Abstract
Type III machines of pathogenic Yersinia spp. transport Yop proteins across the bacterial envelope into host cells. Translational fusions of yopE to the dihydrofolate reductase gene (dhfr) or the β-galactosidase gene (lacZ) generate hybrid proteins that block type III injection of Yop proteins into host cells, consistent with the canonical view that impassable DHFR and LacZ hybrids jam secretion machines. Mutations in repressors of posttranscriptional gene regulation, Yersinia enterocolitica yscM1 and yscM2 as well as Yersinia pestis lcrQ, relieve the YopE-DHFR-imposed blockade and restore type III injection into host cells. Genetic suppression of the type III blockade does not, however, promote YopE-DHFR secretion. A model is proposed whereby rejection of YopE-DHFR from the secretion pathway inhibits type III gene expression.
Signal peptide-bearing precursor proteins are substrates for protein secretion across the plasma membrane (10, 53). Hydrophobic signal peptides, the recognition elements of secretion catalysts, are both necessary and sufficient to trigger membrane translocation of fused reporter proteins that under physiological conditions cannot travel the secretion pathway (6). However, fusions of signal peptides to proteins that fold rapidly generate impassable hybrids, as the narrow channels of membrane translocation machines can typically accommodate only unfolded protein substrates (58). By default, the fate of all signal peptide-bearing precursors is initiation into the secretion machinery, and impassable hybrids therefore jam the pathway, blocking transport of all other substrates (58). Examples of impassable reporter proteins are dihydrofolate reductase (DHFR), β-galactosidase (LacZ), and ubiquitin, each of which has been shown to block bacterial or eukaryotic secretion machines when fused to transport substrates (29, 37, 58).
Type III secretion of gram-negative pathogens is unique in that the type III machinery transports polypeptides through a narrow needle complex spanning 50 to 100 nm while crossing the bacterial double-membrane envelope and the plasma membrane of host target cells (54, 67). Unlike secretion machines for signal peptide-bearing precursors, the type III pathway fulfills accessory roles and is not needed for bacterial growth under laboratory conditions (7, 51). However, as bacteria encounter immune cells during infection, type III secretion becomes essential in preventing phagocytic killing of the invading microbes (50, 65, 66). The expression of genes encoding type III machines and their substrates is regulated in all bacterial species, and there is increasing evidence not only that each microbial species employs unique sets of genes encoding type III effector proteins for host cell injection but also that the order in which these polypeptides are transported is developmentally determined (1, 32, 39, 59).
Pathogenic yersiniae, Yersinia enterocolitica, Y. pseudotuberculosis, and Y. pestis, depend on the type III secretion system encoded by their respective 70-kb virulence plasmids to transport Yops during host infection (23). When these bacteria are incubated with cultured tissue cells, several type III substrates (effectors) are injected into the eukaryotic cytosol (YopE, YopH, YopM, YopN, YopO, YopP, YopT, YscM1, and YscM2) (22). Moreover, four proteins are also secreted by the type III pathway into the extracellular medium (LcrV, YopB, YopD, and YopR) (42, 44). Yersinia can be induced for type III secretion even without host target cells, as depletion of calcium ions at elevated temperatures causes secretion of Yops into the culture medium (52). Massive induction of type III secretion is associated with a reduction in growth (41). This “low-calcium response” (LCR) phenomenon has been exploited for the identification of genes involved in promoting or regulating type III secretion (33). Because the concentration of free calcium ions in the eukaryotic cytosol is well below the threshold for the in vitro induction of Yop secretion, the low-calcium signal is thought to serve as a physiological inducer for the type III pathway during infection (43).
Fusions of ubiquitin to YopE or YopQ generated hybrids that cannot be transported by the type III pathway (45). This phenotype is caused by reporter assembly into its three-dimensional structure, as mutations that destabilize the ubiquitin fold generate hybrid polypeptides capable of traveling through the needle complex (45). Fusions of dihydrofolate reductase to YopE or YopQ are also not transported by the type III machinery. Moreover, a fusion of full-length YopE to DHFR blocked secretion of Yops, consistent with the canonical view that DHFR hybrids jam the type III secretion machinery (30).
We show here that YopE-DHFR and YopE-LacZ block the Yersinia type III pathway as well as the injection of Yops into host cells. The type III blockade of YopE-DHFR appears to be caused by the reduced expression of yop genes, not by the inhibition of secretion. Consistent with this view, YopE-DHFR blocked the LCR phenotype of Y. pestis and mutations in genes (yopD, lcrH, yscM1 [lcrQ], and yscM2) encoding proteins that function as posttranscriptional repressors of the type III pathway restored Yop secretion. Furthermore, mutations in Y. enterocolitica yscM1 and yscM2 also restored type III injection of host cells even in the presence of YopE-DHFR. Taken together with the observation that YopE-DHFR is not successfully initiated into the secretion pathway, these data suggest a model whereby rejection of YopE-DHFR from secretion machines can inhibit type III gene expression.
MATERIALS AND METHODS
Bacterial strains.
Overnight cultures of Y. pestis KIM D27 and KIM8 (13, 74) and Y. enterocolitica W22703 (24) were grown in heart infusion broth or tryptic soy broth (TSB), respectively, and media were supplemented with 35 μg/ml kanamycin or 30 μg/ml chloramphenicol for plasmid retention. Y. enterocolitica W22703 VTL1 (ΔyopN), MC2 ΔlcrG), VTL2 (ΔyopD), CT133 (ΔlcrH), and EC2 [Δ(yscM1 yscM2)] have been described previously (3, 16, 44, 46). Escherichia coli DH5α (35) was grown in Luria broth in the presence of either 35 μg/ml kanamycin or 20 μg/ml chloramphenicol. Y. pestis MEL2 (ΔlcrQ) was constructed by introducing a stop codon followed by a +1 frameshift mutation after codon 6 in Y. pestis KIM D27 using allelic exchange as described previously (18). Briefly, the suicide pLC28 vector was digested with SalI and PstI. Two fragments of lcrQ were amplified using primers lcrQKO-SalI (5′-AAGTCGACCAATGGAACCCCAACCTCAGGC-3′) and lcrQKO-Eco1 (5′-AAGAATTCTTCAAAGAGTATTGATTTTCATCGAG-3′) as well as lcrQKO-Eco2 (5′-AAGAATTCCAATCGTTAATAAATCAACAAATTACCC-3′) and lcrQKO-PstI (5′-AACTGCAGCGAAAGTTAAGCTTCAGTAAGCG-3′). The resulting fragments were cloned into pCR2.1 (Invitrogen) and then subcloned into pLC28 (18) using a three-way ligation to yield pMM54. pMM54 was transformed directly into Y. pestis KIM D27 and selected on blood base agar (BBA) containing chloramphenicol. The resulting chloramphenicol-resistant colonies were cured of the insert and drug resistance marker by streaking on BBA containing 5% sucrose. Colonies were screened by PCR using primers lcrQKO-SalI and lcrQKO-PstI to amplify lcrQ. The PCR products were digested with EcoRI to screen for the introduction of the EcoRI restriction site.
Molecular biology experiments.
The plasmid pBBR1MCS-2 (38) was used to clone the yopE-dhfr and yopE-lacZ fusions. In order to facilitate cloning, two restriction sites were eliminated (XbaI and BglII). Plasmid pBBR1MCS-2 was digested with BglII, purified, end filled with the Klenow enzyme (New England Biolabs), ligated, and transformed into E. coli DH5α. Plasmid DNA was purified from the resulting colonies and analyzed for the loss of the BglII restriction site by digestion with BglII (pJS91). Elimination of the XbaI restriction site was performed in the same manner using pJS91 to yield pJS92. The yopE-dhfr fusion was cloned from the plasmid pMAF35 (30) into pJS92 by restriction digestion (EcoRI-HindIII) and ligation to yield pJS93. The β-galactosidase gene was PCR amplified from pDA14 (pyopE-lacZ) using the forward primer 5′LacZ-BglII (5′-AAAGATCTATGACTATGATTACGGATTCT-3′) and the reverse primer 3′LacZ-XbaI (5′-AATCTAGATTATTTTTGACACCAGACCAA-3′). The PCR product was digested with BglII and XbaI and cloned into pJS93 precut with BglII and XbaI to yield pJS94. The tac promoter and lacIq gene were cloned into pJS93 from pDA38 (5) using EcoRI-NdeI restriction digestion and ligation to yield pJS95.
Type III secretion assay.
Overnight cultures of Y. pestis KIM8 were diluted 1:50 into fresh M9-Casamino Acids medium (M9-Ca) (42 mM Na2HPO4, 22 mM KH2PO4, 8.6 mM NaCl, 18.6 mM NH4Cl, 0.01 mg/ml FeSO4, 0.001% thiamine, 1 mM MgSO4, 0.4% glucose, and 1% Casamino Acids) containing an antibiotic for plasmid retention. Overnight cultures of Y. enterocolitica W22703 pVL20 (pyopE-dhfr; low copy number) were diluted 1:50 into TSB as described previously (45). Overnight cultures of Y. enterocolitica W22703(pJS93) were diluted 1:50 into brain heart infusion broth (BHI) containing 4 mg/ml glucose. To chelate calcium from BHI and TSB, 20 mM oxalic acid and 20 mM magnesium chloride were added to the media as a supplement. In some cases, 5 mM CaCl2 was added to the media to block type III secretion. Cultures were grown for 2 h at 26°C and then at 37°C for 3 h to induce type III secretion. A 1.4-ml aliquot of culture was removed and centrifuged at 16,000 × g to sediment the bacteria. Seventy-five microliters of 100% trichloroacetic acid (TCA) was added to 1 ml of culture supernatant. Seven hundred microliters of water was added to the bacterial pellet, and 500 μl of the bacterial suspension was precipitated with 500 μl 10% TCA. Precipitated samples were acetone washed, dried, and solubilized in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Protein samples were separated by 15% SDS-PAGE and stained with Coomassie brilliant blue or electrotransferred to polyvinylidene difluoride (PVDF) membranes. Following electrotransfer, proteins were immunoblotted using specific antisera raised against individual Yop proteins and neomycin phosphotransferase (NPT). Horseradish peroxidase-conjugated anti-rabbit secondary antibody and chemiluminescence were used to visualize the samples. Images were quantified using a Fluorchem 8800 imaging system (Alpha Innotech).
