Abstract
We have found, using a newly developed genetic method, a protein (named Cnu, for oriC-binding nucleoid-associated) that binds to a specific 26-base-pair sequence (named cnb) in the origin of replication of Escherichia coli, oriC. Cnu is composed of 71 amino acids (8.4 kDa) and shows extensive amino acid identity to a group of proteins belonging to the Hha/YmoA family. Cnu was previously discovered as a protein that, like Hha, complexes with H-NS in vitro. Our in vivo and in vitro assays confirm the results and further suggest that the complex formation with H-NS is involved in Cnu/Hha binding to cnb. Unlike the hns mutants, elimination of either the cnu or hha gene did not disturb the growth rate, origin content, and synchrony of DNA replication initiation of the mutants compared to the wild-type cells. However, the cnu hha double mutant was moderately reduced in origin content. The Cnu/Hha complex with H-NS thus could play a role in optimal activity of oriC.
The chromosomal DNA replication in Escherichia coli starts from a single locus called oriC that is minimally 258 base pairs (bp) long. This DNA sequence contains DNA-binding sites for many different proteins that participate in DNA replication (Fig. 1). There are eight binding sites (DnaA boxes and I sites) for the initiator protein DnaA (19, 35). The IciA and DpiA proteins bind to the AT-rich 13-mer repeats in oriC. IciA inhibits unwinding of the repeats (11), and overexpression of DpiA can cause SOS response (20). Nucleoid proteins such as IHF and Fis bind specifically to oriC and bend oriC upon binding (27). Another nucleoid protein, HU, binds to oriC nonspecifically but modulates the binding of IHF to oriC (5). Binding of these nucleoid proteins was shown in vitro to assist the action of DnaA protein in the unwinding of oriC (12, 28). The SeqA protein known as a negative modulator of replication initiation (17) binds specifically to two sites in oriC and has higher affinity toward hemimethylated rather than fully methylated oriC (33, 34). Nonspecific acid phosphatase also preferentially binds to hemimethylated oriC (26). Rob binds to the right region of oriC (31), while phosphorylated ArcA protein binds to the left region of oriC (16). Although deletion of the rob gene has no phenotype, phosphorylated ArcA inhibits chromosomal replication in vitro (16). Finally, CspD, a single-stranded DNA-binding protein, was shown to inhibit DNA replication in vitro (37).
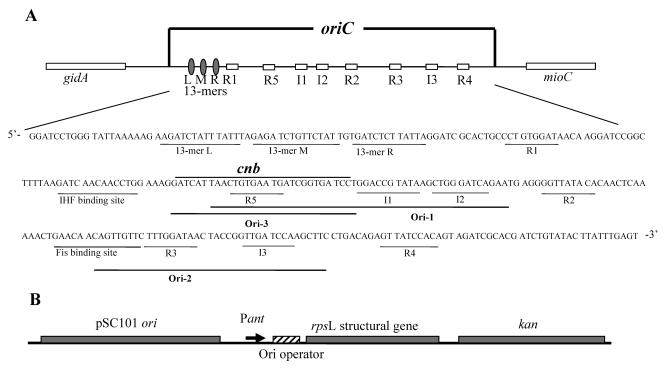
FIG. 1.
(A) Schematic illustration of the oriC region. The important elements of oriC are marked with ovals and empty boxes in the schematic and with underlines in the nucleotide sequence of the minimal oriC. The 13-mers L, M, and R are the AT-rich 13-mer repeats where the origin initially opens. R1, R2, R3, R4, and R5, and I1, I2, and I3 are DnaA boxes and I sites where the initiator protein DnaA binds. (B) Ori-1, Ori-2, and Ori-3 are the DNA sequences used as operators in plasmids with an artificial rpsL operon. The newly discovered protein-binding site cnb is identified over the sequence. We tried to avoid DNA sequences that are known to bind to proteins (except DnaA) while choosing the operator sequences.
The control of chromosomal DNA replication is a complex process in which many proteins are needed to allow initiation at the right time and frequency in accordance with the changing environment. Because the process remains to be satisfactorily understood, we contemplated that there could be more oriC-binding proteins yet to be discovered. In an attempt to find new oriC-binding proteins, we used a genetic strategy that employs transcriptional repression that is caused by DNA binding of a protein to an operator (15). This assay revealed a novel oriC-binding protein, which we have named Cnu (oriC-binding nucleoid-associated). The protein was also previously found independently as one that could complex with H-NS (25). Our in vivo and in vitro assays confirmed that Cnu could interact with H-NS. These assays further showed that oriC binding of Cnu could be dependent on complexation with H-NS. The heteromeric complex appears to bind site-specifically to a 26-bp sequence next to the IHF-binding site, overlapping DnaA box R5 in oriC.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Two well-known E. coli strains and their derivatives, MG1655 and HB101 (supE44 hsdS20 >recA ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1), were used throughout the experiments in this study. MG1655 and its derivatives used in the flow cytometry experiments were grown in either M63 or LB medium at 37°C. HB101 and its derivatives employed in the assay of survival frequency (SF) were grown in LB medium at 37°C.
The cnu, hha, and hns deletion mutants were generated by precisely removing of the gene in question following the procedure of Yu et al. (38). All of the necessary plasmids for the deletion procedure were kindly provided by D. L. Court (NIH, USA). The double mutant cnu hns and the triple mutant cnu hha hns were constructed by the inactivation of the hns gene from the cnu and cnu hha mutants, respectively. An hns gene inactivated by transposon insertion within the gene (osmZ205:Tn10 [9]) was introduced to cnu and cnu hha strains by P1 transduction, and the transductants were selected on tetracycline (12 μg/ml) agar plates. A strain, GM230, carrying the mutant allele was kindly provided by Erhard Bremer. Before transduction, HB101, which is recA, is transformed with a pSC101repA(Ts) plasmid carrying the recA+ gene (pJLR40; provided by Lee Rosner, NIH). Before use, the transductants were cured of the plasmid by overnight growth at 42°C.
Plasmids and construction of an expression library of the E. coli genome.
