Abstract
We describe here the purification and quantification of a water-soluble cyclic form of enterobacterial common antigen (ECACYC) from Escherichia coli K-12 as well as information regarding its subcellular location and the genetic loci involved in its assembly. Structural characterization of purified ECACYC molecules obtained from E. coli K-12 revealed that they uniformly contained four trisaccharide repeat units, and they were substituted with from zero to four O-acetyl groups. Cells from overnight cultures contained approximately 2 μg ECACYC per milligram (dry weight), and cell fractionation studies revealed that these molecules were localized exclusively in the periplasm. The synthesis and assembly of ECACYC were found to require the wzxE and wzyE genes of the wec gene cluster. These genes encode proteins involved in the transmembrane translocation of undecaprenylpyrophosphate-linked ECA trisaccharide repeat units and the polymerization of trisaccharide repeat units, respectively. Surprisingly, synthesis of ECACYC was dependent on the wzzE gene, which is required for the modulation of the polysaccharide chain lengths of phosphoglyceride-linked ECA (ECAPG). The presence of ECACYC in extracts of several other gram-negative enteric organisms was also demonstrated; however, it was not detected in cell extracts of Pseudomonas aeruginosa. These data suggest that in addition to ECAPG, ECACYC may be synthesized in many, if not all, members of the Enterobacteriaceae.
Enterobacterial common antigen (ECA) is a cell surface glycolipid that is present in all gram-negative enteric bacteria thus far examined (19, 23, 25, 38). The carbohydrate portion of the polymer contains the amino sugars N-acetyl-d-glucosamine (GlcNAc), N-acetyl-d-mannosaminuronic acid (ManNAcA), and 4-acetamido-4,6-dideoxy-d-galactose (Fuc4NAc). These amino sugars are linked to one another to form linear polysaccharide chains that are comprised of the trisaccharide repeat unit→ 3-α-d-Fuc4NAc-(1→ 4)-β-d-ManNAcA-(1→ 4)-α-d-GlcNAc-(1→ (22, 24). In addition, the hydroxyl groups at position 6 of the GlcNAc residues are nonstoichiometrically substituted with O-acetyl groups (11, 22). Individual ECA polysaccharide chains are covalently linked to diacylglycerophosphate via glycosidic linkage between the potential reducing terminal GlcNAc residue and the phosphate residue of the aglycone (19, 20, 36). The polysaccharide chains of ECA obtained from Escherichia coli K-12 comprise a modal population of polymers ranging in chain length from 1 to 14 repeat units, with a modal value of 6 to 7 repeat units (2). The phosphoglyceride portion serves to anchor the polymers in the outer leaflet of the outer membrane (1, 39). Accordingly, phosphoglyceride-linked ECA is referred to as ECAPG, and it has been estimated that it accounts for approximately 0.2% of the cellular dry weight of E. coli K-12 (21, 24).
The trisaccharide repeat unit of ECAPG is assembled as the lipid carrier-linked intermediate Fuc4NAc-ManNAcA-GlcNAc-pyrophosphorylundecaprenol (lipid III; Fuc4NAc-ManNAcA-GlcNAc-PP-Und) (3, 4, 37, 38). The genes involved in the assembly of lipid III, as well many of the other genes involved in the utilization of lipid III for the assembly of ECAPG, are located in the wec gene cluster at approximately 85.4 min on the E. coli chromosome (2, 7, 24, 32). Our current understanding of the pathway of ECAPG biosynthesis and the function of the genes that comprise the wec gene cluster have been recently described (12). Steps of the assembly process that are pertinent to this communication are summarized in Fig. 1.
FIG. 1.
Pathway for the assembly of ECAPG. The genes that encode the enzymes that catalyze the individual reactions of the pathway are indicated in italics. Abbreviations are as follows: C55-P, undecaprenylphosphate; C55-PP, undecaprenylpyrophosphate; TDP, dTDP.
In addition to containing ECAPG on the cell surface, cell extracts prepared from Shigella sonnei phase I (11, 22), Yersinia pestis (43), and Plesiomonas shigelloides contain water-soluble cyclic polysaccharides comprised of ECA trisaccharide repeat units (6, 42). These polysaccharides have been designated cyclic ECA (ECACYC). ECACYC polysaccharides lack the phosphoglyceride aglycone of ECAPG, and they are shorter and more uniform with respect to their degree of polymerization than are the polysaccharides of ECAPG. For example, ECACYC from Shigella sonnei phase I contains four to six trisaccharide repeat units (11). More-recent studies have also demonstrated the occurrence of ECACYC in cell extracts of E. coli B and E. coli K-12 (12).