Tissue culture infections.
HeLa cells (ATCC CCL-2) were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine at 37°C and 5% CO2. Two hours prior to infection, the overnight yersinia culture was diluted 1:20 in fresh heart infusion broth (Y. pestis) or fresh TSB (Y. enterocolitica) with an antibiotic in two separate tubes. Ten minutes prior to infection, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 1 mM in one of the two tubes to induce expression of yopE-dhfr. One hour prior to infection, 80 to 90% confluent HeLa cell monolayers were washed twice with phosphate-buffered saline (PBS) and 10 ml DMEM without FBS/glutamine was added. HeLa cells were infected with Y. pestis KIM8 or Y. enterocolitica W22703 transformed with pJS95 at a multiplicity of infection of 10 in the presence of the antibiotic and 1 mM IPTG where indicated in Fig. 4 and 9. After 3 h, the medium fraction was decanted and centrifuged at 10,000 × g for 15 min. Protein in 7 ml of media supernatant was precipitated with methanol-chloroform, and the remaining medium sample was discarded. Ten milliliters of PBS containing 1% SDS was added to the medium pellet, and protein in 7 ml of this sample was precipitated with methanol-chloroform. To the adherent tissue culture cells and their attached bacteria, 1% digitonin in PBS was added to disperse the host cell plasma membrane followed by incubation on a rotary shaker for 20 min (D). Cell remnants and bacteria were detached from the flasks using a cell scraper, and samples were centrifuged and protein was precipitated as described above. Samples were separated by 15% SDS-PAGE and electrotransferred to PVDF membranes. Following electrotransfer, proteins were immunoblotted using specific antisera raised against individual Yop proteins, NPT, chloramphenicol acetyltransferase, and IκB. Horseradish peroxidase-conjugated anti-rabbit secondary antibody and chemiluminescence were used to visualize the samples.
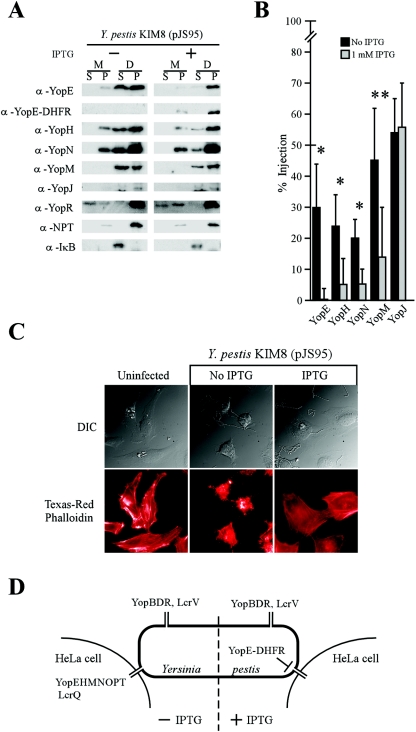
FIG. 4.
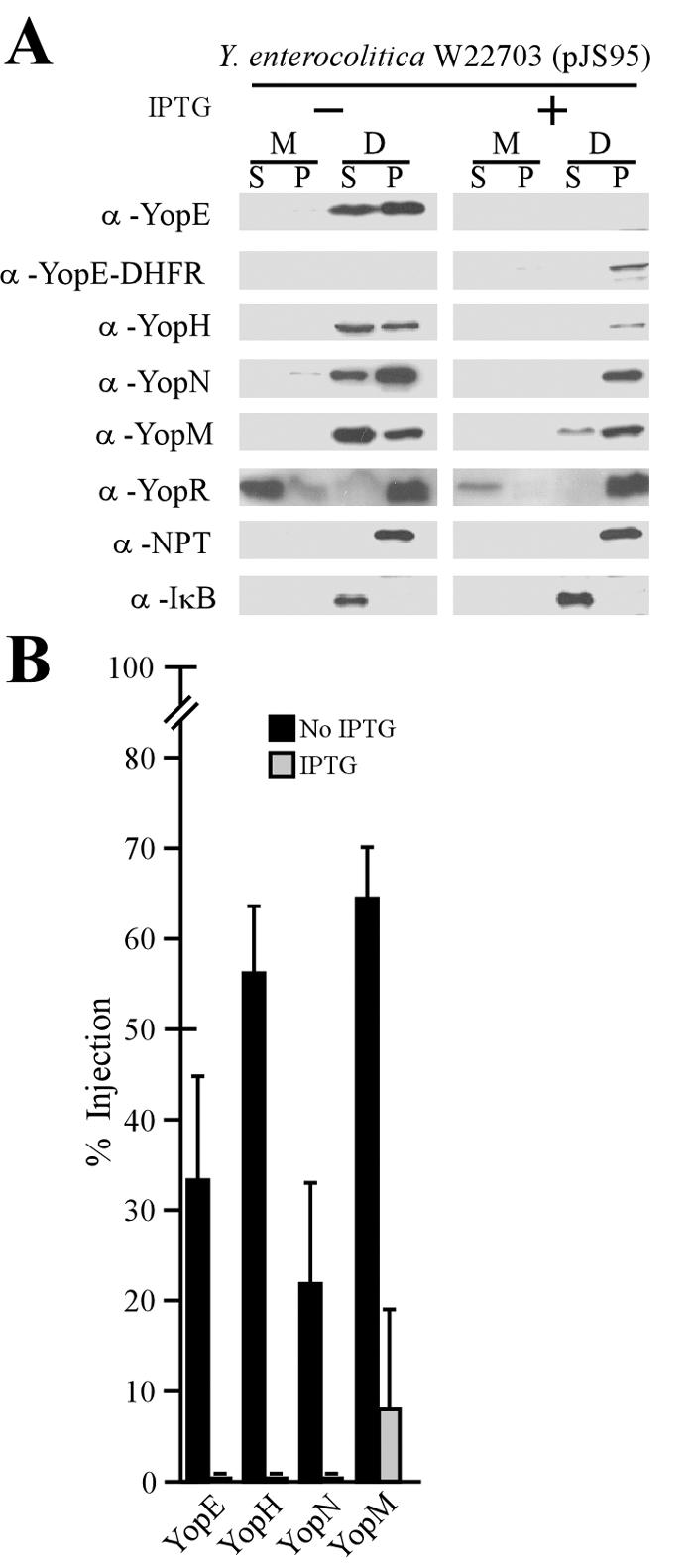
YopE-DHFR blocks Y. pestis type III injection into HeLa cells. (A) Y. pestis strain KIM8(pJS95) was inoculated into HeLa cell monolayers for 3 h at 37°C. Infected tissue cultures were fractionated as described in Materials and Methods, and protein localization was analyzed by immunoblotting with specific antisera. M, medium fraction; D, digitonin fraction; S, supernatant fraction; P, pellet fraction; α, anti. (B) Data in panel A were analyzed to determine the percent amount of injected polypeptide. The bars indicate the means of three independent experiments. The error bars indicate 1 standard deviation of the mean. Significance was calculated using Student's t test (*, P < 0.01; **, P < 0.05). (C) Y. pestis cytotoxicity for HeLa cells was measured by staining F-actin with Texas red-conjugated phalloidin. Differential interference contrast (DIC) images were captured at a magnification of ×600, and fluorescence was measured at 608-nm emission. Samples were compared with a slide that received no bacteria (uninfected). (D) Model for YopE-DHFR-mediated block of type III injection.
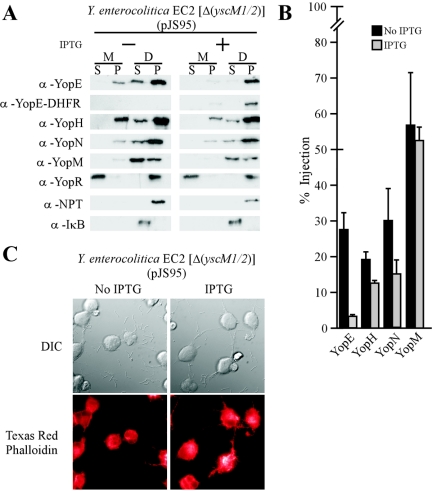
FIG. 9.
YopE-DHFR cannot block type III injection of class II mutant Y. enterocolitica. (A) Strain EC2 [Δ(yscM1 yscM2)] carrying pJS93 was analyzed for type III injection into HeLa cells as described in the legend to Fig. 4. α, anti. (B) Data in panel A were analyzed to determine the percent amount of injected polypeptide. (C) Y. enterocolitica cytotoxicity for HeLa cells was measured by fluorescence microscopy and Texas red-phalloidin staining.
Cytotoxicity assay.