The plasmids used as bait to fish out proteins that bind to oriC were pOri-1, -2, and -3 (Fig. 1B). These plasmids each contain a piece of oriC fragment named Ori-1, Ori-2, or Ori-3 (Fig. 1A) as an operator of the rpsL gene. These plasmids are identical to pHL149 (15) in which the Hin-binding site (hix) is the operator. The pOri-1, -2, and -3 plasmids were constructed by replacing the hix operator with Ori-1, Ori-2, and Ori-3 fragments, respectively. First, the bottom and top strands of each DNA fragment were synthesized and annealed to each other. The resulting double-stranded oligonucleotides were cloned into the SmaI site of pHL343 (a derivative of pPY190) (10), making pHL344, -345, and -350, respectively. The EcoRI-EcoRV restriction fragment of pHL149 (15) was replaced with the EcoRI-EcoRV restriction fragment of pHL344, -345, and -350, completing the assembly of the substrate plasmids pOri-1, pOri-2, and pOri-3, respectively. An E. coli expression genomic library was constructed using plasmid pHL355 (see Fig. 3). Basically this plasmid is a derivative of pBluescript (Stratagene) that contains a piece of DNA in its SspI site that is composed of the lacIq gene from pKH66 (13), the promoter tac, a multiple cloning site, and the transcriptional stop signal (rrnBT1T2) from the plasmid pKK223-3 (Pharmacia). E. coli genomic DNA was isolated by the method described by Chen and Kuo (6). The genomic DNA was partially digested with Sau3AI, and DNA fragments of 500 to 1,500 base pairs were isolated by agarose gel electrophoresis. These DNA fragments were ligated to the BamHI site of pHL355. There were 107 clones in the library, and 60% of the plasmids contained inserts.
FIG. 3.
Description of pHL355 used to construct an expression library of the E. coil genome. pHL355 has been derived from pBluescript and contains the gene for the lac repressor (lacIq), the ampicillin-resistant determinant (amp), the promoter tac (Ptac), and the transcriptional stop signal (rrnBT1T2). This diagram also shows a piece of genomic DNA cloned in the plasmid that gave a high SF value to the host cells. Serial deletions of the insert DNA (shown in this figure) and the corresponding SF values indicated that the putative b1625 open reading frame is the determinant for the high SF value.
Screening for genes whose products can bind to oriC.
The genomic library was introduced into HB101 harboring three different plasmids, HB101/pOri-1, HB101/pOri-2, and HB101/pOri-3, by electroporation. The transformed cells were grown in 3 ml of LB medium containing ampicillin (Amp; 100 μg/ml), kanamycin (Kan; 50 μg/ml), and 20 μM isopropylthiogalactoside (IPTG) for 12 h at 37°C. Cells were spun down, resuspended in 300 μl of LB medium, and plated on LB agar plates containing Amp, Kan, IPTG, and streptomycin (Str; 100 μg/ml). Plates were incubated at 37°C for 72 h. Plasmids (pHL355 containing inserts) were prepared from the fast-growing Str-resistant colonies and analyzed further.
Measurement of survival frequency: an in vivo assay for DNA binding.
A single colony of HB101 harboring both an expression plasmid (Amp resistant) and the rpsL-carrying plasmid (Kan resistant) was grown in 2 ml LB/Amp/Kan/IPTG medium at 37°C for 12 h. The culture was diluted appropriately and plated on LB/Amp/Kan/IPTG agar and also on LB/Amp/Kan/Str/IPTG agar. The plates were incubated at 37°C for 72 h. The numbers of colonies were counted every 24 h. The SF defined as a ratio of the number of Ampr Kanr Strr colonies/ml to the number of Ampr Kanr colonies/ml was calculated. Growth in liquid cultures was assayed by A600 measurements.
Expression and purification of Cnu and H-NS proteins.
The cnu gene was PCR amplified from pHL355-36 and cloned between the NdeI and BamHI sites of pET-15b (Novagen), generating pETCnu. For the expression of 6×His-Cnu (Cnu protein tagged with six histidines at the N terminus), BL21 (DE3)/pETCnu cells were grown in M9 minimal medium containing Amp (50 mg/ml) at 37°C for 16 h. A one-tenth dilution of this culture was made in fresh M9 medium (1 liter), and cells were grown to an A600 of 0.7 at 37°C. IPTG (final concentration, 0.4 mM) was added, and the culture was grown for an additional 6 h. Cells were pelleted and resuspended in 35 ml of phosphate-buffered saline (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4). Phenylmethylsulfonyl fluoride (final concentration, 0.2 mM) was added. Cells were disrupted by sonication (15 cycles of 10 s on and 60 s off). The cell lysate was cleared by centrifugation (12,000 × g) for 45 min at 4°C. The pellet was washed twice with a washing buffer (20 mM sodium phosphate, pH 7.3, 200 mM NaCl, 1% Triton X-100) and dissolved in a buffer (40 mM sodium acetate, pH 7.3, 400 mM NaCl) containing 6 M guanidium chloride. Denatured proteins were refolded by rapid dilution using a refolding buffer (50 mM sodium phosphate, pH 8.0, 0.5 M NaCl, 5 mM imidazole, 1 M urea, 10% d-glucose, 2 mM MgCl2). We kept the final concentration of proteins below an A280 of 0.2/ml. The resulting protein solution was centrifuged (8,000 × g) for 30 min, and the supernatant was loaded onto a 5 ml Ni-NTA Superflow column (QIAGEN) and preequilibrated with the binding buffer (50 mM sodium phosphate, pH 8.0, 0.5 M NaCl, 5 mM imidazole). The column was washed with 50 ml of the binding buffer, followed by 30 ml of the washing buffer (50 mM sodium phosphate, pH 8.0, 0.5 M NaCl, 50 mM imidazole), and the target protein (6×His-Cnu) was eluted from the column with 50 ml of the elution buffer (50 mM sodium phosphate, pH 8.0, 0.5 M NaCl, 1 M imidazole). Fractions containing the target protein were pooled and concentrated to 10 ml using a stirred concentration cell (Amicon). Nuclear magnetic resonance spectroscopic study of the refolded 6×His-Cnu and the native Cnu indicated that there are no structural differences between these two proteins (data not shown).
For the H-NS protein, the plasmid pHOP11 (kindly provided by Shindo, Tokyo University) encoding H-NS was used. E. coli strain BL21 carrying pHOP11 was grown in LB medium at 37°C, and H-NS protein was induced by adding IPTG (final concentration, 0.4 mM) when the culture reached an A600 of 0.6. Cells were grown for an additional 4 h and harvested by centrifugation (8,000 × g) for 15 min. Purification of H-NS protein was carried out as described previously (30).
