Abstract
WOR5 is the fifth and last member of the family of tungsten-containing oxidoreductases purified from the hyperthermophilic archaeon Pyrococcus furiosus. It is a homodimeric protein (subunit, 65 kDa) that contains one [4Fe-4S] cluster and one tungstobispterin cofactor per subunit. It has a broad substrate specificity with a high affinity for several substituted and nonsubstituted aliphatic and aromatic aldehydes with various chain lengths. The highest catalytic efficiency of WOR5 is found for the oxidation of hexanal (Vmax = 15.6 U/mg, Km = 0.18 mM at 60°C). Hexanal-incubated enzyme exhibits S = 1/2 electron paramagnetic resonance signals from [4Fe-4S]1+ (g values of 2.08, 1.93, and 1.87) and W5+ (g values of 1.977, 1.906, and 1.855). Cyclic voltammetry of ferredoxin and WOR5 on an activated glassy carbon electrode shows a catalytic wave upon addition of hexanal, suggesting that ferredoxin can be a physiological redox partner. The combination of WOR5, formaldehyde oxidoreductase, and aldehyde oxidoreductase forms an efficient catalyst for the oxidation of a broad range of aldehydes in P. furiosus.
Pyrococcus furiosus is a strictly anaerobic, fermentative microorganism that grows optimally at 100°C. It can use either peptides or carbohydrates as its carbon and energy source and it reduces elemental sulfur (S0) to H2S if present. The growth of P. furiosus is strictly dependent on the presence of tungsten (18). Four tungsten enzymes have previously been purified from this organism, all of which are members of the aldehyde oxidoreductase (AOR) family. Complete genome analysis has revealed the presence of a gene encoding a putative fifth member of this family and two genes for two more W or Mo enzymes assigned as putative formate dehydrogenases.
Three of the four AOR family enzymes have been purified and characterized in some detail: aldehyde ferredoxin oxidoreductase (AOR) (11), glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) (12) and formaldehyde ferredoxin oxidoreductase (FOR) (17). AOR has a broad substrate specificity but appears to be most active on aldehydes derived from amino acids (11). FOR has the highest activity on to aldehydes with one to three carbons (17), and both AOR and FOR are thought to play a role in peptide fermentation. In contrast, GAPOR is only known to convert the substrate glyceraldehyde-3-phosphate. It functions in glycolysis where it converts glyceraldehyde-3-phosphate to 3-phosphoglycerate, replacing glyceraldehyde-3 phosphate dehydrogenase and phosphoglycerate kinase in an unusual Emden-Meyerhof glycolysis (12).
A fourth tungsten-containing enzyme, WOR4, was more recently purified from P. furiosus grown in the presence of S0 (16). No activity has been identified yet, but the protein may play a role in S0 reduction because it could not be purified in the absence of S0 in the growth medium. From microarray analysis it is known that the expression of WOR4 at the mRNA level is upregulated in cold-adapted cells that were grown for many generations at 72°C. Cells that were incubated for shorter periods at 72°C (1 to 5 h) showed a fivefold increase in the expression of the putative fifth tungsten-containing enzyme, WOR5 (19). Also, the adjacent open reading frame (ORF) PF1479, coding for a 19-kDa protein with 16 cysteine residues that could bind multiple iron sulfur clusters, is upregulated to the same order of magnitude. This suggests coregulation of both proteins. The presence of four possible iron sulfur clusters in this protein associated with WOR5 indicates a role in electron transfer for the 19-kDa protein similar to the role of ferredoxin in the reactions catalyzed by the other enzymes from the AOR family.
In the present study we describe the purification and characterization of WOR5. This enzyme was discovered in a side fraction during a standard FOR purification. During this purification all fractions were examined for formaldehyde and crotonaldehyde oxidation activity, to discriminate between fractions that contain FOR and those that contain AOR. Some fractions showed an unexpected ratio for these two activities. Further examination has led us to the identification of WOR5 as a fifth aldehyde oxidoreductase with very broad substrate specificity.
MATERIALS AND METHODS
Growth of the organism and protein purification.
P. furiosus (DSM 3638) was grown in an 8-liter fermentor at 90°C, under anaerobic conditions with starch as the carbon source as previously described (2). After 18 h running in batch mode, the culture was switched to continuous mode with a dilution rate of 0.3 h−1 (15) resulting in a wet weight of approximately 2 g/liter. WOR5 was purified from 100 g cells (wet weight) under anaerobic conditions at 23°C. All buffers were repeatedly degassed and flushed with argon and contained 1 mM cysteine to scavenge traces of O2. Cells were broken by osmotic shock upon dilution with 5 volumes 30 mM Tris-HCl, pH 8, containing 1 mM cysteine, 5 mM MgCl2, 0.1 mg/liter DNase I, and 0.1 mg/liter RNase. The cell extract was loaded on a column of DEAE (Vc = 300 ml) fast flow Sepharose, equilibrated with 20 mM Tris-HCl, pH 8.
