the periplasm of gram-negative bacteria. Adv. Microb. Physiol. 45:51-112. [DOI] [PubMed] [Google Scholar]
- 45.Powell, B. S., M. P. Rivas, D. L. Court, Y. Nakamura, and C. L. Turnbough, Jr. 1994. Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 22:5765-5766. (Erratum, 23: 1278, 1985.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabin, R. S., and V. Stewart. 1993. Dual response regulators (NarL and NarP) interact with dual sensors (NarX and NarQ) to control nitrate- and nitrite-regulated gene expression in Escherichia coli K-12. J. Bacteriol. 175:3259-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaefer, B. C. 1995. Revolutions in rapid amplification of cDNA ends: new strategies for polymerase chain reaction cloning of full-length cDNA ends. Anal. Biochem. 227:255-273. [DOI] [PubMed] [Google Scholar]
- 48.Scott, C., J. D. Partridge, J. R. Stephenson, and J. Green. 2003. DNA target sequence and FNR-dependent gene expression. FEBS Lett. 541:97-101. [DOI] [PubMed] [Google Scholar]
- 49.Self, W. T., A. M. Grunden, A. Hasona, and K. T. Shanmugam. 2001. Molybdate transport. Res. Microbiol. 152:311-321. [DOI] [PubMed] [Google Scholar]
- 50.Silverstone, A. E., R. R. Arditti, and B. Magasanik. 1970. Catabolite-insensitive revertants of lac promoter mutants. Proc. Natl. Acad. Sci. USA 66:773-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 52.Sinclair, P. R., and D. C. White. 1970. Effect of nitrate, fumarate, and oxygen on the formation of the membrane-bound electron transport system of Haemophilus parainfluenzae. J. Bacteriol. 101:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart, V. 2003. Nitrate- and nitrite-responsive sensors NarX and NarQ of proteobacteria. Biochem. Soc. Trans. 31:1-10. [DOI] [PubMed] [Google Scholar]
- 54.Stewart, V., P. J. Bledsoe, and S. B. Williams. 2003. Dual overlapping promoters control napF (periplasmic nitrate reductase) operon expression in Escherichia coli K-12. J. Bacteriol. 185:5862-5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart, V., Y. Lu, and A. J. Darwin. 2002. Periplasmic nitrate reductase (NapABC enzyme) supports anaerobic respiration by Escherichia coli K-12. J. Bacteriol. 184:1314-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart, V., and J. Parales. 1988. Identification and expression of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J. Bacteriol. 170:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart, V., and R. S. Rabin. 1995. Dual sensors and dual response regulators interact to control nitrate- and nitrite-responsive gene expression in Escherichia coli, p. 233-252. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, D.C.
- 58.Studholme, D. J., and R. N. Pau. 2003. A DNA element recognised by the molybdenum-responsive transcription factor ModE is conserved in proteobacteria, green sulphur bacteria and archaea. BMC Microbiol. 3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unden, G., and J. Bongaerts. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim. Biophys. Acta 1320:217-234. [DOI] [PubMed] [Google Scholar]
- 60.Walker, M. S., and J. A. DeMoss. 1992. Role of alternative promoter elements in transcription from the nar promoter of Escherichia coli. J. Bacteriol. 174:1119-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker, M. S., and J. A. DeMoss. 1994. NarL-phosphate must bind to multiple upstream sites to activate transcription from the narG promoter of Escherichia coli. Mol. Microbiol. 14:633-641. [DOI] [PubMed] [Google Scholar]
- 62.White, D. C. 1963. Respiratory systems in hemin-requiring Haemophilus species. J. Bacteriol. 85:84-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White, D. C., and P. R. Sinclair. 1971. Branched electron-transport systems in bacteria. Adv. Microb. Physiol. 5:173-211. [DOI] [PubMed] [Google Scholar]
- 64.Williams, S. M., N. J. Savery, S. J. W. Busby, and H. J. Wing. 1997. Transcription activation at class I FNR-dependent promoters: identification of the activating surface of FNR and the corresponding contact site in the C-terminal domain of the RNA polymerase alpha subunit. Nucleic Acids Res. 25:4028-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wing, H. J., S. M. Williams, and S. J. W. Busby. 1995. Spacing requirements for transcription activation by Escherichia coli FNR protein. J. Bacteriol. 177:6704-6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woodbury, C. P., Jr., O. Hagenbuchle, and P. H. von Hippel. 1980. DNA site recognition and reduced specificity of the EcoRI endonuclease. J. Biol. Chem. 255:11534-11548. [PubMed] [Google Scholar]
- 67.Wu, H.-C., K. L. Tyson, J. A. Cole, and S. J. W. Busby. 1998. Regulation of transcription initiation at the Escherichia coli nir operon promoter: a new mechanism to account for co-dependence on two transcription factors. Mol. Microbiol. 27:493-505. [DOI] [PubMed] [Google Scholar]