Protein-protein interaction assay using Ni-NTA agarose or glutaraldehyde.
Ni-NTA agarose (50% slurry; QIAGEN) was equilibrated with the binding buffer containing 0.1% Triton X-100. H-NS protein (0.2 ml of 36 μM), the 6×His-Cnu protein (0.2 ml of 26 μM), and 0.4 ml of the binding buffer were mixed. To this, 120 μl of Ni-NTA (see above) was added, and the resulting mixture was incubated at 4°C for 1 h. The Ni-NTA agarose was washed twice with 0.5 ml of the binding buffer and three times with 0.2 ml of the washing buffer containing 0.1% Triton X-100. Bound proteins were eluted four times with 0.1 ml of the elution buffer. Fractions were analyzed by 12% Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE [29]).
For the glutaraldehyde cross-linking experiments, 9 μM 6×His-Cnu and 5 μM H-NS were mixed in a buffer containing 20 mM sodium phosphate (pH 7.3) and 200 mM NaCl. To these protein mixtures, glutaraldehyde solution was added to make final concentrations of 0.00025, 0.005, 0.025, and 0.125%. The mixtures were incubated at 25°C for 30 min, and the proteins were analyzed by 10% SDS-PAGE.
Flow cytometry.
The number of origins per cell was determined by the replication runoff method (32). Freshly saturated cultures were diluted about 200-fold to an optical density at 600 nm (OD600) of 0.01 and grown to an OD600 of ≤0.4 either in LB medium or in 1× M63 medium (KD Biochemicals) supplemented with 2 mM MgSO4, 0.1 mM CaCl2, 1 μg/ml thiamine, and 0.2% glucose. Additionally, 0.2% Casamino Acids (CAA) was added in some experiments (the hns mutant grew poorly without CAA since the strain is weakly auxotrophic for leucine, isoleucine, and valine) (14). For replication runoff, rifampin at 150 μg/ml and cephalexin at 10 μg/ml were added to prevent replication initiation and cell division, and the cultures were incubated for 3 h and chilled on ice. All subsequent manipulations were done at 4°C. Cells were pelleted from 1.8 ml of the culture, washed twice with 1 ml TE buffer (10 mM Tris HCl, pH 7.4 + 1 mM EDTA), and resuspended in 0.1 ml TE buffer, to which 0.9 ml 77% ethanol was added. The cells were stored at −20°C overnight or longer. Before flow cytometric analysis, the cells were resuspended twice in 1 ml TM buffer (10 mM Tris HCl, pH 7.4, + 10 mM MgSO4), and the OD600of the cell suspension was determined. An aliquot representing 107 cells was removed and added to a final volume of 0.9 ml TM, assuming that an OD600 of 1 equals 109 cells/ml. To this, 1,000 μl of Hoechst 33342 (Molecular Probes) solution in TM buffer was added to a final concentration of 0.5 μg/ml, and the staining was continued for 1 to 2 h before the cells were analyzed by flow cytometry.
Flow cytometry was performed using BD LSR II (Becton Dickinson). It has a Coherent Sapphire 488-nm laser that generates forward and side scatter signals and a Lightwave Xcite 355-nm (UV) laser for generating fluorescence signals. For collecting Hoechst 33342 fluorescence, a combination of a 505 LP dichroic mirror and a 440/40 band-pass filter were used in front of the detector.
Western blot analysis and site-directed point mutations and deletions.
The Western analysis of Cnu protein was done by Tricine-SDS-PAGE that could separate proteins smaller than 10 kDa (29). The polyclonal antibody of Cnu was raised by injection of purified Cnu protein into a rabbit (Peptron, Korea). Single nucleotide changes and a few base pair deletions were made by using PCR as described previously by Barik and Galinski (3).
Nucleotide sequence accession number.
The DNA sequence of the cnu gene and the surrounding sequence have been deposited in GenBank under accession number AY442175.
RESULTS
Strategy for finding oriC-binding proteins.
A newly developed system was used to identify cloned genes whose products could bind to oriC of E. coli. The system uses a low-copy-number plasmid that has an artificial rpsL operon, in which one of several different oriC fragments was inserted as an operator (Fig. 1B). If a streptomycin-resistant E. coli strain (because of the presence of the rpsL20 allele in the chromosome), such as HB101, harbors this plasmid, the host cells become streptomycin sensitive (8). If the binding of a protein to the oriC operator caused transcriptional repression of the rpsL gene, the host cell could then survive in a medium containing Str (15). In a previous study, the DNA-binding activity of a specific protein to an operator sequence of the rpsL operon was evaluated by measuring SF. SF is defined as a ratio between the number of cells that made colonies on Str agar plates and the total number of plated cells (see Materials and Methods). For example, binding of the Hin recombinase to the hix operator caused 2% of plated host cells to make colonies on Str agar plates. Thus, the SF of cells in which the DNA-binding activity of the Hin recombinase was measured was 2 × 10−2 (15).
We have used three different DNA fragments of oriC as operators (Fig. 1A). They were named Ori-1, Ori-2, and Ori-3, and the plasmids bearing these DNA fragments as operators were called pOri-1, pOri-2, and pOri-3, respectively. HB101 containing pOri-1 and pOri-2 showed no Str-resistant colonies. However, HB101 harboring pOri-3 showed an SF of 3 × 10−2, suggesting that an endogenous protein(s) binds to the Ori-3 sequence.
Growth phase dependence of Ori-3-binding activity.