WOR5 eluted from the column between 175 and 270 mM NaCl with a gradient (1,400 ml) from 0 to 500 mM NaCl. Fractions with WOR5 activity were combined and loaded onto a hydroxyapatite (HAP) column (Vc = 120 ml) equilibrated with 5 mM potassium phosphate buffer, pH 7.5. WOR5 eluted from the column as 110 to 270 mM potassium phosphate was applied, using a gradient from 5 to 300 mM potassium phosphate in 500 ml. Fractions containing WOR5 activity were pooled and concentrated by ultrafiltration using an Amicon PM-30 membrane. The concentrated sample of WOR5 was applied to a Superdex-200 column (Vc = 320 ml), equilibrated with 20 mM Tris-HCl, pH 8, and 150 mM NaCl. Fractions containing WOR5 were combined, concentrated, and washed to a maximal salt concentration of 50 mM NaCl before application to a Mono-Q column (Vc = 1 ml) equilibrated with 20 mM PIPES (piperazine-diethanesulfonic acid, pH 6.8). WOR5 eluted from the column at 72 to 85 mM NaCl using a gradient (60 ml) from 70 to 200 mM NaCl.
Enzyme assays.
WOR5 activity was routinely assayed at 60°C, under anaerobic conditions, with 5 mM hexanal as the substrate and 1 mM methyl viologen as the electron acceptor in 50 mM EPPS [4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid] buffer, pH 8.4. Hexanal and other aldehydes tested were added to the assay mixture as a solution in 100% ethanol. The activities of AOR, GAPOR, and FOR were determined as previously described (11, 12, 17). The specific activities are on the basis of protein concentration.
Other assays.
Protein concentration was determined using the bicinchoninic acid assay method with bovine serum albumin as the standard. The tungsten content of the purified WOR5 protein was determined by catalytic adsorptive stripping voltammetry (6). Subunit molecular weight and degree of purity were determined with sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on a Phast System (GE Healthcare) in 8 to 25% SDS. Iron and acid-labile sulfur were determined colorimetrically (3, 14). Metal analysis was carried out by diluting the protein sample up to a volume of 1.5 ml (0.2% HNO3) and introducing it in an inductively coupled plasma optical emission spectrometer Optima 4300 DV (Perkin Elmer, Norwalk, Conn.). Total element content was determined at 280.271 nm (Mg) and 393.366 nm (Ca). Standard calibration curves between 0 and 1 mg/liter were measured immediately after the sample and were used for final calculations.
Cyclic voltammetry.
Cyclic voltammograms of P. furiosus ferredoxin and WOR5 were recorded with an Autolab PSTAT10 potentiostat. The electrochemical experiments were performed with a three electrode microcell using the method previously described (7). The working electrode was a nitric acid activated glassy carbon disk. A micro platinum electrode was used as counter electrode and the potential was measured with reference to an Ag/AgCl electrode. A droplet with a volume of 25 μl containing 50 μM of ferredoxin in 25 mM morpholinepropanesulfonic acid (MOPS) buffer, pH 7.2, and 7 mM of neomycin was placed on the working electrode. WOR5 was added to a final concentration of 5 μM and hexanal was added to a final concentration of 50 mM. The voltammograms were recorded at a scan rate of 10 mV/s at 60°C.
Spectroscopy.
X-band electron paramagnetic resonance spectra were recorded on a Bruker ER 200D spectrometer, using facilities and data handling as detailed elsewhere (13). For the reduction of enzyme with substrate, a sample of WOR5 (120 μM) was incubated with hexanal (10 mM) for up to 1 hour at 60°C. The UV-visible spectrum was recorded with a Hewlett Packard 8452A diode array spectrophotometer.
RESULTS AND DISCUSSION
Purification of WOR5.
WOR5 was purified from a cell extract of P. furiosus by monitoring the ability of column fractions to catalyze the hexanal-dependent reduction of methyl viologen. Cell extract contained 0.36 U/mg of hexanal oxidizing activity (Table 1) and this is of the same order as the oxidizing activity of formaldehyde (0.34 U/mg) and crotonaldehyde (0.55 U/mg).
TABLE 1.