HeLa tissue cultures, 4 × 105 cells, were grown on glass coverslips in a 12-well tissue culture dish and infected as described above. After 3 h of infection, media were removed and the cells were fixed with 3.7% formaldehyde in PBS for 20 min on a rotary shaker. Fixation was quenched with 0.1 M glycine in PBS for 5 min. Cells were permeabilized with 0.1% Triton X-100 in PBS for 30 min and then blocked for 15 min in PBS containing 5% skim milk and 0.05% Tween 20. Filamentous actin was labeled with 165 nM Texas red-conjugated phalloidin in PBS containing 5% skim milk and 0.05% Tween 20 for 20 min. The labeling solution was removed, each well was washed four times with 1 ml of PBS and buffer aspirated, and samples were dried for 1 h. Coverslips were affixed to glass slides and visualized with an Olympus AX RL module microscope. Texas red-phalloidin visualization was achieved through excitation (591 nm) and emission (608 nm) with a U-MNG cube. Images were captured with a Hamamatsu Ocra digital camera.
RNA isolation and RT-PCR analysis.
Total RNA from Y. enterocolitica W22703(pJS95), uninduced or IPTG induced for type III secretion as described above, was isolated using an RNeasy minikit (QIAGEN) by following the manufacturer's instructions. DNA was removed by incubating with TURBO DNase (Ambion), and RNA was repurified by phenol extraction and ethanol precipitation. For reverse transcriptase PCR (RT-PCR) analysis, 300 ng of total RNA was used to synthesize cDNA of yopB, lcrV, and rpoA using the primers 3′YopB (5′-ATGGGGTCTGCCGGCCAAATTA-3′), 3′LcrV (5′-TCATATTTCTGAATGAAACGGTTCAGTGCTTCA-3′), and 3′RpoA (5′-TCTAGGCGCATGCCTAAAGAAAGACCA-3′). Primers were annealed to RNA by incubation at 65°C for 5 min and then placed on ice for 5 min. Cloned avian myeloblastosis virus reverse transcriptase (Invitrogen) was added together with 0.5 mM deoxynucleoside triphosphates and incubated at 42°C for 60 min. The reaction was stopped by incubation at 95°C for 5 min, and then the reaction mixture was placed on ice. The resulting cDNA was used as a template for PCR amplification using primer sets 5′YopB (5′-GCATCAAAGATTTTTGGCTGGCTCA-3′) and 3′YopB, 5′LcrV (5′-ATGATTAGAGCCTACGAACAAAACCCACAAC-3′) and 3′LcrV, and 5′RpoA (5′-ATGCAGGGTTCTGTGACAGAGTTTCT-3′) and 3′RpoA. The resulting PCR product was separated by electrophoresis on 0.8% agarose gel.
Pulse-labeling of Y. enterocolitica and immunoprecipitation.
Overnight cultures of Y. enterocolitica W22703(pJS95) and EC2(pJS95) [Δ(yscM1 yscM2)] were grown in M9-Ca medium supplemented with an antibiotic for plasmid retention. Cultures were diluted into two separate tubes that contained M9-Ca lacking methionine and cysteine (18) and grown for 2 h at 26°C. IPTG was added to one of the tubes to a concentration of 1 mM. An 800-μl aliquot of each culture was labeled with 8 μCi of Pro-Mix for 2 min at 37°C. Three 250-μl aliquots were precipitated with 250 μl ice-cold 10% TCA and then acetone washed. Precipitated samples were suspended in SDS-sample buffer and immunoprecipitated with specific YopB, LcrV, or RpoA antibodies. Immunoprecipitated samples were solubilized in SDS-sample buffer, separated by 15% SDS-PAGE, and quantified by PhosphorImager.
RESULTS
YopE-DHFR and YopE-LacZ block Yersinia type III secretion
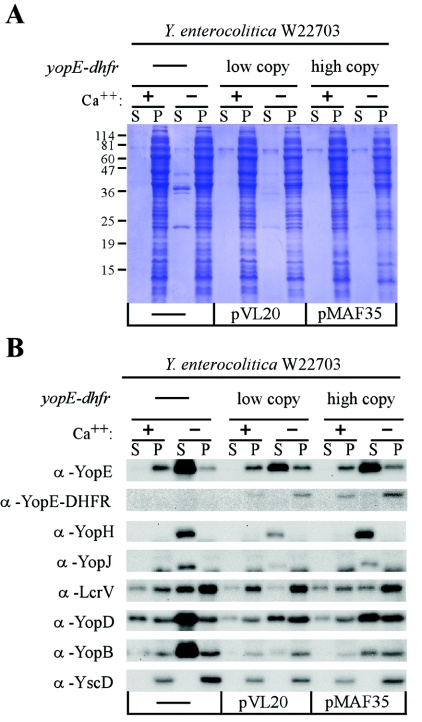
We sought to determine whether expression of yopE-dhfr from plasmids produces distinct type III secretion phenotypes in yersiniae. Previous work showed that expression of yopE-dhfr from a low-copy-number plasmid (pVL20, a pSC101 derivative with one to five copies) in Y. enterocolitica strain W22703 did not block the secretion of YopR, as measured by immunoblotting (45). In contrast, expression of yopE-dhfr from a medium-copy-number plasmid (pMAF35, a pBBR1MCS-2 derivative with 20 copies) reduced Yop secretion into the culture medium, as measured by Coomassie-stained SDS-polyacrylamide gels (30). Plasmids pVL20 and pMAF35 were transformed into Y. enterocolitica W22703 (24). Transformants were grown in broth culture at 37°C in the presence or absence of calcium ions, which serve as a gratuitous inducer of type III secretion (52). Cultures were centrifuged, supernatants were separated from bacterial pellets, and proteins in both fractions were precipitated with TCA and then separated by SDS-PAGE and stained with Coomassie brilliant blue (Fig. 1). Y. enterocolitica harboring no plasmid secreted Yop proteins into the extracellular medium, as evidenced by the abundant appearance of Yop proteins in supernatant samples (Fig. 1A). Only small amounts of Yop proteins were detected in the culture medium of yersiniae harboring pVL20 or MAF35, indicating that temperature-induced expression of yopE-dhfr on low- and medium-copy-number plasmids both reduced secretion and produced indistinguishable type III phenotypes (Fig. 1A).
FIG. 1.
Effect of low-copy-number (pVL20) and medium-copy-number (pMAF35) plasmids carrying yopE-dhfr on type III secretion of Y. enterocolitica W22703. Type III secretion was measured by separating the medium supernatant (S) and the bacterial pellet (P) of centrifuged cultures that had been induced by temperature shift and low calcium (+ or −). (A) Proteins in both fractions were separated by SDS-PAGE and stained with Coomassie brilliant blue. The positions of molecular weight markers are indicated. (B) Proteins were also electroblotted onto a PVDF membrane and detected with specific antisera. α, anti.
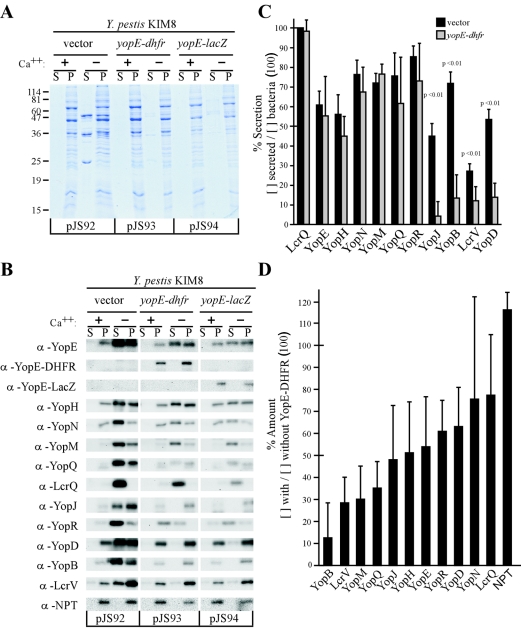
Y. pestis strain KIM8 is a nonpigmented variant with a 100-kb chromosomal deletion of the high-pathogenicity island that also lacks pPCP1, the 7.5-kb virulence plasmid encoding the surface protease Pla (13, 14, 25). Strain KIM8 harbors the pCD1 virulence plasmid, however, and promotes type III secretion in a manner that is indistinguishable from that of Y. pestis wild-type strain KIM (71). yopE-dhfr and yopE-lacZ, a yopE fusion to the E. coli β-galactosidase gene, were cloned into pJS92, a variant of pBBR1MCS-2 (38), to generate pJS93 and pJS94, respectively. Y. pestis strain KIM8 was transformed with pJS92, pJS93, and pJS94, and type III secretion was examined as described above for Y. enterocolitica. Depletion of calcium from culture media induced Yop secretion of Y. pestis KIM8 carrying the vector control plasmid pJS92 (Fig. 2A). In contrast, expression of yopE-dhfr (pJS93) or yopE-lacZ (pJS94) blocked Y. pestis type III secretion as only very small amounts of Yop proteins could be detected by Coomassie staining and SDS-PAGE (Fig. 2A).
FIG. 2.