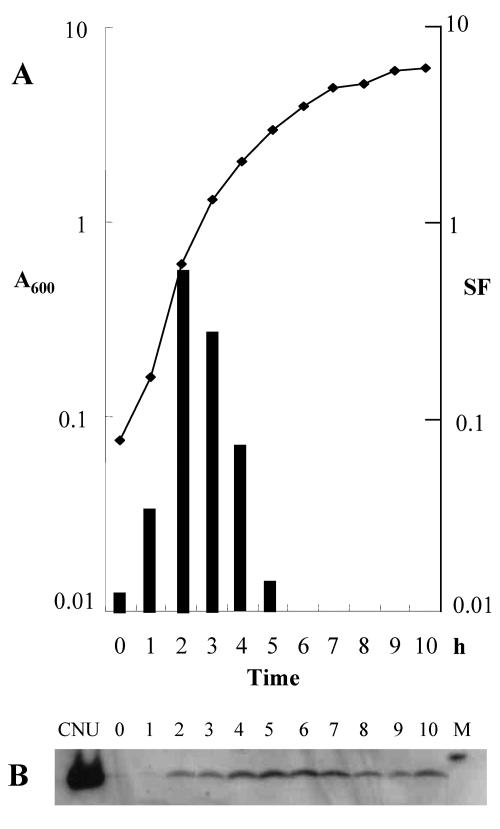
The SF assay described above was used to measure Ori-3 binding at different growth phases of the host cells. SF was measured at several time points during the growth of the HB101/pOri-3 culture. As shown in Fig. 2A, SF was around 0.03 when overnight grown cells were freshly diluted to a new medium, and it increased to 0.65 when the cells were at the log phase (A600 of 0.7). SF decreased to 0.02 when the cells were entering stationary phase. These results suggested that the binding activity of an endogenous protein(s) onto Ori-3 is maximal at the early log phase and minimal at stationary phase.
FIG. 2.
(A) SF measurements at various times during growth of HB101/pOri-3. The line graph (growth curve) shows A600 values at different time points. The bars represent SF values at the corresponding time points. (B) Western blots of Cnu at various times during growth of HB101. The first lane (Cnu) is the purified Cnu protein (1 μg). The numbers indicate the time points in panel A. Crude extracts (20 μg of protein) from the culture at each time point were loaded. Lane M is the 10-kDa Western size marker (ELPis-Biotech, Taejean, Korea).
Identification of the cnu gene.
When an expression plasmid library of the E. coli genome (in vector pHL355 [see Materials and Methods]) was introduced to HB101 harboring pOri-1, pOri-2, or pOri-3, only HB101/pOri-3 produced Str-resistant colonies. They were mostly small (0.5 mm in diameter), but a few were big (2 mm in diameter). The small colonies were Str resistant, apparently because of the binding of an endogenous protein(s) to Ori-3 as described above. The big colonies most likely resulted from cells that received a plasmid from the library that overexpressed the same oriC-binding protein(s). Plasmids were isolated from the big colonies and were introduced to fresh HB101/pOri-3 cells, and the SF of the resultant strains was measured. Out of 36 candidates, one showed an SF of over 0.5, indicating that the promoter for rpsL is well repressed in this case. DNA sequencing of the insert of the corresponding plasmid, pHL355-36, revealed two open reading frames (b1625 and b1626) of unknown functions. Serial deletion of the insert and measurement of the SF of cells carrying each of the deleted plasmids indicated that b1625 is responsible for the high SF of the candidate (Fig. 3). DNA sequence analysis showed that the b1625 gene codes for a small protein (71 amino acids, 8.4 kDa) homologous to the Hha protein of E. coli (21), RmoA from the plasmid R100-1 (22), and YmoA of Yersinia enterocolitica (Fig. 4) (7). These proteins, including the one made from b1625 (YgdT) were known to be associated with the nucleoid protein H-NS (23, 24, 25). We named the b1625 gene cnu.
FIG. 4.
Comparison of amino acid sequences of proteins homologous to Cnu. The identical amino acids are shown in boldface.
Analysis of open reading frames surrounding cnu in the genome of E. coli K-12 (4) revealed that there are six consecutive open reading frames following cnu. It is likely that these open reading frames comprise an operon in which cnu is the first gene. Primer extension analysis of the RNA prepared from pHL355-36/HB101 indicated that there are at least three promoters in the 5′-untranslated region of the cnu gene (data not shown).
Cnu protein interaction with H-NS.
Both Cnu and H-NS proteins were purified to homogeneity (see Materials and Methods). The Cnu protein was tagged with six histidines at the N terminus (6×His-Cnu). Purified H-NS and 6×His-Cnu were mixed and incubated at 4°C for 1 h with Ni-NTA agarose beads. The agarose beads were washed, and bound proteins were eluted. H-NS and 6×His-Cnu were found to elute simultaneously (Fig. 5B). Since H-NS alone does not bind to Ni-NTA agarose beads (Fig. 5A), these results suggest that H-NS binds to Cnu. The physical association of these two proteins was further probed by glutaraldehyde cross-linking. The proteins were mixed and incubated in the presence of glutaraldehyde, and changes in their oligomeric states were visualized by SDS-PAGE. As shown in Fig. 5C, higher concentrations of glutaraldehyde barely changed the oligomeric state of the 6×His-Cnu alone, but when the two proteins were mixed, the oligomeric states of both 6×His-Cnu and H-NS changed. Since the mobility of cross-linked 6×His-Cnu and H-NS complexes differs from that of cross-linked H-NS alone (Fig. 5C), these results show that Cnu could complex with H-NS in vitro.
FIG. 5.
(A and B) SDS-PAGE showing proteins eluted from Ni-NTA agarose after interaction with H-NS alone (A) or a mixture of 6×His-Cnu and H-NS (B). (C) SDS-PAGE of proteins after cross-linking with increasing concentrations of glutaraldehyde (0.00025, 0.005, 0.025, and 0.125%). (D) SDS-PAGE of crude extract of BL21/pETCnu after Cnu overexpression, loading onto a Ni-NTA agarose column, washing of the loaded column, and elution from the column.
To assess protein-protein interactions in vivo, total proteins from HB101 harboring pETCnu that overproduces 6×His-Cnu were loaded onto a Ni-NTA agarose column. The column was washed, and bound proteins were eluted. As shown in Fig. 5D, H-NS was eluded with 6×His-Cnu. A mass spectroscopic analysis of the higher-molecular-weight band above H-NS showed that it is dimeric 6×His-Cnu. These results along with the findings of Paytubi et al. (25) suggested that Cnu could make a protein complex with H-NS in vivo.
Alternate Ori-3-binding proteins.