Purification of WOR5 from P. furiosus
| Step | Protein (mg) | Activity (U) | Sp act (U/mg) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| Cell extract | 3,394 | 1,219 | 0.36 | 100 | 1 |
| DEAE | 1,454 | 1,202 | 0.83 | 99 | 2.3 |
| HAP | 411 | 811 | 1.97 | 67 | 5.5 |
| SD-200 | 46 | 480 | 10.3 | 39 | 29 |
| Mono-Q | 5.3 | 83 | 15.6 | 6.8 | 43 |
FOR and AOR (but not GAPOR) are also able to catalyze the oxidation of hexanal although at a much lower rate (Table 2). Therefore, the apparent WOR5 activity observed in the cell extract is the sum of the hexanal oxidizing activities of FOR, AOR and WOR5. The specific activities of FOR and AOR for the oxidation of hexanal were used in combination with the measured activity on formaldehyde and crotonaldehyde, to identify the presence of the three enzymes in fractions eluting from the columns. After the first DEAE column, FOR (eluting at 100 mM NaCl) was readily separated from AOR and WOR5, and after the HAP column AOR (7 mM KPi) and WOR5 (110 mM KPi) could also be separated.
TABLE 2.
Specific activity of AOR, FOR, and WOR5 for the oxidation of aldehydes, determined at 80°C and with methyl viologen as electron acceptor unless indicated otherwise
Approximately 5 mg of WOR5 was purified from 100 g wet weight of frozen P. furiosus cells. For comparison, the estimated yields of AOR, FOR, GAPOR, and WOR4 per 100 g of cells were 23, 12, 6, and 2.5 mg, respectively (16).
Molecular properties of WOR5.
Purified WOR5 gave a single band in SDS-polyacrylamide gel electrophoresis that corresponded to a size of 67 ± 2 kDa (Fig. 1). The apparent size as determined by native PAGE was 135 ± 5 kDa, suggesting that the enzyme is a homodimer (data not shown). The presence of a single subunit was confirmed by N-terminal sequence analysis, which resulted in a single sequence (MYAYNGKLLDVDLTREKVKEV) that matched 100% with the N terminus of the ORF (PF1480, wor5) in the genome sequence of P. furiosus. ORF wor5 encodes a 64.9-kDa protein based on the deduced amino acid sequence, which is in good agreement with experimental data (67 kDa). WOR5 has a high sequence identity with the four other members of the family: AOR (30%), FOR (34%), GAPOR (27%), and WOR4 (31%).
