Abstract
This study concerns the cloning, characterization, and expression of the lysin (LysK) from staphylococcal phage K in Lactococcus lactis. Lactococcal lysates containing recombinant LysK were found to inhibit a range of different species of staphylococci isolated from bovine and human infection sources, including methicillin-resistant Staphylococcus aureus. LysK thus has potential as an antimicrobial for applications in the prevention and/or treatment of infections caused by staphylococci.
Staphylococci are a major cause of human and animal diseases and are particularly problematic due to their ability to acquire resistance to commonly used antibiotics (7). Phage lysins have attracted considerable interest as novel antimicrobials against gram-positive bacteria and have been used to control a wide range of pathogens such as group A streptococci (9), Streptococcus pneumoniae (4), Bacillus anthracis (13), and Enterococcus faecalis (15). In the 1950s, a lytic enzyme, “virolysin,” obtained from phage lysates after phage K infection of Staphylococcus aureus strain K1, was reported (12). However, virolysin was only active against dead and not live cells (12). A second lysin, PAL (phage-associated lysin), was also described which lysed dead and live S. aureus cells (14). In addition, phage lytic enzymes from staphylococcal phages Twort (6), 187 (5), phi11 (8), and 80α (1) have previously been described but neither their ability to kill live cells nor therapeutic capabilities have been reported.
In this study, we cloned and heterologously overexpressed the lysin LysK, identified from the genome of phage K, in Lactococcus lactis. Phage K (American Type Culture Collection, 19685-B1) is a polyvalent broad-host-range antistaphylococcal phage. Its genome has been previously sequenced (10), and it has been shown to kill a broad range of newly isolated pathogenic staphylococci, including both human and veterinary strains (11). Initially LysK was cloned and heterologously overexpressed in Escherichia coli (as a His-tagged fusion protein under the control of the T7 promoter); however, recombinant LysK was consistently located in the insoluble fraction as inclusion bodies (data not shown). For this reason, we chose to express the lysin in the gram-positive organism L. lactis NZ9800, using the nisin-inducible expression system (2). In addition to lysing dead staphylococci, a lactococcal lysate containing recombinant LysK inhibited live cultures of a number of pathogenic strains, demonstrating the lytic capabilities of this lysin in controlling staphylococcal numbers.
Sequence analysis, cloning, and overexpression of LysK.
To amplify lysK for cloning and plasmid constructions, cDNA was used as the template as the lysin gene is interrupted by an intron (10). RNA was isolated and cDNA synthesized as described previously (10). Reverse transcription-PCR results demonstrated that the lysK transcript appears between 10 and 20 min after phage infection (data not shown). The lysK gene was amplified from phage K cDNA using the following primers: LysinF (5′ CGGCATGCAGGAGGAAAAAAAAAATGGCTAAGACTCAAGCAGAAATAAATAAAC 3′) and LysinR (5′ GCTCTAGACTATTTGAATACTCCCCAGGC 3′) and cloned into the SphI/XbaI sites (underlined) of the nisin expression vector pNZ8048, generating the plasmid pSOFlysK. This construct was introduced into E. coli XL-1 Blue and following confirmation of the correct sequence subsequently introduced into L. lactis NZ9800, an MG1614 derivative containing the nisRK signal transduction genes integrated on the chromosome. When compared with sequences in the database, LysK was found to contain both a domain from the amidase-2 (N-acetylmuramoyl-l-alanine amidase) family and a CHAP (cysteine, histidine-dependent amidohydrolases/peptidases) domain.
LysK inhibits MRSA strain DPC5645 in zymographic analysis.
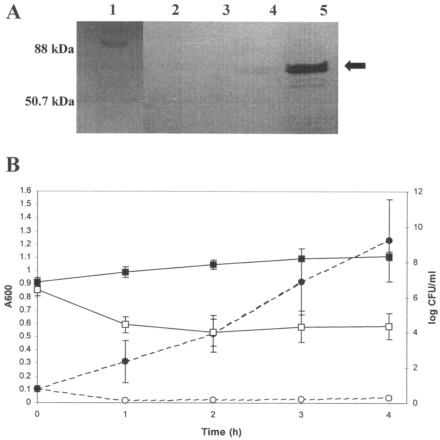
To investigate lysin activity and expression, zymographic analysis was performed as described previously (3) with heat-killed strain DPC5645 (a methicillin-resistant S. aureus [MRSA] strain isolated from an Irish hospital) incorporated in the resolving gel. Mid-log-phase (A600, 0.5) cells of L. lactis NZ9800.(pSOFLysK) and the control L. lactis NZ9800.(pNZ8048) were induced for 4 h with 50 ng of nisin/ml of culture, after which 1.5-ml samples were collected. Following sonication, the samples were subjected to zymographic analysis using polyacrylamide gel electrophoresis, with gels containing autoclaved DPC5645 cells. Upon renaturing, a lytic zone of clearing was evident at 54 kDa in the lane containing pSOFLysK induced with nisin (Fig. 1A, lane 5), corresponding to the predicted molecular mass of LysK, unlike the uninduced control, where the zone was much fainter (Fig. 1A, lane 4), and no lytic zones were evident in the lanes containing the vector control (Fig. 1A, lanes 2 and 3). These results confirmed that recombinant LysK from lactococci is enzymatically active and capable of degrading staphylococcal cell walls.