Since the Hha protein is homologous to Cnu, the question of whether Cnu is the only protein that binds to Ori-3 arises. To test this, we constructed the following series of HB101 derivatives: HB101cnu, HB101hha, and HB101hns, in which the cnu, hha, and hns genes were deleted, respectively. We also generated HB101cnuhha, in which both the cnu and hha genes were deleted. Our rationale was that if one of the proteins preferentially binds to Ori-3, then the corresponding deletion mutant would grow slower or even die in the presence of pOri-3 in Str-containing medium. First, as a control, the generation times of these pOri-3-containing cells were determined in kanamycin medium, and they were not significantly different from each other (Table 1). The results in Str-containing medium were as follows, and they are summarized in Table 1.
TABLE 1.
Generation times of cells in LB medium with different antibiotics
| Strain/plasmida | Generation time (min) in:
|
||
|---|---|---|---|
| Kan | Kan + Str | Kan + Str/Kan | |
| HB101/pHL149 | 64 | No growth | 0 |
| HB101/pOri-3 | 62 | 93 | 1.48 (1.00)b |
| HB101hns/pOri-3 | 69 | 129 | 1.87 (1.26) |
| HB101cnu/pOri-3 | 66 | 102 | 1.55 (1.05) |
| HB101hha/pOri-3 | 65 | 96 | 1.48 (1.00) |
| HB101cnuhha/pOri-3 | 64 | 114 | 1.78 (1.20) |
| HB101cnuhns/pOri-3 | 69 | 133 | 1.93 (1.30) |
| HB101cnuhhahns/pOri-3 | 78 | 172 | 2.21 (1.49) |
The SF of all the deletion mutants of HB101 carrying a Hin-producing plasmid and a hix operator plasmid was same as that of HB101, suggesting that the deletion mutants were as useful as HB101 for the measurement of SF.
The numbers in parentheses indicate the relative growth rate of each strain to that of HB101/pOri-3 grown in kanamycin and streptomycin medium. A value higher than 1.00 means growth slower than that of HB101/pOri-3.
The plasmid pHL149 (15) is identical to pOri-3 except that it has a Hin-binding site, hix, instead of Ori-3. Therefore, HB101/pHL149 is not supposed to grow in the presence of streptomycin, and it did not. This strain served as a control for no Ori-3 binding (negative control). The generation time of HB101/pOri-3 in Str medium was 93 min, suggesting that the binding of a protein(s) to the Ori-3 site in pOri-3 allowed growth of the strain. This strain served as a positive control. Deletion of either cnu or hha from the chromosome did not affect the growth of the corresponding strain with pOri-3 in Str medium, suggesting that either cnu or hha suffices for binding to Ori-3. However, when both genes were deleted, the generation time of the corresponding strain (HB101cnuhha/pOri-3, 114 min) was significantly longer than that of the positive control strain (HB101/pOri-3, 93 min). This result suggested that both Cnu and Hha proteins are required for Ori-3 binding.
Deletion of the hns gene alone significantly affected the generation time of the corresponding strain (HB101hns) with pOri-3 in Str medium (129 min). Deletion of both cnu and hns genes resulted in the same generation time as that of HB101hns (133 min). These results suggested the involvement of H-NS in the Ori-3 binding in vivo and were consistent with our in vitro data showing that Cnu complexes with H-NS. Deletion of all of the three genes resulted in the corresponding strain, HB101cnuhhahns, having the slowest generation time (172 min), suggesting that the three proteins are required for the Ori-3 binding in vivo. Since the triple deletion mutant (HB101cnuhhahns) did not result in zero growth in Str medium, it is likely that there are factors other than Cnu, Hha, or H-NS that could bind to Ori-3.
The concentration of Cnu at the different phases of growth of the HB101 culture was measured by Western blotting (Fig. 2B). The result showed that Cnu expression becomes maximal as cells enter the stationary phase but decreases later in the stationary phase. Since the SF of HB101/pOri-3 was highest in log phase (Fig. 2A), this result supported the notion, discussed above, that there could be other growth-phase-dependent factors involved in Ori-3 binding.
Sequence specificity of Ori-3 binding.
The SF of HB101/pOri-1 was zero, suggesting little to no binding to the Ori-1 sequence. In contrast, the SF of HB101/pOri-3 was 0.03, although Ori-3 has only six more bases in the 5′ end than Ori-1 (Fig. 1). We wanted to determine whether the six bases were critical for Ori-3 binding. We employed the plasmid pHL355-36, which overproduces Cnu, and the plasmid pHL355Hha, which overproduces Hha. The SF of both HB101/pOri-3/pHL355-36 and HB101/pOri-3/pHL355Hha were over 0.5 (Table 2), suggesting that overproduction of either protein alone could elicit more binding to the Ori-3 site, leading to higher SF.
TABLE 2.
Operator sequence change versus SF
| Parameter or change from Ori-3 | Operator sequence | SF
|
|
|---|---|---|---|
| CNU | Hha | ||
| Position | 1 5 1015 202530 | ||
| Ori-3 | 5′-CCC-GATC ATTAACTGTGAATGATCGGTGATCCTG - GGG -3′ | 0.54 | 0.58 |
| 5′ 2-bp deletion | TCATTAACTGTGAATGATCGGTGATCCTG | 0.56 | 0.59 |
| 5′ 3-bp deletion | CATTAACTGTGAATGATCGGTGATCCTG | 0.50 | 0.57 |
| 5′ 4-bp deletion | ATTAACTGTGAATGATCGGTGATCCTG | 0.50 | 0.51 |
| 5′ 5-bp deletion | TTAACTGTGAATGATCGGTGATCCTG | 0 | 0 |
| 5′ 10-bp deletion | TGTGAATGATCGGTGATCCTG | 0 | 0 |
| 5′ 2-bp addition | AGGAT CATTAACTGTGAATGATCGGTGATCCTG | 0.52 | 0.54 |
| 3′ 2-bp deletiona | GAT CATTAACTGTGAATGATCGGTGATCC | 0 | 0 |
| T to A at position 30 | GAT CATTAACTGTGAATGATCGGTGATCCAG | 0 | 0 |
| T to C at position 30 | GAT CATTAACTGTGAATGATCGGTGATCCCG | 0 | 0 |
| A to G at position 19 | GAT CATTAACTGTGAATGGTCGGTGATCCTG | 0.49 | 0.57 |
| 3′ 10-bp deletionb | GAT CATTAACTGTGAATGATC | 0 | 0 |
| cnb | ATTAACTGTGAATGATCGGTGATCCT | ||
This deletion results in the same DNA sequence as a change from T to G at position 30 due to the GGG sequence of the vector at the 3′ end of Ori-3.