FIG. 1.
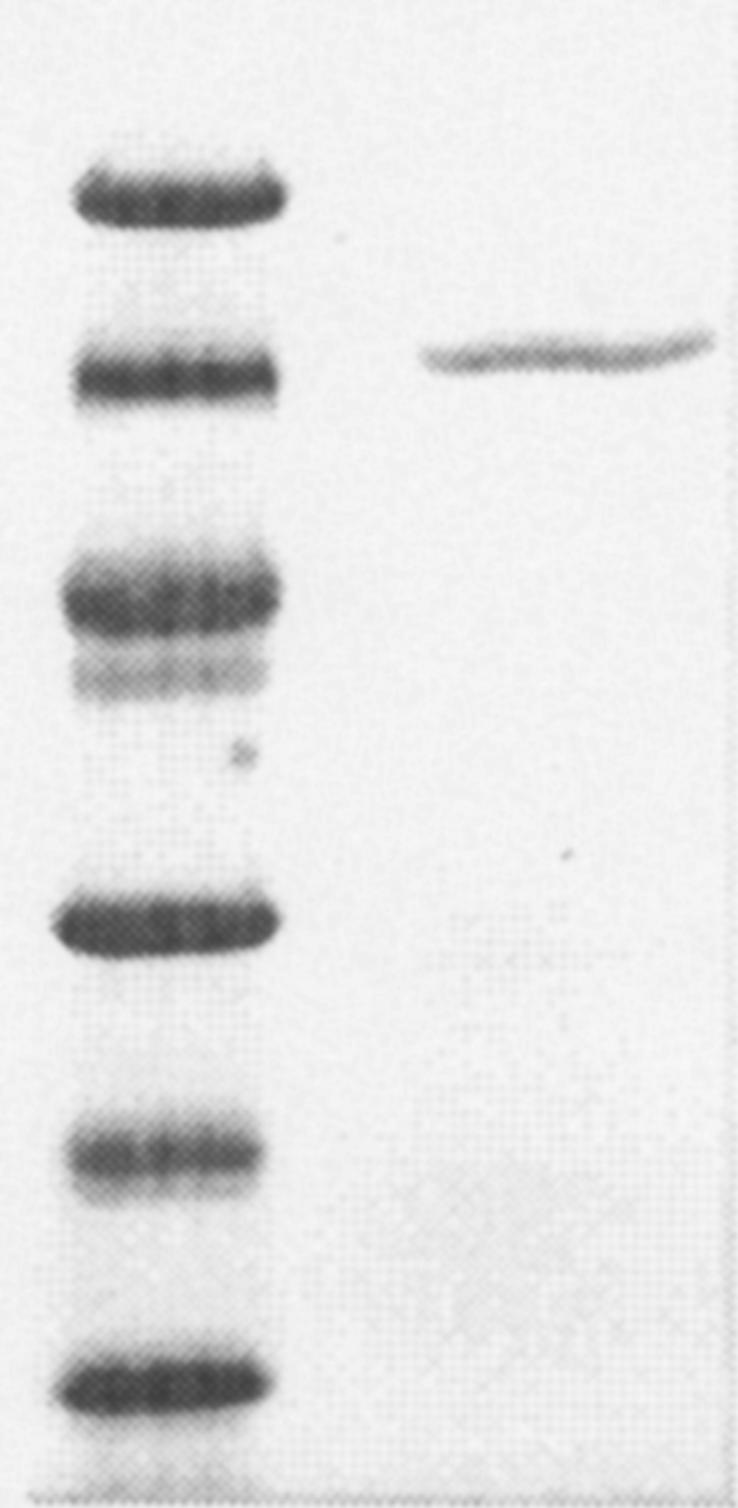
SDS-PAGE of purified WOR 5, lane 1: Low molecular weight markers from top: 94, 67, 43, 30, 20,14 kDa, lane 2: purified WOR 5.
Purified WOR5 contained 0.13 ± 0.05 g-atom of tungsten, 0.7 g-atom of magnesium, 1.4 g-atom of calcium, 2.8 ± 0.3 g-atoms of acid-labile sulfur, and 3.0 ± 0.1 g-atoms of iron per g-atom subunit. Based on the translated protein sequence of WOR5 and its homology to the other members of the family, one [4Fe-4S] cluster and one tungstobispterin center per subunit are expected for WOR5. The experimentally determined 0.13 g-atom of tungsten per subunit is perhaps due to loss of the cofactor during the purification.
Spectroscopy.
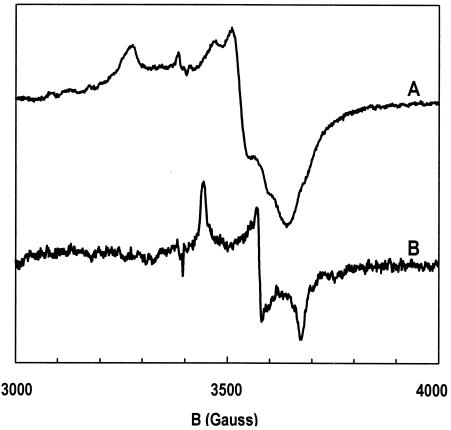
The as-isolated WOR5 enzyme was electron paramagnetic resonance silent. Incubation with 10 mM hexanal substrate for 1 hour at 60°C resulted in partial reduction of the prosthetic groups showing up as W(V) and [4Fe-4S]1+ in the electron paramagnetic resonance, as shown in Fig. 2. At low temperature, 15 K, the spectrum was dominated by the iron-sulfur cluster; weak features of the partially saturated and overmodulated tungsten signal were also detected. The iron-sulfur signal had approximate g values of 2.08, 1.93, and 1.87, however, the spectral shape was rather broad and exhibited small extra peaks, perhaps as the result of magnetic interaction. Possible origins of dipolar interaction are coupling between two cubanes of a protein dimer or coupling between a cubane and high-spin W(IV) within a subunit. No high-spin signals were detected, suggesting that the cluster was purely S = 1/2. In GAPOR (5) and FOR (our unpublished observation), the [4Fe-4S]1+ cluster exists as a mixture of an S = 1/2 and an S = 3/2 ground state, and the cubane in AOR has been found to occur essentially only in the high spin state (10). In oxidized WOR4 an unusual signal was assigned to a high-potential iron protein type of [4Fe-4S]3+ cluster (16).
FIG. 2.