Plasmids carrying yopE-dhfr (pJS93) or yopE-lacZ (pJS94), but not the vector control (pJS92), block type III secretion of Y. pestis KIM8. (A) Coomassie brilliant blue-stained, SDS-PAGE-separated proteins in the Yersinia medium supernatant (S) and the bacterial pellet (P). (B) Immunoblot analysis of protein localization in the medium supernatant and the bacterial pellet. α, anti. (C) Quantification of immunoblot analysis in panel B for the percent amount of secreted protein and comparison between bacteria harboring pJS92 (vector control) and pJS93 (yopE-dhfr). Bars represent the means of three independent experiments. The error bars represent the standard errors of the means. Significance was determined using Student's t test. (D) Total amounts of Yersinia proteins were determined by immunoblotting of proteins from whole-culture samples and compared between bacteria expressing YopE-DHFR (pJS93) and the vector control (pJS92).
Type III substrate recognition in Yersinia expressing yopE-dhfr.
We wondered whether expression of yopE-dhfr fusions impacted substrate recognition and transport by the secretion machinery. In previous studies, substrate recognition was analyzed by comparing the amount of secreted protein to the total amount of protein in the supernatant ([S]) and pellet ([P]) according to the following equation: percent amount of substrate recognized or secreted polypeptide = {[S]/([S] + [P])} × 100% (18). Yersinia cultures induced for type III secretion were centrifuged, and TCA-precipitated proteins in supernatant and pellet samples were analyzed by immunoblotting with specific antisera (Fig. 1B and 2B). Although YopE-DHFR and YopE-LacZ were not secreted by Y. pestis strain KIM8, expression of these hybrids did not abrogate substrate recognition and transport of several Yop proteins (Fig. 2B). In three independent experiments, secretion, i.e., substrate recognition and transport, in yopE-dhfr-expressing Y. pestis was not affected for YopE, YopH, YopM, YopN, YopQ, YopR, and LcrQ (Fig. 2C). However, significant defects in substrate recognition or transport were detected for LcrV, YopB, YopD, and YopJ (Fig. 2C).
Our experimental design cannot distinguish between defects in substrate recognition, for example, rejection of proteins from entering the type III secretion pathway, and defects in transport, for example, obstruction of the pathway by proteins that are too bulky for the narrow needle complex. yopE-dhfr expression hindered the secretion of only four proteins, and it would therefore seem unlikely that the YopE-DHFR hybrid would obstruct the needle complex, as this would be expected to precipitate a general blockade in type III secretion. Secretion, i.e., the sum of substrate recognition and transport events, was also measured in Y. enterocolitica (Fig. 1B). Expression of yopE-dhfr did not affect substrate recognition and transport of YopE and YopH; however, that of LcrV, YopB, YopD, and YopJ was reduced (Fig. 1B). Thus, yopE-dhfr expression seems to exert similar effects on substrate secretion in different Yersinia species. Importantly, yopE-dhfr expression also did not impose a general block of the Y. enterocolitica type III machinery. When cells were induced in the absence of calcium, the overall abundance of YopE-DHFR was 18% of that of YopE (data not shown).
YopE-DHFR reduces the expression of yop genes.
Close inspection of immunoblots in Fig. 1 and 2 revealed that the overall amount of Yop proteins was reduced in yersiniae expressing yopE-dhfr. To substantiate this hypothesis, we measured the total amount of Yop protein produced in Yersinia cultures by immunoblotting of TCA-precipitated samples. Expression of yopE-dhfr only slightly reduced or left unaffected the production of YopN and LcrQ. However, YopE-DHFR significantly reduced the immune-reactive signals of YopB, YopM, YopQ, YopJ, YopH, YopR, YopD, and LcrV, further corroborating the notion that the hybrid reduced the total amount of a subset of type III substrates (Fig. 2D).
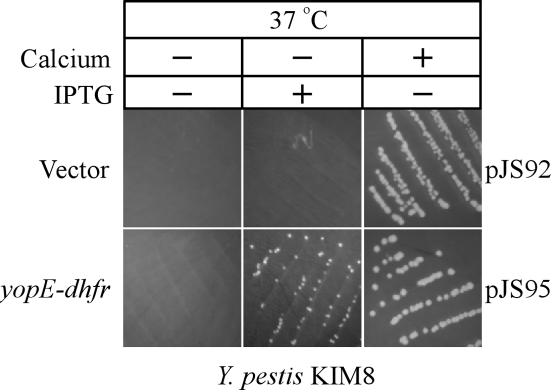
If YopE-DHFR reduced the expression of type III genes, one would expect that the hybrid protein may also affect the LCR of Y. pestis. Removal of calcium ions from agar medium prevents growth and colony formation of Y. pestis (41), and it is now appreciated that massive transport of Yop proteins via the type III pathway hinders bacterial growth under these conditions (52). To test whether expression of yopE-dhfr blocks the LCR, Y. pestis strains carrying pJS92 or pJS95, a plasmid expressing yopE-dhfr from the IPTG-inducible tac promoter (2), were streaked on agar media lacking calcium (Fig. 3). In the presence of calcium, Y. pestis KIM8(pJS92) and Y. pestis KIM8(pJS95) both formed colonies. However, when streaked on agar medium lacking calcium, neither of the two strains multiplied to form colonies. This result indicates that strain KIM8 is subject to LCR suppression of bacterial growth irrespective of the presence or absence of the plasmid. When streaked on calcium-depleted agar medium containing IPTG, Y. pestis KIM8(pJS95), but not Y. pestis KIM8(pJS92), formed colonies. Thus, IPTG-induced expression of yopE-dhfr blocks the LCR of Y. pestis.
FIG. 3.
YopE-DHFR blocks the Y. pestis LCR. Y. pestis strain KIM8 was transformed with a vector control plasmid or with the same vector expressing yopE-dhfr from the IPTG-inducible tac promoter (pJS95). Strains were streaked on HIA plates and incubated at 37°C with or without calcium, the latter of which is an inducer of the type III pathway, and with or without IPTG, an inducer of the tac promoter.
YopE-DHFR hinders type III injection of Yop proteins.
To test whether YopE-DHFR blocks bacterial type III injection, HeLa tissue culture cells were inoculated with Y. pestis strain KIM8(pJS95) and incubated for 3 hours. The culture medium was decanted and centrifuged to separate extracellular medium in the supernatant from nonadherent bacteria in the pellet. Tissue cultures were then extracted with digitonin, a detergent that disrupts the cholesterol-containing plasma membrane of HeLa cells but not the bacterial envelope (42). Digitonin extracts were centrifuged to separate cytosolic contents in the supernatant from bacterial sediment and cellular organelles and cytosolic debris in the pellet. After precipitation with chloroform-methanol, proteins were separated by SDS-PAGE and analyzed by immunoblotting with specific antisera. As expected, yersiniae that did not express yopE-dhfr injected YopE, YopH, YopJ, YopM, and YopN into HeLa cells, and YopR was found secreted into the extracellular medium (Fig. 4A). As a control for proper fractionation, digitonin extraction solubilized IκB from the HeLa cell cytosol but not NPT from the bacterial cytoplasm. In contrast, after addition of the IPTG inducer, Y. pestis synthesized YopE-DHFR, which abolished all type III injection of YopE and significantly reduced the injection of YopH, YopM, and YopN (Fig. 4A). However, neither the type III injection of YopJ nor the secretion of YopR was significantly reduced (Fig. 4A and B).
If YopE-DHFR mediated at least a partial blockade of the type III pathway, as suggested by data in Fig. 4A and B, we would expect to observe defects in Yersinia type III pathway-mediated HeLa cell cytotoxicity (65). To test this prediction, Yersinia-infected tissue culture cells were stained with Texas red-phalloidin and viewed by fluorescence microscopy. Y. pestis strain KIM8 infection of HeLa cultures caused rounding of cells and redistribution of actin filaments (Fig. 4C). In contrast, the YopE-DHFR-mediated blockade of the type III pathway abolished Yop protein cytotoxicity, consistent with the hypothesis that the hybrid blocked Y. pestis injection of Yops (Fig. 4D). To examine whether the YopE-DHFR blockade occurred also in Y. enterocolitica, HeLa tissue culture cells were inoculated with strain W22703(pJS95). Without IPTG induction of yopE-dhfr, Y. enterocolitica injected YopE, YopH, YopM, and YopN, whereas YopR was secreted into the extracellular medium (Fig. 5A). IPTG-induced expression of yopE-dhfr led to the synthesis of YopE-DHFR, which was not transported by the type III machinery but remained in the pellet fraction (Fig. 5A). YopE-DHFR not only blocked all Y. enterocolitica type III injection of YopE, YopH, and YopN but also reduced the injection of YopM and secretion of YopR (Fig. 5A and B).
FIG. 5.
YopE-DHFR blocks Y. enterocolitica type III injection into HeLa cells. (A) Y. enterocolitica strain W22703(pJS95) was inoculated into HeLa cell monolayers for 3 h at 37°C and subjected to digitonin fractionation as described in Materials and Methods. α, anti. (B) Data in panel A were analyzed to determine the percent amount of injected polypeptide.