Sequence contains only the inverted repeat.
Single-base deletions from the 5′ end up to the fourth residue of the Ori-3 operator had no effect on the binding. However, the deletion up to the fifth adenine abolished the binding activity completely, suggesting that the fifth adenine is critical for the Ori-3 binding (Table 2). The addition of two bases to the 5′ end had no deleterious effect. The deletion of two bases from the 3′ end of Ori-3 eliminated the binding activity. Though deciphering the exact boundary of the binding site was complicated by the surrounding DNA sequences from the vector, the deletion and the addition experiments suggested that Cnu binds to a 26-bp sequence referred to as cnb in Table 2. The cnb site is located next to the IHF-binding site, overlapping DnaA box R5 in oriC (Fig. 1). The binding specificity of Cnu seemed extraordinary. Replacing the thirtieth T of the Ori-3 sequence with either A, G, or C completely eliminated binding. There are two adenine methylation sites (GATC) in cnb. Elimination of the second methylation site in cnb by changing the nineteenth A to G did not affect binding, suggesting that DNA methylation has no effect on DNA-binding activity of Cnu. The 21-bp inverted repeat sequence in Ori-3 could not serve as the binding site for the proteins. Interestingly, the SF values with Hha overproduction were almost identical to those of Cnu overproduction (Table 2). Taken together, these results suggest that the Ori-3 binding of Cnu or Hha protein is sequence specific.
Phenotypes of a cnu mutant.
Searching the E. coli genome with the cnu gene sequence revealed that hha is the only homolog of cnu. The Hha-HNS complex was suggested to be involved in gene regulation to cope with environmental changes, such as changes in osmolarity and temperature (2, 18). Thus, to see if the Hha homologue Cnu is engaged in the modulation of gene expression necessary to cope with environmental changes, we measured the growth of the cnu mutant at different temperatures and salt concentrations. There was no noticeable difference between MG1655 and MG1655cnu in growth at the temperatures (25, 32, 37, and 42°C), and NaCl concentrations (0, 0.17, 0.5, and 1.0 M) tested, suggesting that cnu is not involved in the gene regulation to cope with environmental changes (data not shown). A possibility remains that Cnu could be engaged in regulation of genes other than the environmentally regulated ones. In fact, another oriC-binding protein, Rob, functions as a transcriptional activator rather than a replication regulator (13).
Our results indicating that the Cnu and Hha proteins, possibly complexed with H-NS, bind to Ori-3 suggested that the phenotype of a cnu or hha deletion mutant in chromosome replication would be the same as that of an hns mutant. One of the prominent phenotypes of hns mutants is a reduced number of chromosomes per cell (ploidy) (1, 14). We have constructed a series of MG1655 strains, MG1655cnu, MG1655hha, and MG1655hns, in which the cnu, hha, and hns genes, respectively, are deleted. We also generated MG1655cnuhha, in which both the cnu and hha genes are deleted. These cells were analyzed for variation of the chromosomal number using flow cytometry. As shown in Fig. 6 and Table 3, unlike the isogenic hns mutant, the MG1655cnu cells had chromosome numbers comparable to those of the wild-type or isogenic hha cells. The double mutant cnu hha, however, showed reduced ploidy. Replication initiation was synchronous in all cases. The phenotype of the double mutant thus approaches but is not as strong as that of the hns mutant. This suggests that H-NS has roles in DNA replication other than through Cnu/Hha binding or that these proteins could be replaced by another unknown factor(s).
FIG. 6.
Determination of cell size (A) and chromosome content (B-D) by flow cytometry. Cells used were exponentially growing in different growth media: LB broth (A-B), minimal glucose + CAA (C), and minimal glucose (D). Depending upon the growth medium, the two major peaks of Hoechst fluorescence represent cells with either four and eight (B) or two and four (C-D) chromosomes, but their ratio varied depending upon the genotype of the cells as identified to the right of the figure (see Table 3 for details). The cells used were either MG1655 (WT) or its isogenic derivatives. The dam mutant (BR2786) is from our lab collection and is not derived from MG1655.
TABLE 3.
Generation times and chromosome content of cells in different growth mediaa
| Strain | LB medium
|
MinGlu CAA
|
MinGlu
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| τ | % of cells with:
|
τ | % of cells with:
|
τ | % of cells with:
|
||||
| 4 chromosomes per cell | 8 chromosomes per cell | 2 chromosomes per cell | 4 chromosomes per cell | 2 chromosomes per cell | 4 chromosomes per cell | ||||
| WT | 23 | 48 | 33 | 35 | 35 | 57 | 68 | 74 | 19 |
| hns | 29 | 67 | 14 | 48 | 83 | 10 | 48b | 81 | 11 |
| cnu | 23 | 36 | 42 | 35 | 40 | 55 | 68 | 81 | 12 |
| hha | 23 | 38 | 41 | 35 | 43 | 51 | 68 | 75 | 20 |
| cnu hha | 25 | 57 | 29 | 38 | 55 | 40 | 71 | 78 | 16 |
MinGluCAA, minimal glucose with CAA; MinGlu, minimal glucose; τ, generation time in minutes; WT, wild type.
The hns cultures grown in MinGlu also had 0.2% CAA.
Two additional mutant strains of MG1655, cnu hns and cnu hha hns (see Materials and Methods) were constructed and subjected to flow cytometry analysis along with other mutant strains (Fig. 7). The results showed that addition of hns reduced ploidy as expected in all cases: instead of cells primarily with four origins, a significant population of cells had two origins at the time of rifampin addition. Again, the effect of cnu deletion was marginal, but addition of cnu hha double mutations significantly increased the fraction of two-origin cells at the expense of four-origin cells (Fig. 7). The replication was still synchronous in cells with cnu hha hns triple mutations. The growth of these cells was also unaffected under our experimental conditions. Therefore, these genes cannot be essential for viability even when combined.
FIG. 7.
Flow cytometry of triple mutant MG1655cnuhhahns along with other MG1655 derivatives. Genotypes were indicated in each box. Cells used were exponentially growing in LB medium. The generation times of all six strains tested were in the range of 24 to 28 min.