FIG. 1.
(A) A zymogram which contains autoclaved MRSA (DPC5645) cells. Lane 1, prestained low-range molecular mass marker (Bio-Rad); lane 2, NZ9800.(pNZ8048) minus nisin; lane 3, NZ9800.(pNZ8048) plus nisin; lane 4, NZ9800.(pSOFLysK) minus nisin; lane 5, NZ9800.(pSOFLysK) plus nisin. LysK activity is indicated by a black arrow. (B) Killing of S. aureus DPC5645 with lactococcal lysates containing LysK. Lysates obtained from NZ9800.(pSOFLysK) plus nisin were used as the source for LysK, and lysates obtained from NZ9800.(pNZ8048) plus nisin were used a control. Symbols represent the following: ▪, cell numbers of DPC5645 plus lysate from induced NZ9800.(pNZ8048); □, cell numbers of DPC5645 plus lysate from induced NZ9800.(pSOFLysK); •, OD values of DPC5645 plus lysate from induced NZ9800.(pNZ8048); and ○, OD values of DPC5645 plus lysate from induced NZ9800.(pSOFLysK). Values are the means of three independent experiments with standard deviation indicated by vertical bars.
Lactococcal lysates containing LysK kill a wide range of staphylococci.
To obtain lactococcal lysates containing staphylococcal LysK, mid-log-phase (A600, 0.5) cells of L. lactis NZ9800.(pSOFLysK) and the control strain, L. lactis NZ9800.(pNZ8048), were induced for 4 h with 50 ng of nisin/ml of culture. Cells were washed twice in sterile distilled water, and the final pellet from a 200-ml culture was then resuspended in 5 ml of sterile distilled water. One-milliliter volumes of cells were ribolysed three times for 45 s (setting 4.5 with 2-min intervals on ice; Hybaid, Middlesex, United Kingdom) to obtain crude lysate. Following lysis, samples were centrifuged at 10,000 × g for 10 min at 4°C and supernatants were stored at −20°C.
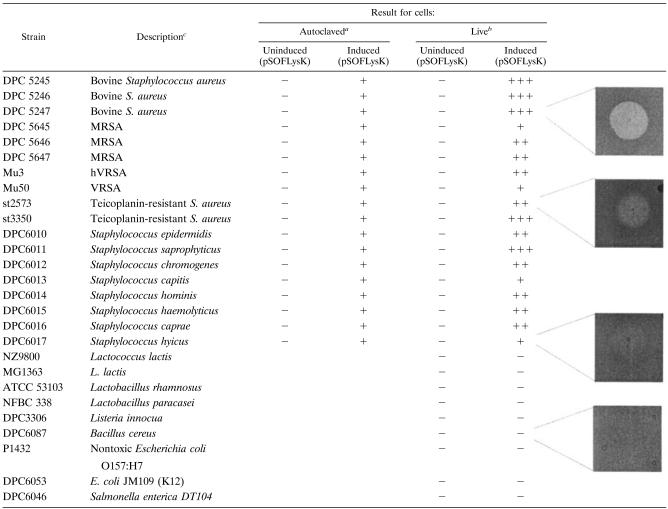
Initially crude LysK activity was assessed for its ability to form lytic zones on autoclaved staphylococci. Bacterial strains used for host range analysis are held in the Dairy Products Research Centre culture collection and are listed in Table 1. An overnight autoclaved 50-ml culture of each staphylococcal strain (Table 1) was centrifuged, and the pellet was added to a 10-ml molten agar (0.7% [wt/vol]) overlay based on brain heart infusion medium. The mixture was poured into two petri dishes to make a “zymogram plate.” After the overlay had solidified, 10-μl aliquots of lysates were applied as spots to the surface and the plates were scored for lytic activity. Both coagulase-positive and -negative staphylococci as well as drug-resistant strains were inhibited by lysin-containing lactococcal extract (Table 1).
TABLE 1.
Lytic spectrum of LysK
Lytic zone (+) or no lytic zone (−) on zymogram plates.
Strong (+++), medium (++), or weak (+) lytic zones as indicated by the pictures on the right. − no lytic zone.
hVRSA, heterogeneous vancomycin-resistant S. aureus. VRSA, vancomycin-resistant S. aureus.
Subsequently, lactococcal lysates containing LysK were assessed for their ability to form a clearing on live staphylococcal strains (Table 1). In addition, strains belonging to other genera (Table 1) were tested for sensitivity to crude LysK. Lysates from untreated L. lactis NZ9800.(pSOFlysK) and induced, untreated L. lactis NZ9800.(pNZ8048) cells were used as controls. Lytic activity was scored by the level of clearing of the zone after overnight incubation at 37°C. In addition to lysing dead staphylococcal cells, lactococcal lysates were active against a wide variety of live staphylococci, including bovine mastitis strains, MRSA strains from Irish hospitals, heterogeneous vancomycin-resistant and vancomycin-resistant S. aureus strains, and also teicoplanin-resistant strains (Table 1). A variation in lytic capabilities was evident against these staphylococcal strains. The lysin-containing lactococcal extract was incapable of lysing other gram-positive bacteria, such as Listeria innocua, Bacillus cereus, Lactobacillus rhamnosus, and Lactobacillus paracasei.