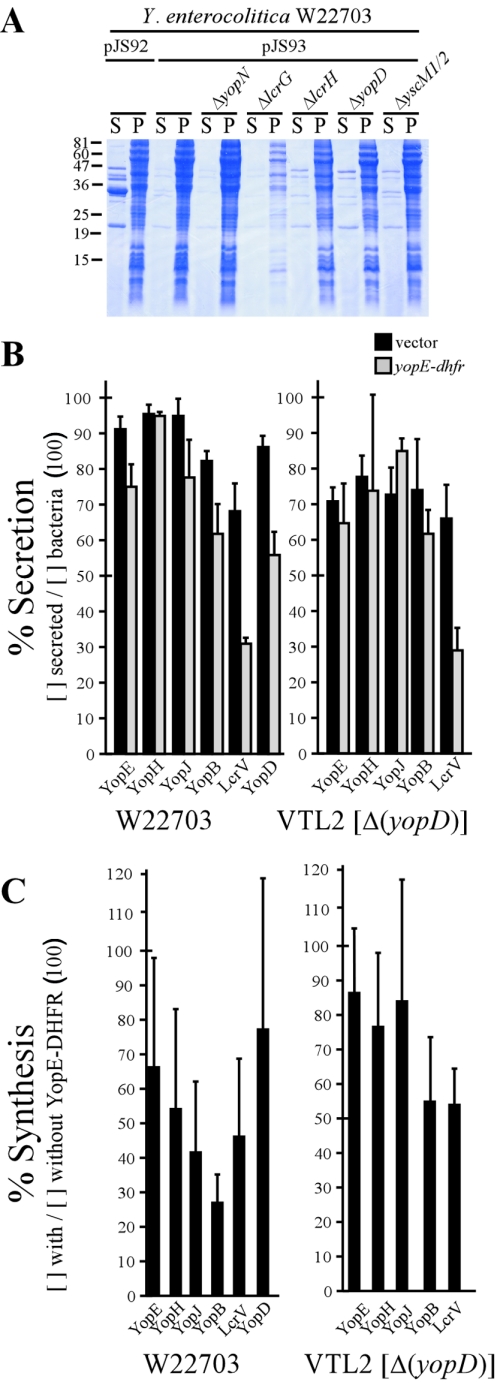
Mutations in class II genes relieve the YopE-DHFR block of type III secretion.
Class I and II genes of the Y. enterocolitica yop virulon function as repressors of the type III pathway (3). Under physiological conditions, this repression can be relieved by environmental signals that trigger specific secretion reactions (43). Class I genes (yopN, tyeA, sycN, yscB, and lcrG) regulate the low-calcium response of yersiniae, and mutations in these genes cause premature secretion of all effector Yops into the extracellular medium (19, 26, 31, 36). In contrast, class II genes (yopD, lcrH, yscM1, and yscM2) repress yop expression at a posttranscriptional step unless bacteria perceive extracellular signals (16, 17, 43, 74). When measured by Coomassie staining and SDS-PAGE, deletions in class I genes, e.g., lcrG and yopN, did not affect the YopE-DHFR-mediated blockade in type III secretion. In contrast, mutations in lcrH (3) and yopD (74) or deletions of the functionally redundant yscM1 and yscM2 (70) increased type III secretion in the presence of YopE-DHFR (Fig. 6A). The amount of Yop protein secretion and synthesis in strains W22703 and VTL2 (ΔyopD) was quantified by immunoblotting. Expression of yopE-dhfr did not affect the secretion of YopE, YopH, YopJ, or YopB in the wild type or the class II mutant strain VTL2. However, the observed increase in the amount of type III secreted proteins occurred through increases in the synthesis of YopE, YopH, YopJ, YopB, and LcrV (Fig. 6C).
FIG. 6.
Mutations in class II genes relieve the type III blockade of YopE-DHFR. Y. enterocolitica W22703(pJS92) (vector control) and strains W22703, VTL1 (ΔyopN), MC2 (ΔlcrG), VTL2 (ΔyopD), CT133 (ΔlcrH), and EC2 [Δ(yscM1 yscM2)], each carrying pJS93, were analyzed for type III secretion in the absence of calcium. (A) Coomassie brilliant blue-stained SDS-PAGE gel for separation of proteins in the medium supernatant (S) and the bacterial pellet (P). The positions of molecular weight markers are indicated. (B) Proteins in the supernatant and bacterial pellet fractions were also electroblotted onto a PVDF membrane, analyzed with specific antisera for strains W22703 (wild type) and VTL2 (ΔyopD), and quantified for the percent amount of secreted polypeptide. (C) Total amounts of Yersinia proteins were determined by immunoblotting proteins from whole-culture samples and compared between bacteria expressing YopE-DHFR (pJS93) and the vector control (pJS92).
Expression of yopE-dhfr inhibits synthesis of type III secretion substrates.
We sought to determine whether the yopE-dhfr-mediated reduction in the overall concentration of type III secretion substrates occurred via regulatory effects at the level of transcription or protein synthesis or degradation. Y. enterocolitica W22703 cultures were induced for type III secretion in the presence or absence of yopE-dhfr. Total RNA was isolated, and transcript levels of yopB and lcrV were analyzed. As a control for expression of unrelated genes, we measured the transcription of rpoA, encoding an essential subunit of RNA polymerase. cDNA was synthesized using avian myeloblastosis virus reverse transcriptase, which was then used as a template for PCR. The omission of reverse transcriptase in duplicate reactions was used as a control for cDNA synthesis. Data in Fig. 7A show that the transcript levels of yopB, lcrV, and rpoA remained unaltered regardless of the expression of yopE-dhfr.
FIG. 7.
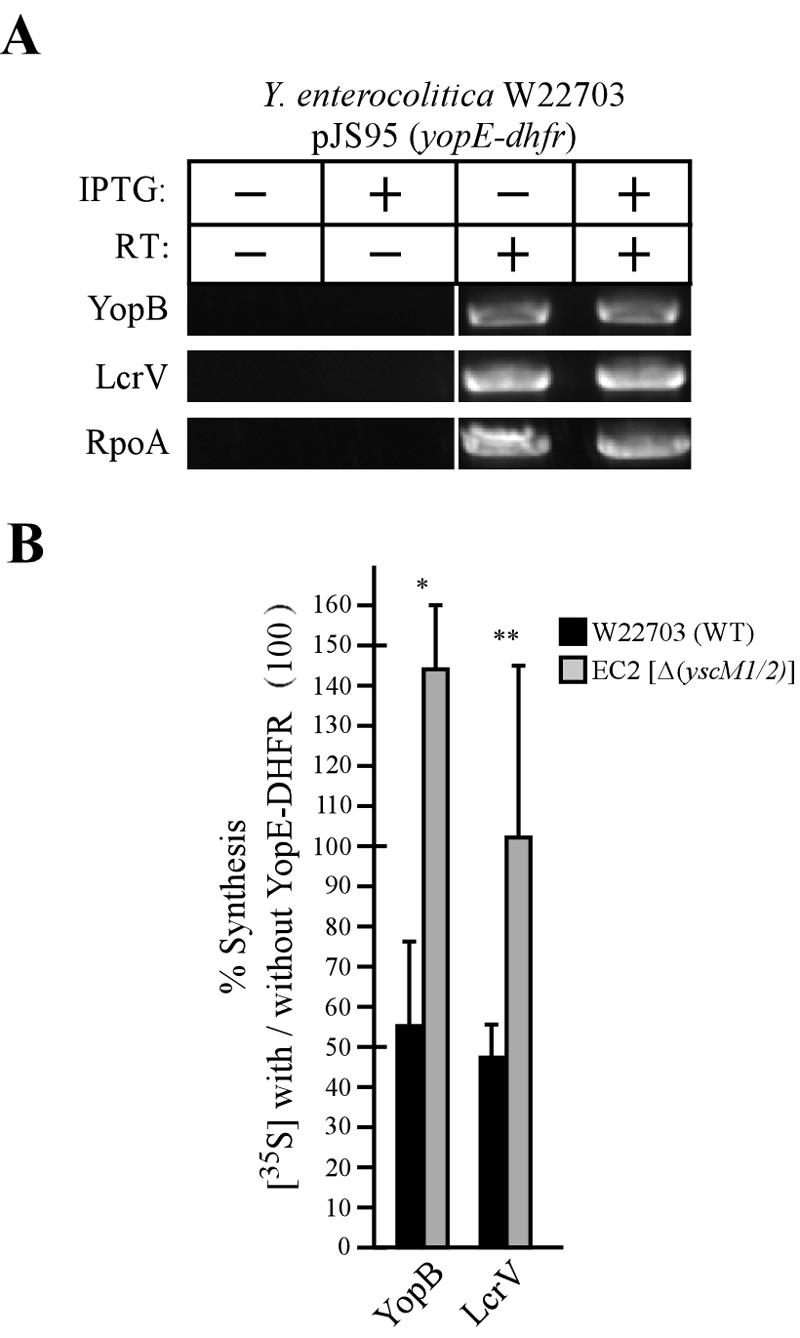
Expression of yopE-dhfr does not affect yop transcript levels but results in an inhibition of Yop protein synthesis. (A) Total RNA was isolated from Y. enterocolitica W22703(pJS95) that had been induced for type III secretion. Where indicated, IPTG was added to the culture to induce the expression of yopE-dhfr. Transcript levels of yopB, lcrV, and rpoA were analyzed by RT-PCR. As a control for cDNA synthesis, reverse transcriptase (RT) was omitted from the reaction where indicated. (B) Y. enterocolitica W22703(pJS95) (black bars) and EC2(pJS95) [Δ(yscM1 yscM2)] (gray bars) cultures induced for type III secretion were pulse-labeled with 10 μCi/ml Pro-Mix, TCA precipitated, immunoprecipitated, and analyzed by SDS-PAGE and phosphorimaging. Relative amounts of protein synthesis were calculated by dividing the total amount of YopB and LcrV synthesized in strains expressing yopE-dhfr by the total amount of YopB and LcrV synthesized in strains not expressing yopE-dhfr. Values were normalized to the expression of RpoA and multiplied by 100%. Black and gray bars indicate the means of four independent experiments. Error bars indicate 1 standard deviation from the mean. Statistical significance was calculated using Student's t test. *, P < 0.01; **, P < 0.05.