DISCUSSION
The genetic screen that identified the cnu gene is based on transcriptional repression activity of DNA-binding proteins. If the binding activity to an operator does not elicit transcriptional repression, the binding cannot be detected. This may explain why we could not detect the binding of DnaA protein to any of the operator fragments used, even though all of the fragments contained a binding site for DnaA. Thus, the mode of binding to Ori-3 by DnaA and the proteins studied here (Cnu/Hha) could be quite different. The DNA-binding activity of Cnu/Hha proteins when overproduced seems strong and stable because the binding caused more than 50% of host cells to survive in an Str-containing medium. In comparison, the specific DNA binding of the Hin recombinase (when overproduced) to its recombination site made only 3% of host cells Str resistant (15).
We tried to demonstrate binding of purified Cnu, Hha, and H-NS to Ori-3 by EMSA. Neither Cnu or Hha alone nor Cnu-HNS or Hha-HNS complex showed binding to Ori-3 (data not shown). Our result indicating that HB101cnuhha/pOri-3 did show a decent growth rate (albeit slower than that of other mutants) in Str medium would argue that the Ori-3-binding activity is still there in the cnu hha double mutant of HB101 (Table 1). Our failure to detect binding of Cnu-HNS complex to Ori-3 in vitro could therefore be due to the requirement of factors other than Cnu, Hha, and H-NS proteins in the cell. We tried a DNase I footprinting assay with supercoiled pOri-3, but again, the binding activity to Ori-3 was not detected. Thus, the possibility of the requirement for a specific DNA topology in Ori-3 binding seems not to be the case.
Although the identity of the binding complex remains unclear, the sequence specificity of the binding was unambiguous (Table 2). H-NS is known as a nonspecific DNA-binding protein, although it binds preferentially to curved DNAs (36). The sequence specificity of binding thus suggests that Cnu, Hha, or some unidentified proteins are making H-NS a specific DNA-binding protein. Alternatively, H-NS alters DNA structure that allows these proteins to contact DNA.
The flow cytometry analysis of the deletion mutants (Fig. 6) suggested possible involvement of Cnu and Hha in chromosomal DNA replication. Since the deletion of either cnu or hha did not show any changes in DNA contents, and the double mutants (cnu hha) showed moderately reduced ploidy compared to that of the wild type, it is likely that either protein alone can be functional in DNA replication. These results suggest that the interaction of H-NS with a specific site in oriC, mediated either by Cnu or Hha, is important for chromosomal replication.
Acknowledgments
We thank Sukhyun Kang and Deog Su Hwang at Seoul National University for the construction of the E. coli genomic library. We also thank the students of Biology at Chungnam National University, Young Lang Lee, and Jeong Jun Ji, for the in vivo assays, Yong Bhum Song for the Western analysis, and Bora Kim for the preparation of the manuscript.
This study was supported by a grant (R01-1999-000-00115-0) from the Basic Research Program of the Korea Science and Engineering Foundation, Republic of Korea, and by the Korea Research Foundation grant (KRF-2004-R05-2004-000-10423-0).
REFERENCES
- 1.Atlung, T., and F. G. Hansen. 2002. Effect of different concentrations of H-NS protein on chromosome replication and the cell cycle in Escherichia coli. J. Bacteriol. 184:1843-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balsalobre, C., J. Johansson, B. E. Uhlin, A. Juarez, and F. J. Munoa. 1999. Alteration in protein expression caused by the hha mutation in Escherichia coli: influence of growth medium osmolarity. J. Bacteriol. 181:3018-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barik, S., and M. S. Galinski. 1991. “Megaprimer” method of PCR: increased template concentration improves yield. BioTechniques 10:489-490. [PubMed] [Google Scholar]
- 4.Blatter, F. R., G. Plunkett, III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. D. Collado-Vides, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davids, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Bonnefoy, E., and J. Rouviere-Yaniv. 1992. HU, the major histone-like protein of E. coli, modulates the binding of IHF to oriC. EMBO J. 11:4489-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, W., and T. Kuo. 1993. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 21:2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis, G. R., C. Sluiters, L. Delor, D. Geib, K. Kniga, C. Lambert-de-Rouvroit, M. P. Sory, J. C. Vanooteghem, and T. Michiels. 1991. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol. Microbiol. 5:1023-1034. [DOI] [PubMed] [Google Scholar]
- 8.Dean, D. 1981. A plasmid cloning vector for the direct selection of strains carrying recombinant plasmids. Gene 15:3240-3244. [DOI] [PubMed] [Google Scholar]
- 9.Higgins, C. F., C. J. Dorman, D. A. Stirling, L. Waddell, I. R. Booth, G. May, and E. Bremer. 1988. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell 52:569-584. [DOI] [PubMed] [Google Scholar]
- 10.Hughes, K. T., P. Youderian, and M. I. Simon. 1988. Phase variation in Salmonella: analysis of Hin recombinase and hix recombination site interaction in vivo. Genes Dev. 2:937-948. [DOI] [PubMed] [Google Scholar]
- 11.Hwang, D. S., and A. Kornberg. 1992. Opening of the replication origin of Escherichia coli by DnaA protein with protein HU or IHF. J. Biol. Chem. 267:23083-23086. [PubMed] [Google Scholar]
- 12.Hwang, D. S., and A. Kornberg. 1992. Opposed actions of regulatory proteins, DnaA and IciA, in opening the replication origin of Escherichia coli. J. Biol. Chem. 267:23087-23091. [PubMed] [Google Scholar]