Electron paramagnetic resonance spectra of the tungsten center and the iron-sulfur cluster in substrate-reduced WOR5. The enzyme, 8 mg/ml, was incubated with 10 mM hexanal for 1 h at 60°C. Trace A is a low-temperature spectrum (15 K) dominated by the signal from the [4Fe-4S]1+ cluster; trace B is a high-temperature spectrum (50 K) from W(V) in tungstobispterin. Electron paramagnetic resonance conditions: microwave frequency, 9,533 MHz; microwave power, 50 mW (A) and 200 mW (B); modulation frequency, 100 kHz; modulation amplitude, 6.3 Gauss (A) and 3.2 Gauss (B).
Increasing the temperature from 15 K to 30 K resulted in the virtual broadening away of the iron-sulfur signal consistent with its being from a [4Fe-4S]1+ cluster (not shown). A further increase to 50 K affords a single S = 1/2 signal of W(V), which was nonsaturable with microwave powers up to 200 mW. The g values were 1.977, 1.906, and 1.855. A 183W hyperfine interaction (14.4%; I = 1/2) of approximate strength A ≅ 40 G in all directions is just detectable as shoulders in Fig. 2B. Spin quantitation gave 0.45 spins per monomer of WOR5 for the cubane and 0.07 spins for W(V). The g values of the tungsten signal are comparable to the g values found for a tungsten signal at low redox potential in AOR (1.989, 1.901, and 1.863) (10). These comparable g values indicate that the tungsten ion is present in a similar coordination in the bispterin cofactor. The tungsten signal at low redox potential in GAPOR has significantly lower g values (1.948, 1.887, and 1.831) (5). In the electron paramagnetic resonance spectrum of WOR4 no tungsten signal could be detected (16).
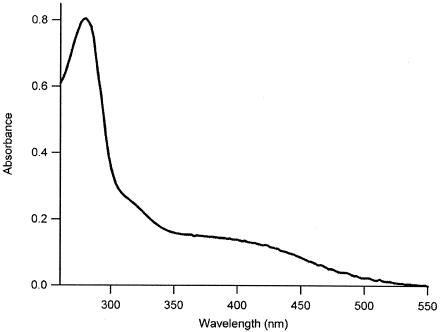
The optical spectrum of WOR5 as isolated (Fig. 3) shows a protein peak at 280 nm and a broad feature with a maximum at circa 390 nm, characteristic for iron-sulfur clusters of higher nuclearity such as cubanes. A shoulder is observed at approximately 320 nm. Extinction coefficients of ɛ390 = 1.15 mM−1 cm−1 and ɛ280 = 6.44 mM−1 cm−1 were determined from the UV-visible spectrum of WOR5 defining a purity index of A390/A280 = 0.18.
FIG. 3.
UV-visible absorption spectrum of P. furiosus WOR5. The protein concentration was 8 mg/ml in 20 mM Tris, pH 8.
Catalytic properties of WOR5.
The enzyme was purified by monitoring the ability of fractions to catalyze the oxidation of hexanal and the reduction of methylviologen. In addition to hexanal WOR5 was able to utilize a range of aliphatic and aromatic aldehydes as substrates summarized in Table 3. The specific activity and Km values were obtained by measuring the activity with methyl viologen as electron acceptor at 60°C at three different aldehyde concentrations (0.5, 5, and 25 mM) in duplicate, and fitting a Michaelis-Menten curve. The substrates can be divided in three classes based on Km values. Km < 0.5 mM was found for aldehydes where the carbonyl is attached to an aliphatic C atom. The degree of substitution of this beta C atom does not significantly influence the affinity or activity neither does the size of the aldehyde or the length of the side chain. The aromatic aldehydes with the carbonyl group directly attached to the phenyl-ring had Km values between 1 and 5 mM. Crotonaldehyde and formaldehyde (both approximately Km = 45 mM) were only oxidized at high concentrations.
TABLE 3.
Oxidation of aldehydes by WOR5 at 60°C and methyl viologen as the electron acceptora
| Substrate | Vmax (U/mg) | Km (mM) |
|---|---|---|
| Formaldehydeb | 8.5 ± 1.0 | 45 ± 12 |
| Crotonaldehydeb | 1.1 ± 0.1 | 46 ± 6 |
| Acetaldehydeb | 0.34 ± 0.05 | 1.5 ± 0.2 |
| Glutaraldehydea | 1.4 ± 0.1 | 9.4 ± 0.2 |
| 2-Methoxybenzaldehyde | 15.1 ± 0.6 | 4.8 ± 0.6 |
| Cinnamaldehyde | 7.4 ± 1.6 | 1.6 ± 0.1 |
| 2-Naphthaldehyde(β) | 7.7 ± 0.8 | 1.3 ± 0.1 |
| Hexanala | 15.6 ± 1.8 | 0.18 ± 0.02 |
| Hydratropaldehydea | 9.3 ± 0.7 | 0.12 ± 0.04 |
| 3-Phenylbutyraldehydea | 8.0 ± 0.6 | 0.42 ± 0.12 |
| 2-Ethylhexanala | 8.3 ± 1.5 | 0.17 ± 0.02 |
| Isobutyraldehyde | 11.8 ± 0.9 | 0.79 ± 0.03 |
| 2-Methylbutyraldehyde | 7.7 ± 0.4 | 0.43 ± 0.09 |
| 2-Methylvaleraldehyde | 12.7 ± 1.3 | 0.27 ± 0.03 |
| Glyceraldehyde-3-phosphate | 0.0 |
Substrate concentrations of 0.05, 0.5, and 5 mM were used to determine Michaelis constants. Higher concentrations caused solubility problems.