Y. enterocolitica W22703(pJS95) and EC2(pJS95) [Δ(yscM1 yscM2)] were induced for type III secretion in the presence and absence of IPTG. After 3 h of induction an aliquot of culture was removed and newly synthesized proteins were pulse-labeled with 10 μCi/ml of [35S]methionine and [35S]cysteine for 2 min. Equal amounts of culture were TCA precipitated, and proteins were immunoprecipitated and then separated by SDS-PAGE. The amount of synthesized protein was quantified using phosphorimaging, and protein levels were normalized to the non-type III control protein RpoA. The observed rates of synthesis are depicted as ratios of the total amount of synthesis in strains expressing yopE-dhfr versus strains not expressing yopE-dhfr (Fig. 7B). Expression of yopE-dhfr in wild-type Y. enterocolitica led to a significant reduction in the amount of newly synthesized YopB and LcrV (Fig. 7B). Expression of yopE-dhfr in the class II mutant led to a restoration of the rate of YopB and LcrV synthesis (Fig. 7B). Pulse-labeling of YopB and LcrV and a chase of cultures with unlabeled methionine/cysteine permitted analysis of the turnover of immunoprecipitated polypeptides. We did not detect changes in the turnover rates of YopB and LcrV in the presence of yopE-dhfr expression. Together these data suggest that the expression of yopE-dhfr results in an inhibition of Yop protein synthesis and not in a decrease in yop transcription. Further, the observed feedback inhibition in the synthesis of YopB and LcrV was suppressed by a class II regulatory mutant.
Mutations in lcrQ relieve the block of Y. pestis LCR.
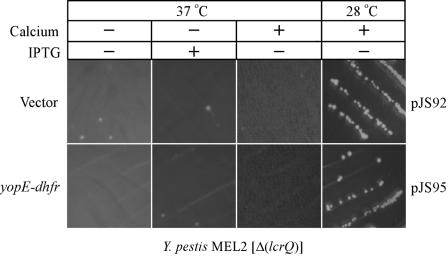
Y. pestis expresses lcrQ, a class II gene and posttranscriptional repressor with homology to the functionally redundant yscM1 and yscM2 of Y. enterocolitica (15, 64, 76). To test whether or not the YopE-DHFR-mediated block in the LCR could be overcome by lcrQ mutations, Y. pestis strain MEL2 (ΔlcrQ) was transformed with pJS95 or with the pJS92 vector control. Transformants were streaked on heart infusion agar (HIA) plates with or without IPTG and incubated at 28 or 37°C for 2 days. Y. pestis strain MEL2 grew at 28°C even in the absence of IPTG and irrespective of the presence or absence of yopE-dhfr (Fig. 8). When the temperature was shifted to 37°C, IPTG induction of yopE-dhfr could not restore growth on agar medium. The addition of calcium to agar media also could not restore the growth of Y. pestis strain MEL2, as class II mutations abolish posttranscriptional repression of yop genes in the presence or absence of environmental calcium ions (16).
FIG. 8.
YopE-DHFR cannot block temperature-sensitive growth of class II mutant Y. pestis. Y. pestis strain MEL2 (ΔlcrQ) was transformed with a vector control plasmid or with a plasmid expressing yopE-dhfr from the IPTG-inducible tac promoter (pJS95). Strains were streaked on HIA plates and incubated at 28 or 37°C with or without calcium and with or without IPTG, an inducer of the tac promoter driving yopE-dhfr expression.
Mutations in yscM1 and yscM2 relieve the block of Yersinia type III injection.
If class II mutations abolish the YopE-DHFR blockade and thereby restore a temperature-sensitive-growth phenotype, can lcrQ or yscM1 and yscM2 mutations also restore type III injection of effector Yops in yersiniae expressing yopE-dhfr? To test this, Y. enterocolitica EC2(pJS95) [Δ(yscM1 yscM2)] was inoculated into HeLa cell cultures and expression of yopE-dhfr was controlled by the addition or omission of IPTG. In the absence of IPTG, YopE-DHFR was not synthesized and the Δ(yscM1 yscM2) mutant strain EC2 injected YopE, YopH, YopM, and YopN into HeLa cells and YopR was secreted into the extracellular medium. Upon addition of IPTG, the expression of YopE-DHFR could be detected in bacterial cells. As expected, the hybrid protein was not injected into HeLa cells but instead sedimented with the bacteria into the pellet fraction. However, digitonin fractionation could detect type III injection for YopH, YopM, and YopN and the secretion of YopR. YopE, on the other hand was neither secreted nor injected (Fig. 9B). This result can be explained by the YopE-DHFR-mediated sequestration of SycE, a cytoplasmic chaperone and YopE binding protein that is absolutely required for its type III injection into mammalian cells (20, 73). Restoration of effector Yop injection in the presence of YopE-DHFR could be observed by fluorescence microscopy, as cells displayed rounding and Texas red-phalloidin staining revealed actin redistribution throughout the cytoplasm in both the presence and absence of IPTG (Fig. 9C). Thus, Δ(yscM1 yscM2) mutations in Y. enterocolitica restore type III injection of effector Yops even in the presence of the impassable substrate YopE-DHFR.
Role of sycE in the yopE-dhfr blockade of the type III pathway.
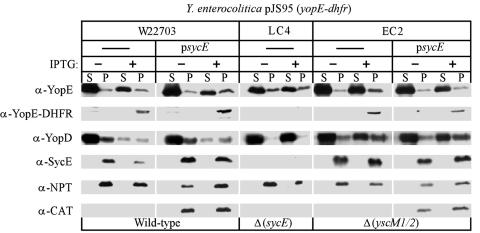
Previous work indicated that SycE, a small cytoplasmic chaperone that binds YopE residues 15 to 78 (8, 20, 73, 75), is required for the yopE-dhfr-mediated blockade of type III secretion (30). To test whether overexpression of sycE can rescue YopE-DHFR secretion or relieve the type III blockade, a high-copy-number plasmid carrying sycE (20) was transformed into wild-type and sycE and yscM1 yscM2 mutant strains. As expected, overexpression of sycE resulted in an increase in the total amount of SycE and an increase in the total amount of YopE and YopE-DHFR. The latter is likely due to the stabilization of both wild-type YopE and YopE-DHFR due to increases in the concentration of the SycE chaperone (20). However, overexpression of sycE did not restore the decrease in YopD synthesis in the presence of yopE-dhfr expression (Fig. 10). Previous experiments have shown that expression of yopE-dhfr does not result in a blockade of type III secretion in a sycE mutant background (30). Interestingly, deletion of the sycE gene led to a drastic decrease in the overall concentration of YopE-DHFR (Fig. 10). Pulse-chase analysis with a [35S]methionine-labeled polypeptide revealed that deletion of sycE also caused an increase in the degradation of the YopE-DHFR, similar to wild-type YopE (18) (data not shown). As expected, overexpression of sycE within the yscM1 yscM2 mutant did not affect YopD synthesis (Fig. 10). Taken together, these results confirm the requirement for SycE in the YopE-DHFR-mediated inhibition of synthesis of type III substrates. However, overexpression of sycE does not affect the YopE-DHFR-mediated blockade of the type III pathway.
FIG. 10.
Overexpression of sycE does not rescue the inhibition of Yop synthesis in strains expressing yopE-dhfr. Strains of Y. enterocolitica expressing pJS95 (yopE-dhfr) were transformed with a high-copy-number plasmid that expresses sycE from the native sycE promoter. The resulting strains were induced for type III secretion by chelation of calcium. Cultures were centrifuged to separate medium in the supernatant (S) from bacterial sediment (P, pellet), and proteins were detected by immunoblotting. Where indicated, IPTG was added to the culture to induce the expression of yopE-dhfr. α, anti.
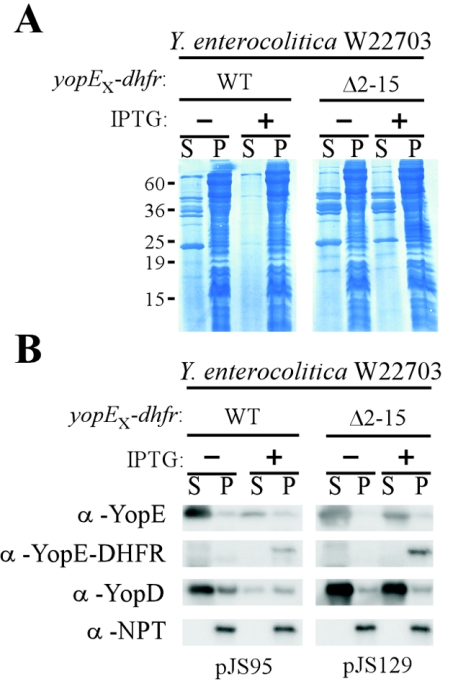
Type III secretion blockade requires the secretion signal of YopE-DHFR.
Previous studies on the requirement of sycE for the YopE-DHFR blockade and data in Fig. 10 suggest that the hybrid protein must at some time be initiated into the type III pathway. Studies on the secretion signal of yopE have outlined that the first 15 amino acids or codons are important for the secretion of YopE (4, 18, 61, 69). Therefore, removal of amino acids 2 to 15 in YopE-DHFR should not only prevent efficient initiation of the hybrid into type III secretion but may also prevent a blockade in this pathway. As expected, expression of yopEΔ2-15-dhfr did not affect the secretion of Yop proteins, as measured by Coomassie staining and SDS-PAGE (Fig. 11A). When these samples were analyzed by immunoblotting, it was found that expression of yopE-dhfr leads to a decrease in the amount of YopD synthesized although small amounts of type III transport of this polypeptide still occurred. However, expression of yopEΔ2-15-dhfr did not affect YopD synthesis or promote a block in type III secretion (Fig. 11B). Together these data suggest that YopE-DHFR initiation into and YopE-DHFR rejection from the type III pathway trigger reduced synthesis of type III secretion substrates.