The effect of crude LysK from induced (as described above) L. lactis NZ9800.(pSOFlysK) was tested against an exponentially growing S. aureus strain, DPC5645. Crude lysates from the induced L. lactis NZ9800.(pNZ8048) strain were included as a negative control. S. aureus strain DPC5645 (3 ml) was grown to an optical density (OD) of approximately 0.1 at 600 nm, when 500 μl of the lactococcal extract containing LysK was added. In kill curves using a human MRSA strain (DPC5645), a 99% reduction in staphylococcal cell numbers was observed 1 h after the addition of lysates containing LysK (Fig. 1B), demonstrating that recombinant LysK is capable of killing live pathogenic staphylococci.
In summary, while a number of studies have characterized staphylococcal lysins (6), to our knowledge none has been reported to have a broad spectrum of activity across the genus against live cells. In the present study, a genetically modified lactic acid bacterium overexpressing LysK was constructed. Expression in L. lactis yielded an active protein with an apparent molecular mass of 54 kDa, which corresponds to the predicted molecular mass of LysK. Lysates containing LysK killed a wide range of staphylococci, including problematic strains such as MRSA and pathogenic S. aureus strains associated with bovine mastitis. A difference in lytic ability was observed with different staphylococcal strains, possibly reflecting differences in the cell wall composition between strains. However, other gram-positive bacteria from different genera including beneficial probiotic strains were not affected by lysates containing LysK, suggesting LysK is specific to the genus Staphylococcus. This specificity of LysK is potentially advantageous for prophylactic and/or therapeutic purposes. In conclusion, the recombinant protein retains the broad spectrum within the Staphylococcus genus of the phage itself, suggesting that it could have widespread applications as a therapeutic agent for infections associated with staphylococci.
Acknowledgments
This research was funded by the Irish Government under the FIRM Programme as part of the National Development Plan 2000-2006, by EU structural funds and the Science Foundation of Ireland. Sarah O'Flaherty is in receipt of a Teagasc Walsh Fellowship.
REFERENCES
- 1.Bon, J., N. Mani, and R. K. Jayaswal. 1997. Molecular analysis of lytic genes of bacteriophage 80 alpha of Staphylococcus aureus. Can. J. Microbiol. 43:612-616. [DOI] [PubMed] [Google Scholar]
- 2.de Ruyter, P. G. G. A., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickey, R. M., D. P. Twomey, R. P. Ross, and C. Hill. 2003. Production of enterolysin A by a raw milk enterococcal isolate exhibiting multiple virulence factors. Microbiology 149:655-664. [DOI] [PubMed] [Google Scholar]
- 4.Loeffler, J. M., and V. A. Fischetti. 2003. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 47:375-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loessner, M. J., S. Gaeng, and S. Scherer. 1999. Evidence for a holin-like protein gene fully embedded out of frame in the endolysin gene of Staphylococcus aureus bacteriophage 187. J. Bacteriol. 181:4452-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loessner, M. J., S. Gaeng, G. Wendlinger, S. K. Maier, and S. Scherer. 1998. The two-component lysis system of Staphylococcus aureus bacteriophage Twort: a large TTG-start holin and an associated amidase endolysin. FEMS Microbiol. Lett. 162:265-274. [DOI] [PubMed] [Google Scholar]
- 7.Lowy, F. D. 2003. Antimicrobial resistance: the example of Staphylococcus aureus. J. Clin. Investig. 111:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarre, W. W., H. Ton-That, K. F. Faull, and O. Schneewind. 1999. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage phi11. Identification of a D-alanyl-glycine endopeptidase activity. J. Biol. Chem. 274:15847-15856. [DOI] [PubMed] [Google Scholar]
- 9.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Flaherty, S., A. Coffey, R. Edwards, W. Meaney, G. F. Fitzgerald, and R. P. Ross. 2004. Genome of staphylococcal phage K: a new lineage of Myoviridae infecting gram-positive bacteria with a low G+C content. J. Bacteriol. 186:2862-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Flaherty, S., R. P. Ross, W. Meaney, G. F. Fitzgerald, M. F. Elbreki, and A. Coffey. 2005. Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl. Environ. Microbiol. 71:1836-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ralston, D. J., B. S. Baer, M. Lieberman, and A. P. Krueger. 1955. Virolysin: a virus-induced lysin from staphylococcal phage lysates. Proc. Soc. Exp. Biol. Med. 89:502-507. [DOI] [PubMed] [Google Scholar]
- 13.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 14.Sonstein, S. A., J. M. Hammel, and A. Bondi. 1971. Staphylococcal bacteriophage-associated lysin: a lytic agent active against Staphylococcus aureus. J. Bacteriol. 107:499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoong, P., R. Schuch, D. Nelson, and V. A. Fischetti. 2004. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J. Bacteriol. 186:4808-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]