Substrate concentrations of 5, 25, 50 and 100 mM were used to determine Michaelis constants.
These activities and Km values reflect a clear difference in substrate specificity between WOR5 and the other tungsten-containing aldehyde oxidoreductases (Table 2). In its broad substrate specificity WOR5 clearly distinguishes itself from FOR and GAPOR, and the main difference between WOR5 and AOR is the low activity and affinity of the former for crotonaldehyde, which is one of the best substrates for AOR.
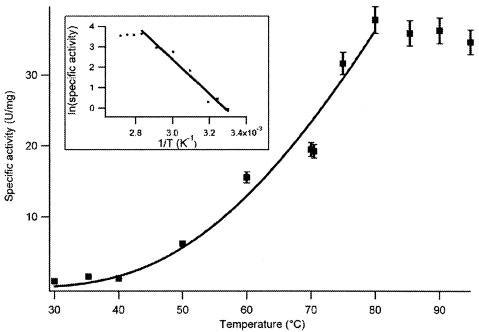
The temperature dependence of the specific activity of WOR5 for the oxidation of hexanal was determined from 30°C to 100°C (Fig. 4). Up to 80°C the activities were fitted with an Arrhenius equation that describes reaction rate as a function of temperature. The maximum specific activity was measured at 80°C. At temperatures higher than 80°C the specific activity rapidly decreased in time, probably due to instability of the protein.
FIG. 4.
Temperature dependence of the hexanal-oxidizing activity of WOR5. Activities have been fitted to the Arrhenius equation with an activation energy Ea of 70 ± 7 kJ/mol.
In vitro reconstitution of electron transfer chain.
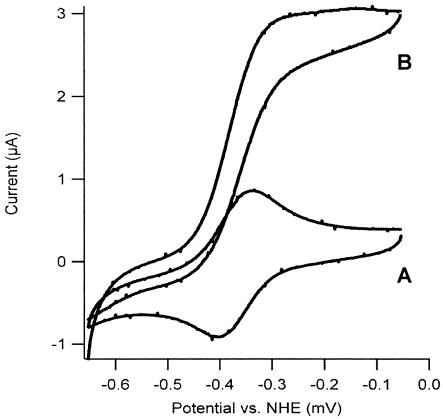
The cyclic voltammogram of P. furiosus ferredoxin shows a reversible electron transfer between the ferredoxin and the activated glassy carbon electrode (Fig. 5) as observed earlier (5). Addition of WOR5 or hexanal separately did not significantly change the voltammogram of ferredoxin. However, when hexanal plus WOR5 was added at 60°C a catalytic wave appeared, showing that the enzyme is able to oxidize hexanal and transfer the electrons through the ferredoxin to the electrode. These results suggest that ferredoxin can be a physiological redox partner of WOR5 as for the other enzymes from the AOR family. However, the ORF PF1479 next to wor5 on the genome encodes a protein with multiple iron-sulfur cluster binding motifs, which could also function as physiological partner protein.
FIG. 5.
Cyclic voltammogram of P. furiosus ferredoxin in the presence of WOR5 without hexanal (A) and with hexanal at 60°C (B). The droplet volume was 25 μl and contained 25 mM MOPS buffer, pH 7.2, 7 mM neomycin, 50 μM ferredoxin, 5 μM WOR5, and 50 mM hexanal. The potential scan rate was 10 mV/s.
Sequence comparisons.