FIG. 11.
Removal of the YopE secretion signal abolished the YopE-DHFR blockade of the type III pathway. Y. enterocolitica W22703 expressing yopE-dhfr or yopEΔ2-15-dhfr was induced for low-calcium type III secretion in the presence or the absence of IPTG. Cultures were centrifuged to separate medium in the supernatant (S) from bacterial sediment (P, pellet), and proteins were separated on a 15% SDS-PAGE gel and stained with Coomassie brilliant blue (A) or transferred to a PVDF membrane and immunoblotted with specific YopE, YopD, and NPT antibodies (B). α, anti.
DISCUSSION
With the exception of the twin-arginine translocation pathway (27), transport of polypeptides across plasma membranes involves passage of unfolded polypeptides through narrow translocation channels (9). Translocation can be fueled by protein synthesis (72), by chaperone binding of substrates in a trans compartment (pulling) (12), or by piston-like movements of translocation factors through membrane channels (pushing) (28). Because signal peptide-mediated initiation of secretion substrates into translocation machines occurs by default and is irrevocable (9), fusion of signal peptides to rapidly folding polypeptides generates substrates that are impassable for membrane translocation channels and thus jam the machinery. Type III secretion does not conform to the classic paradigms of membrane translocation machines, because the physical dimensions of the secretion machinery, a 2-nm-wide and 50- to 100-nm-long channel, are not consistent with the classical fueling reactions for protein translocation (11, 40). Nevertheless, the mechanisms whereby the type III secretion machinery transports proteins through its hollow needle are not yet known.
Mapping of secretion signals has shed some light on the unique properties of type III machines. While the first 7 to 12 codons of yop genes include the signals necessary and sufficient for secretion of hybrid proteins generated from fused reporter genes, three arguments question the functionality of classical signal peptides (48, 62, 69). First, bioinformatic analysis failed to reveal a universal feature (amino acid conservation or peptide motif) of Yop proteins that could function as a secretion signal for the type III machine (21). Second, frameshift mutations in yop coding sequences do not abolish the function of secretion signals (4), whereas some synonymous mutations in yop secretion signals can abolish substrate recognition (34, 63). Third, multiple sequence elements of yop genes, in a successive manner beginning at the 5′ end (N-terminal end) and including amino acid binding sites for secretion chaperones, contribute to substrate recognition and translocation efficiency in a manner that is unique for each substrate, suggesting that each Yop protein assumes a defined position in a cascade of secretion reactions (18, 61). These observations are not limited to yersiniae. For example, the secretion signal properties of Xanthomonas sp. type III substrates are similar to those of yersiniae (55). Further, export of heterologous proteins by the flagellar basal body complex of Escherichia coli occurs when the 5′ untranslated region of the flagellin gene is fused to reporter gene coding sequence (49). Taken together, these results suggest that substrate recognition in type III secretion of gram-negative bacteria involves at least some features of the mRNAs that encode proteins destined to travel the pathway (60, 68).
In this report, we add to the unique features of type III secretion machines. Although the type III machinery cannot transport impassable substrates, as has been shown for the TAT pathway, these hybrids do not jam the pathway and indeed do not even interfere with its function. Because YopE-DHFR is neither inserted into type III needles nor associated with the membrane envelope (45), we conclude that the hybrid polypeptide must be rejected from the type III secretion pathway. To our knowledge, type III secretion is the first example for the rejection of an impassable transport substrate for any membrane translocator. The notion that impassable substrates inhibit gene expression of other substrates, presumably via feedback inhibition loops to prevent cellular accumulation of polypeptides, has been reported for several secretion systems including Sec transport of signal peptide-bearing precursors across the plasma membrane (56, 57). Genetic relief of the YopE-DHFR blockade of type III secretion points to the class II genes, whose products are known to regulate gene expression in the yop virulon by a posttranscriptional mechanism that involves a short nucleotide sequence in yop untranslated regions as a target (16).
The hypothesis that impassable substrates of type III pathways can be rejected provides new insight into the workings of this fascinating translocation machine. The irrevocable fate of Yop proteins does not seem to be initiation into the type III pathway and transport. If it were, substrates such as YopE-DHFR would be continuously initiated and would thereby clog up the machine. However, it appears as if YopE-DHFR molecules that were rejected could no longer be considered substrates by the secretion machine. Such separation of secretion signal-controlled fate and the destiny of mature polypeptides can provide fertile experimental grounds for testing the mRNA signal hypothesis in type III secretion. However, we realize that a myriad of mechanistic possibilities, even those involving polypeptide properties (47), could lead to the permanent rejection of substrate from the pathway, and additional experimentation is needed to study the fate of proteins that are rejected from the type III pathway. Nevertheless, the data presented here provide several new opportunities, namely, to map the signal of yopE or of other yop genes required for implementing feedback inhibition via class II gene repression and the possible identification of yopE elements required for rejection of substrate from the type III pathway.
Acknowledgments
We thank Guy Cornelis (Biozentrum Basel) for plasmid pMAF35 and members of our laboratory for helpful discussions and critical reading of the manuscript.
This work was supported by United States Public Health Awards from the National Institute of Allergy and Infectious Diseases, Infectious Diseases Branch AI42797, to O. Schneewind. J. Sorg acknowledges support from the Molecular Cell Biology Training Grant T32GM007183. M. Marketon and O. Schneewind acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (GLRCE, National Institute of Allergy and Infectious Diseases Award 1-U54-AI-057153).
REFERENCES
- 1.Agrain, C., I. Callebaut, L. Journet, I. Sorg, C. Paroz, L. J. Mota, and G. R. Cornelis. 2005. Characterization of a type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol. Microbiol. 56:54-67. [DOI] [PubMed] [Google Scholar]
- 2.Amann, E., J. Brosius, and M. Ptashne. 1983. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene 25:167-178. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, D. M., K. S. Ramamurthi, C. Tam, and O. Schneewind. 2002. YopD and LcrH regulate the expression of Yersinia enterocolitica YopQ at a posttranscriptional step and bind to yopQ mRNA. J. Bacteriol. 184:1287-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, D. M., and O. Schneewind. 1997. An mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278:1140-1143. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, D. M., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol. Microbiol. 31:1139-1148. [DOI] [PubMed] [Google Scholar]
- 6.Bassford, P. J., Jr., T. J. Silhavy, and J. Beckwith. 1979. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J. Bacteriol. 139:19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmann, T., K. Erickson, E. Galyov, C. Persson, and H. Wolf-Watz. 1994. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J. Bacteriol. 176:2619-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birtalan, S. C., R. M. Phillips, and P. Ghosh. 2002. Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol. Cell 9:971-980. [DOI] [PubMed] [Google Scholar]
- 9.Blobel, G. 1980. Intracellular protein topogenesis. Proc. Natl. Acad. Sci. USA 77:1496-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blobel, G., and B. Dobberstein. 1975. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 67:835-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri ‘needle complex’, a part of its type III secreton. Mol. Microbiol. 39:652-663. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky, J. L., J. Goeckeler, and R. Schekman. 1995. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 92:9643-9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brubaker, R. R. 1969. Mutation rate to nonpigmentation in Pasteurella pestis. J. Bacteriol. 98:1404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brubaker, R. R., A. K. Sample, D. Z. Yu, R. J. Zahorchak, P. C. Hu, and J. M. Fowler. 1987. Proteolysis of V antigen from Yersinia pestis. Microb. Pathog. 2:49-62. [DOI] [PubMed] [Google Scholar]
- 15.Cambronne, E. D., L. W. Cheng, and O. Schneewind. 2000. LcrQ/YscM1, regulators of the Yersinia yop virulon, are injected into host cells by a chaperone dependent mechanism. Mol. Microbiol. 37:263-273. [DOI] [PubMed] [Google Scholar]
- 16.Cambronne, E. D., and O. Schneewind. 2002. Yersinia enterocolitica type III secretion: yscM1 and yscM2 regulate yop gene expression by a posttranscriptional mechanism that targets the 5′-untranslated region of yop mRNA. J. Bacteriol. 184:5880-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cambronne, E. D., J. A. Sorg, and O. Schneewind. 2004. Binding of SycH chaperone to YscM1 and YscM2 activates effector yop expression in Yersinia enterocolitica. J. Bacteriol. 186:829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng, L. W., D. M. Anderson, and O. Schneewind. 1997. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol. Microbiol. 24:757-765. [DOI] [PubMed] [Google Scholar]