- 13.Jair, K. W., X. Yu, K. Skarstad, B. Thöny, N. Fujita, A. Ishihama, and R. E. Wolf, Jr. 1996. Transcriptional activation of promoters of the superoxide and multiple antibiotic resistance regulons by Rob, a binding protein of the Escherichia coli origin of chromosomal replication. J. Bacteriol. 178:2507-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaidow, A., M. Wachi, J. Nakamura, J. Magae, and K. Nagai. 1995. Anucleate cell production by Escherichia coli Δhns mutant lacking a histone-like protein, H-NS. J. Bacteriol. 177:3589-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, S. Y., H. J. Lee, H. Lee, S. H. Kim, E. H. Cho, and H. M. Lim. 1998. In vivo assay of protein-protein interactions in Hin-mediated DNA inversion. J. Bacteriol. 180:5954-5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, Y. S., J. S. Han, Y. Jeon, and D. S. Hwang. 2001. The Arc two-component signal transduction system inhibits in vitro Escherichia coli chromosomal initiation. J. Biol. Chem. 276:9917-9923. [DOI] [PubMed] [Google Scholar]
- 17.Lu, M., J. Campbell, E. Boye, and N. Kleckner. 1994. SeqA: a negative modulator of replication initiation in E. coli. Cell 77:413-426. [DOI] [PubMed] [Google Scholar]
- 18.Madrid, C., J. M. Neito, M. Paytubi, M. Falconi, C. Gualerzi, and A. Juarez. 2002. Temperature- and H-NS-dependent regulation of a plasmid-encoded virulence operon expressing Escherichia coli hemolysin. J. Bacteriol. 184:5058-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGarry, K. C., V. T. Ryan, J. E. Grimwade, and A. C. Leonard. 2004. Two discriminatory binding sites in the Escherichia coli replication origin are required for DNA strand opening by initiator DnaA-ATP. Proc. Natl. Acad. Sci. USA 101:2811-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, C., H. Ingmer, L. E. Thomsen, K. Skarstad, and S. N. Cohen. 2003. DpiA binding to the replication origin of Escherichia coli plasmids and chromosomes destabilizes plasmid inheritance and induces the bacterial SOS response. J. Bacteriol. 185:6025-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieto, J. M., M. Carmona, S. Bolland, Y. Jubete, F. de la Cruz, and A. Juarez. 1991. The hha gene modulates haemolysin expression in Escherichia coli. Mol. Microbiol. 5:1285-1293. [DOI] [PubMed] [Google Scholar]
- 22.Nieto, J. M., A. Prenafeta, E. Miquelay, A. Torrades, and A. Juarez. 1998. Sequence, identification and effect on conjugation of the rmoA gene of plasmid R 100-1. FEMS Microbiol. Lett. 169:59-66. [DOI] [PubMed] [Google Scholar]
- 23.Nieto, J. M., C. Madrid, A. Prenafeta, E. Miquelay, C. Balsalobre, M. Carrascal, and A. Juarez. 2000. Expression of the hemolysin operon in Escherichia coli is modulated by a nucleoid-protein complex that includes the proteins Hha and H-NS. Mol. Gen. Genet. 263:349-358. [DOI] [PubMed] [Google Scholar]
- 24.Nieto, J. M., C. Madrid, E. Miquelay, J. L. Parra, S. Rodriguez, and A. Juarez. 2002. Evidence for direct protein-protein interaction between members of the enterobacterial Hha/YmoA and H-NS families of proteins. J. Bacteriol. 184:629-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paytubi, S., C. Madrid, N. Forns, J. M. Nieto, C. Balsalobre, B. E. Uhlin, and A. Juarez. 2004. YdgT, the Hha paralogue in Escherichia coli, forms heteromeric complexes with H-NS and StpA. Mol. Microbiol. 54:251-263. [DOI] [PubMed] [Google Scholar]
- 26.Reshetnyak, E., E. d'Alencon, R. Kern, A. Taghbalout, P. Guillaud, and M. Kohiyama. 1999. Hemi-methylated oriC DNA binding activity found in non-specific acid phosphatase. Mol. Microbiol. 31:167-175. [DOI] [PubMed] [Google Scholar]
- 27.Roth, A., B. Urmoneit, and W. Messer. 1994. Functions of histone-like proteins in the initiation of DNA replication at oriC of Escherichia coli. Biochimie 76:917-923. [DOI] [PubMed] [Google Scholar]
- 28.Ryan, V. T., J. E. Grimwade, J. E. Camarra, E. Crooke, and C. Leonard. 2004. Escherichia coli prereplication complex assembly is regulated by dynamic interplay among Fis, IHF and DnaA. Mol. Microbiol. 51:1347-1359. [DOI] [PubMed] [Google Scholar]
- 29.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 30.Shindo, H., T. Iwaki, R. Ieda, H. Kurumizaka, C. Ueguchi, T. Mizuno, S. Morikawa, H. Nakamura, and H. Kuboniwa. 1995. Solution structure of the DNA binding domain of a nucleoid-associated protein, H-NS, from Escherichia coli. FEBS Lett. 360:125-131. [DOI] [PubMed] [Google Scholar]
- 31.Skarstad, K., B. Thöny, D. S. Hwang, and A. Kornberg. 1993. A novel binding protein of the origin of the Escherichia coli chromosome. J. Biol. Chem. 268:5365-5370. [PubMed] [Google Scholar]
- 32.Skarstad, K., R. Bernander, and E. Boye. 1995. Analysis of DNA replication in vivo by flow cytometry. Methods Enzymol. 262:604-613. [DOI] [PubMed] [Google Scholar]
- 33.Skarstad, K., G. Lueder, R. Lurz, C. Speck, and W. Messer. 2000. The Escherichia coli SeqA protein binds specifically and co-operatively to two sites in hemimethylated and fully methylated oriC. Mol. Microbiol. 36:1319-1326. [DOI] [PubMed] [Google Scholar]
- 34.Slater, S., S. Wold, M. Lu, E. Boye, K. Skarstad, and N. Kleckner. 1995. E. coli SeqA protein binds oriC in two different methyl-modulated reactions appropriate to its roles in DNA replication initiation and origin sequestration. Cell 82:927-936. [DOI] [PubMed] [Google Scholar]
- 35.Speck, C., and W. Messer. 2001. Mechanism of origin unwinding: sequential binding of DnaA to double- and single-stranded DNA. EMBO J. 20:1469-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada, H., T. Yoshida, K. Tanaka, C. Sasakawa, and T. Mizuno. 1991. Molecular analysis of the Escherichia coli hns gene encoding a DNA sequences. Mol. Gen. Genet. 230:332-336. [DOI] [PubMed] [Google Scholar]
- 37.Yamanaka, K., W. Zheng, E. Crooke, Y.-H. Wang, and M. Inouye. 2001. CspD, a novel DNA replication inhibitor induced during the stationary phase in Escherichia coli. Mol. Microbiol. 39:1572-1584. [DOI] [PubMed] [Google Scholar]
- 38.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]