A BLAST search (1) of the sequences of the aldehyde oxidoreductases from P. furiosus against the genomes of two other Pyrococcus species, Pyrococcus horikoshii (9) and Pyrococcus abyssi (http://www.genoscope.cns.fr/pab/) identifies homologs for AOR, FOR, GAPOR and FOR (Table 4). These four proteins all have homologs with sequence identities greater than 73%. All species have a putative fifth oxidoreductase, but these are mutually not very similar. In P. horikoshii there appear to be two additional proteins, but their genes are adjacent on the genome and probably form an αα′ dimer and can therefore be considered as one protein. In Table 4, these fifth aldehyde oxidoreductases are compared with WOR5 to visualize the low homology between these enzymes and WOR5. In fact this fifth putative aldehyde oxidoreductase in both P. horikoshii and P. abyssi has more homology with P. furiosus AOR (approximately 40% sequence identity). From this genome comparison we can conclude that WOR5 is the only P. furiosus oxidoreductase that has no true homolog in one of the other Pyrococcus species. There are also no true homologues (identity greater than 40%) identified when the sequence of WOR5 is blasted against all the genomes collected in the Expasy database (http://www.expasy.org/tools/BLAST/).
TABLE 4.
Genes homologous to the five oxidoreductases from P. furiosus present in the genome sequences of P. horikoshii (9) and P. abyssi (http://www.genoscope.cns.fr/pab/) expressed as the sequence identity between the deduced amino acid sequences
| P. furiosus gene | % Identity (gene)
|
|
|---|---|---|
| P. horikoshii | P. abyssi | |
| AOR | 77 (PH1019) | 77 (PAB0647) |
| FOR | 88 (PH1274) | 88 (PAB0798) |
| GAPOR | 80 (PH0457) | 80 (PAB1315) |
| WOR4 | 73 (PH0028) | 76 (PAB2330) |
| WOR5 | 37 (PH0891) | 37 (PAB2085) |
| 26 (PH0892) | ||
Another remarkable feature of WOR5 is the sequence of its [4Fe-4S] binding motif. The motifs in FOR, AOR, and GAPOR all contain four cysteine residues (Cxx[x]CxxxCxnC) that coordinate the [4Fe-4S] cluster. Only the last three are conserved in the sequence of WOR5, which could imply the presence of a [3Fe-4S] cluster. However, the electron paramagnetic resonance spectrum of WOR5 exhibits a clear signal of a [4Fe-4S]1+ cubane cluster. Instead of the first cysteine there is an aspartate residue in the sequence of WOR5. Replacement of cysteine by an aspartate ligand was earlier observed in P. furiosus proteins, namely, the second cysteine of the [4Fe-4S] cluster of ferredoxin (4) and the first cysteine of the [2Fe-2S] cluster in sulfide dehydrogenase (or ferredoxin:NAPD oxidoreductase) (8).
Concluding remarks.
With the purification of the fifth and presumably last member of the tungsten-containing family of oxidoreductases from P. furiosus, a next challenge will be to elucidate their functions and mutual relations. It is an intriguing question why the cell needs at least four aldehyde oxidoreductase enzymes with relatively broad substrate specificities expressed under similar conditions.
Acknowledgments
We thank Nahid Hasan for supplying the P. furiosus ferredoxin and Gerard Krijger of the Department of Radiation, Radionuclides and Reactors at the Delft University of Technology, for performing the inductively coupled plasma optical emission spectrometer analysis.
This research was supported by a grant from the Council for Chemical Sciences of the Netherlands Organization for Scientific Research (700.51.301).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendsen, A. F., P. T. Veenhuizen, and W. R. Hagen. 1995. Redox properties of the sulfhydrogenase from Pyrococcus furiosus. FEBS Lett. 368:117-121. [DOI] [PubMed] [Google Scholar]
- 3.Brumby, P. E., R. W. Miller, and V. Massey. 1965. The content and possible catalytic significance of labile sulfide in some metalloflavoproteins. J. Biol. Chem. 240:2222-2228. [PubMed] [Google Scholar]
- 4.Calzolai, L., C. M. Gorst, Z.-H. Zhao, Q. Teng, M. W. W. Adams, and G. N. La Mar. 1995. 1H NMR investigation of the electronic and molecular structure of the four-iron cluster ferredoxin from the hyperthermophile Pyrococcus furiosus. identification of Asp 14 as a cluster ligand in each of the four redox states. Biochemistry 34:11373-11384. [DOI] [PubMed] [Google Scholar]