- 19.Cheng, L. W., O. Kay, and O. Schneewind. 2001. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J. Bacteriol. 183:5293-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng, L. W., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: on the role of SycE in targeting YopE into HeLa cells. J. Biol. Chem. 274:22102-22108. [DOI] [PubMed] [Google Scholar]
- 21.Cornelis, G. R. 2003. How Yop proteins find their way out of Yersinia. Mol. Microbiol. 50:1091-1094. [DOI] [PubMed] [Google Scholar]
- 22.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornelis, G. R., and C. Colson. 1975. Restriction of DNA in Yersinia enterocolitica detected by the recipient ability for a derepressed R factor from Escherichia coli. J. Gen. Microbiol. 87:285-291. [DOI] [PubMed] [Google Scholar]
- 25.Day, J. B., and G. V. Plano. 1998. A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol. Microbiol. 30:777-789. [DOI] [PubMed] [Google Scholar]
- 26.DeBord, K., V. T. Lee, and O. Schneewind. 2001. On the role of LcrG and LcrV during the type III targeting of effector Yops by Yersinia enterocolitica. J. Bacteriol. 183:4588-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLisa, M. P., D. Tullman, and G. Georgiou. 2003. Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc. Natl. Acad. Sci. USA 100:6115-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Economou, A., and W. Wickner. 1994. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell 78:835-843. [DOI] [PubMed] [Google Scholar]
- 29.Eilers, M., and G. Schatz. 1986. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature 322:228-232. [DOI] [PubMed] [Google Scholar]
- 30.Feldman, M. F., S. Muller, E. Wuest, and G. R. Cornelis. 2002. SycE allows secretion of YopE-DHFR hybrids by the Yersinia enterocolitica type III Ysc system. Mol. Microbiol. 46:1183-1197. [DOI] [PubMed] [Google Scholar]
- 31.Forsberg, A., A.-M. Viitanen, M. Skunik, and H. Wolf-Watz. 1991. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5:977-986. [DOI] [PubMed] [Google Scholar]
- 32.Galan, J. E., and A. Collmer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322-1333. [DOI] [PubMed] [Google Scholar]
- 33.Goguen, J. D., J. Yother, and S. C. Straley. 1984. Genetic analysis of the low calcium response in Yersinia pestis Mud1(Ap lac) insertion mutants. J. Bacteriol. 160:842-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goss, J. W., J. A. Sorg, K. S. Ramamurthi, H. Ton-That, and O. Schneewind. 2004. The secretion signal of YopN, a regulatory protein of the Yersinia enterocolitica type III secretion pathway. J. Bacteriol. 186:6320-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-572. [DOI] [PubMed] [Google Scholar]
- 36.Iriarte, M., M.-P. Sory, A. Boland, A. P. Boyd, S. D. Mills, I. Lambermont, and G. R. Cornelis. 1998. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 17:1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnsson, N., and A. Varshavsky. 1994. Ubiquitin-assisted dissection of protein transport across membranes. EMBO J. 13:2686-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, and K. M. Peterson. 1995. Four new derivatives of the broad-host range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 39.Kubori, T., and J. E. Galan. 2003. Temporal regulation of Salmonella virulence effector function by proteasome-dependent protein degradation. Cell 115:333-342. [DOI] [PubMed] [Google Scholar]
- 40.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S.-I. Aizawa. 1998. Supermolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 41.Kupferberg, L. L., and K. Higuchi. 1958. Role of calcium ions in the stimulation of growth of virulent strains of Pasteurella pestis. J. Bacteriol. 76:120-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, V. T., D. M. Anderson, and O. Schneewind. 1998. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol. Microbiol. 28:593-601. [DOI] [PubMed] [Google Scholar]
- 43.Lee, V. T., S. K. Mazmanian, and O. Schneewind. 2001. A program of Yersinia enterocolitica type III secretion reactions is triggered by specific host signals. J. Bacteriol. 183:4970-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, V. T., and O. Schneewind. 1999. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol. Microbiol. 31:1619-1629. [DOI] [PubMed] [Google Scholar]
- 45.Lee, V. T., and O. Schneewind. 2002. Yop fusions to tightly folded protein domains and their effects on Yersinia enterocolitica type III secretion. J. Bacteriol. 184:3740-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, V. T., C. Tam, and O. Schneewind. 2000. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 275:36869-36875. [DOI] [PubMed] [Google Scholar]
- 47.Lloyd, S. A., A. Forsberg, H. Wolf-Watz, and M. S. Francis. 2001. Targeting exported substrates to the Yersinia TTSS: different functions for different signals? Trends Microbiol. 9:367-371. [DOI] [PubMed] [Google Scholar]
- 48.Lloyd, S. A., M. Norman, R. Rosqvist, and H. Wolf-Watz. 2001. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39:520-531. [DOI] [PubMed] [Google Scholar]
- 49.Majander, K., L. Anton, J. Antikainen, H. Lang, M. Brummer, T. K. Korhonen, and B. Westerlund-Wikstrom. 2005. Extracellular secretion of polypeptides using a modified Escherichia coli flagellar secretion apparatus. Nat. Biotechnol. 23:475-481. [DOI] [PubMed] [Google Scholar]
- 50.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. Plague bacteria target immune cells during infection. Science, in press. [DOI] [PMC free article] [PubMed]
- 51.Michiels, T., J.-C. Vanooteghem, C. Lambert de Rouvroit, B. China, A. Gustin, P. Boudry, and G. R. Cornelis. 1991. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J. Bacteriol. 173:4994-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michiels, T., P. Wattiau, R. Brasseur, J.-M. Ruysschaert, and G. Cornelis. 1990. Secretion of Yop proteins by yersiniae. Infect. Immun. 58:2840-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milstein, C., G. G. Brownlee, T. M. Harrison, and M. B. Mathews. 1972. A possible precursor of immunoglobulin light chains. Nat. New Biol. 239:117. [DOI] [PubMed] [Google Scholar]
- 54.Mota, L. J., L. Journet, I. Sorg, C. Agrain, and G. R. Cornelis. 2005. Bacterial injectisomes: needle length does matter. Science 307:1278. [DOI] [PubMed] [Google Scholar]
- 55.Mudgett, M. B., O. Chesnokova, D. Dahlbeck, E. T. Clark, O. Rossier, U. Bonas, and B. J. Staskawicz. 2000. Molecular signal required for type III secretion and translocation of the Xanthomonas campestris AvrBs2 protein to pepper plants. Proc. Natl. Acad. Sci. USA 97:13324-13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakatogawa, H., and K. Ito. 2002. The ribosomal exit tunnel functions as a discriminating gate. Cell 108:629-636. [DOI] [PubMed] [Google Scholar]
- 57.Nakatogawa, H., and K. Ito. 2001. Secretion monitor, SecM, undergoes self-translation arrest in the cytosol. Mol. Cell 7:185-192. [DOI] [PubMed] [Google Scholar]
- 58.Oliver, D. B., and J. Beckwith. 1981. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell 25:765-772. [DOI] [PubMed] [Google Scholar]
- 59.Petterson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 60.Ramamurthi, K. S., and O. Schneewind. 2003. Substrate recognition by the Yersinia type III protein secretion machinery. Mol. Microbiol. 50:1095-1102. [DOI] [PubMed] [Google Scholar]
- 61.Ramamurthi, K. S., and O. Schneewind. 2005. A synonymous mutation in Yersinia enterocolitica yopE affects the function of the YopE type III secretion signal. J. Bacteriol. 187:707-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramamurthi, K. S., and O. Schneewind. 2002. Yersinia enterocolitica type III secretion: mutational analysis of the yopQ secretion signal. J. Bacteriol. 184:3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramamurthi, K. S., and O. Schneewind. 2003. Yersinia yopQ mRNA encodes a bipartite type III secretion signal in the first fifteen codons. Mol. Microbiol. 50:1189-1198. [DOI] [PubMed] [Google Scholar]
- 64.Rimpilainen, M., A. Forsberg, and H. Wolf-Watz. 1992. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J. Bacteriol. 174:3355-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of yersinia obstructs the primary host defense. Mol. Microbiol. 4:657-667. [DOI] [PubMed] [Google Scholar]
- 66.Rosqvist, R., A. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosqvist, R., K.-E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorg, J. A., N. C. Miller, and O. Schneewind. 2005. Substrate recognition of type III secretion machines testing the RNA signal hypothesis. Cell. Microbiol. 7:1217-1225. [DOI] [PubMed] [Google Scholar]
- 69.Sory, M.-P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA 92:11998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stainier, I., M. Iriarte, and G. R. Cornelis. 1997. YscM1 and YscM2, two Yersinia enterocolitica proteins causing downregulation of yop transcription. Mol. Microbiol. 26:833-843. [DOI] [PubMed] [Google Scholar]
- 71.Straley, S. C., G. V. Plano, E. Skrzypek, P. L. Haddix, and K. A. Fields. 1993. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol. Microbiol. 8:1005-1010. [DOI] [PubMed] [Google Scholar]
- 72.Walter, P., R. Gilmore, and G. Blobel. 1984. Protein translocation across the endoplasmic reticulum. Cell 38:5-8. [DOI] [PubMed] [Google Scholar]
- 73.Wattiau, P., and G. R. Cornelis. 1993. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol. Microbiol. 8:123-131. [DOI] [PubMed] [Google Scholar]
- 74.Williams, A. W., and S. C. Straley. 1998. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J. Bacteriol. 180:350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woestyn, S., M.-P. Sory, A. Boland, O. Lequenne, and G. R. Cornelis. 1996. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol. Microbiol. 20:1261-1271. [DOI] [PubMed] [Google Scholar]
- 76.Wulff-Strobel, C. R., A. W. Williams, and S. C. Straley. 2002. LcrQ and SycH function together at the Ysc type III secretion system in Yersinia pestis to impose a hierarchy of secretion. Mol. Microbiol. 43:411-423. [DOI] [PubMed] [Google Scholar]