- 5.Hagedoorn, P. L., J. R. Freije, and W. R. Hagen. 1999. Pyrococcus furiosus glyceraldehyde 3-phosphate oxidoreductase has comparable W6+/5+ and W5+/4+ reduction potentials and unusual [4Fe-4S] EPR properties. FEBS Lett. 462:66-70. [DOI] [PubMed] [Google Scholar]
- 6.Hagedoorn, P. L., P. van't Slot, H. P. van Leeuwen, and W. R. Hagen. 2001. Electroanalytical determination of tungsten and molybdenum in proteins. Anal. Biochem. 297:71-78. [DOI] [PubMed] [Google Scholar]
- 7.Hagen, W. R. 1989. Direct electron transfer of redox proteins at the bare glassy carbon electrode. Eur. J. Biochem. 182:523-530. [DOI] [PubMed] [Google Scholar]
- 8.Hagen, W. R., P. J. Silva, M. A. Amorim, P. L. Hagedoorn, H. Wassink, H. Haaker, and F. T. Robb. 2000. Novel structure and redox chemistry of the prosthetic groups of the iron-sulfur flavoprotein sulfide dehydrogenase from Pyrococcus furiosus; evidence for a [2Fe-2S] cluster with Asp(Cys)3 ligands. J. Biol. Inorg. Chem. 5:527-534. [DOI] [PubMed] [Google Scholar]
- 9.Kawarabayasi, Y., M. Sawada, H. Horikawa, Y. Haikawa, Y. Hino, S. Yamamoto, M. Sekine, S.-I. Baba, H. Kosugi, A. Hosoyama, Y. Nagai, M. Sakai, K. Ogura, R. Otsuka, H. Nakazawa, M. Takamiya, Y. Ohfuku, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K.-I. Aoki, T. Yoshizawa, Y. Nakamura, F. T. Robb, K. Horikoshi, Y. Masuchi, H. Shizuya, and H. Kikuchi. 1998. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 5:55-76. [DOI] [PubMed] [Google Scholar]
- 10.Koehler, B. P., S. Mukund, R. C. Conover, I. K. Dhawan, R. Roy, M. W. W. Adams, and M. K. Johnson. 1996. Spectroscopic characterization of the tungsten and iron centers in aldehyde ferredoxin oxidoreductases from two hyperthermophilic archaea. J. Am. Chem. Soc. 118:12391-12405. [Google Scholar]
- 11.Mukund, S., and M. W. Adams. 1991. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium Pyrococcus furiosus is an aldehyde ferredoxin oxidoreductase. Evidence for its participation in a unique glycolytic pathway. J. Biol. Chem. 266:14208-14216. [PubMed] [Google Scholar]
- 12.Mukund, S., and M. W. W. Adams. 1995. Glyceraldehyde-3-phosphate ferredoxin oxidoreductase, a novel tungsten-containing enzyme with a potential glycolytic role in the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 270:8389-8392. [DOI] [PubMed] [Google Scholar]
- 13.Pierik, A. J., and W. R. Hagen. 1991. S = 9/2 EPR signals are evidence against coupling between the siroheme and the Fe/S cluster prosthetic groups in Desulfovibrio vulgaris (Hildenborough) dissimilatory sulfite reductase. Eur. J. Biochem. 195:505-516. [DOI] [PubMed] [Google Scholar]
- 14.Pierik, A. J., R. B. Wolbert, P. H. Mutsaers, W. R. Hagen, and C. Veeger. 1992. Purification and biochemical characterization of a putative [6Fe-6S] prismane-cluster-containing protein from Desulfovibrio vulgaris (Hildenborough). Eur. J. Biochem. 206:697-704. [DOI] [PubMed] [Google Scholar]
- 15.Raven, N., N. Ladwa, D. Cossar, and R. Sharp. 1992. Continuous culture of the hyperthermophilic archaeon Pyrococcus furiosus. Appl. Microbiol. Biotechnol. 38:263-267. [Google Scholar]
- 16.Roy, R., and M. W. W. Adams. 2002. Characterization of a fourth tungsten-containing enzyme from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 184:6952-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy, R., S. Mukund, G. J. Schut, D. M. Dunn, R. Weiss, and M. W. W. Adams. 1999. Purification and molecular characterization of the tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus: the third of a putative five-member tungstoenzyme family. J. Bacteriol. 181:1171-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schicho, R. N., L. J. Snowden, S. Mukund, J. B. Park, M. W. W. Adams, and R. M. Kelly. 1993. Influence of tungsten on metabolic patterns in Pyrococcus furiosus, a hyperthermophilic archaeon. Arch. Microbiol. 159:380-385. [Google Scholar]
- 19.Weinberg, M. V., G. J. Schut, S. Brehm, S. Datta, and M. W. W. Adams. 2005. Cold shock of a hyperthermophilic archaeon: Pyrococcus furiosus exhibits multiple responses to a suboptimal growth temperature with a key role for membrane-bound glycoproteins. J. Bacteriol. 187:336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]