Recent reports have suggested that the ECAPG of Salmonella enterica serovar Typhimurium functions as a virulence factor for oral infection in mice by contributing to the resistance of the organism to bile salts (33). In contrast, nothing is known concerning the function of ECACYC. Furthermore, although the trisaccharide repeat units of ECAPG and ECACYC are assembled by a common biosynthetic pathway (12), subsequent steps involved in the assembly ECACYC remain to be established. We describe here the separation, purification, and quantification of ECACYC molecules obtained from soluble extracts of E. coli K-12 using reverse-phase high-pressure liquid chromatography (HPLC). Examination of soluble fractions obtained from the cytoplasm and periplasm revealed that ECACYC is a periplasmic component of E. coli K-12. Data are also presented that support the conclusion that the synthesis of ECACYC requires functional wzxE, wzyE, and wzzE genes, which encode proteins involved in the transmembrane translocation of lipid III, the polymerization of ECA trisaccharide repeat units, and the regulation of ECA polysaccharide chain length, respectively. ECACYC was also detected in extracts obtained from several other members of the Enterobacteriaceae, thus suggesting the possibility that ECACYC is a component of all gram-negative enteric bacteria.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. Cultures were grown at 37°C with vigorous aeration in either Luria-Bertani (LB) broth (29), proteose peptone beef extract broth (40), or M9 minimal medium (29) containing 0.2% glucose (M9-glucose medium) and other required nutrients. Tetracycline, ampicillin, kanamycin, gentamicin, and chloramphenicol were added to media when appropriate to give final concentrations of 10 μg/ml, 50 μg/ml, 15 μg/ml, and 30 μg/ml, respectively. Transductions were carried out using phage P1 vir as described by Silhavy et al. (41).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genetic markers or characteristic(s) | Source or referencea |
|---|---|---|
| Strains | ||
| E. coli K-12 | ||
| Sθ864 | F−trp lacZ strA thi upp | 31 |
| Sθ874 | Δ(wzxO16wzxC), P2 eductant of Sθ864 with an extended deletion in the region from udk to his | 31 |
| W3110 | F− λ− IN(rrnD-rrnE) rph-1 | CGSC |
| AB1133 | thr-1 leuB6 Δ(gpt-proA)66 hisG4 argE3 thi-1 rfbd1 lacY1 ara-14 galK2 xyl-5 mtl-1 mgl-51 rpsL31 kdgK51 supE44 | CGSC |
| 21548 | Like AB1133, but wecA::Tn10 | 27 |
| 21568 | Like AB1133, but wecG::Tn10 | 27 |
| 14.5 | λ− F−thr-1 leuB6 tonA31 lacY1 tsx-78 supO eda50 his-4 rfbD1 mgl-51 rpsL136 xyl-5 mtl-1 metF159 thi-1 ara-14 wzxE::cm | P. N. Danese |
| PR4119 | Nonpolar insertion of the gentamicin resistance determinant from pUC19 into the AccI site of the wzyE gene of strain 21548 | This study |
| PR4149 | Like Sθ874, but wecA::Tn10 [21548(P1) × Sθ874] | 35 |
| PR4150 | Like PR4149, but wzxE::cm [14.5(P1) × PR4149] | 35 |
| PR4180 | PR4150/pRL147 (with wild-type wecA under the control of the pBAD promoter) | 35 |
| PR4210 | PR4234/pRL147 (with wild-type wecA under the control of the pBAD promoter) pRL165 (with wild-type wzxE under the control of the lac promoter) | This study |
| PR4214 | PR4237/pRL163 (with wild-type wecG under the control of the pBAD promoter) | This study |
| PR4216 | PR4240/pRL163 (with wild-type wecG under the control of the pBAD promoter) | This study |
| PR4217 | PR4240/pRL164 (with wild-type wzyE-wecG under the control of the pBAD promoter) | This study |
| PR4218 | Like PR4237, but wzzE::km (nonpolar insertion of the aphA-3 kanamycin resistance determinant from pUC18K [31] into wzzE) | This study |
| PR4219 | PR4218/pRL163 (with wild-type wecG under the control of the pBAD promoter) | This study |
| PR4220 | Like W3110, but wzzE::km (nonpolar insertion of the aphA-3 kanamycin resistance determinant from pUC18K [31] into wzzE) | This study |
| PR4221 | PR4220/pCA53 (wild-type wecA-wzzE on a 2.54-kb ClaI fragment cloned in pBR322) | This study |
| PR4222 | Like PR4237, but wzxE::cm [14.5(P1) × PR4237] | This study |
| PR4223 | PR4222/pRL163 (with wild-type wecG under the control of the pBAD promoter) | This study |
| PR4224 | PR4237/pBAD102/D/lacZ | This study |
| PR4225 | PR4234/pACYC184 | This study |
| PR4226 | PR4220/pBR322 | This study |
| PR4227 | W3110/pBR322 | This study |
| PR4228 | Like PR4227, but wecA::Tn10 [21548(P1) × PR4227] | This study |
| PR4233 | PR4234/pRL147 (with wild-type wecA under the control of the pBAD promoter) pRL169 (with wild-type wzxO16 under the control of the lac promoter) | This study |
| PR4234 | Like Sθ874, but wecA::km (nonpolar insertion of the aphA-3 kanamycin resistance determinant from pUC18K [31] into wecA) wzxE::cm [14.5(P1)] | This study |
| PR4236 | PR4234/pRL147 (with wild-type wecA under the control of the pBAD promoter) pRL166 (with wild-type wzxC under the control of the lac promoter) | This study |
| PR4237 | Like W3110, but wecG::Tn10 [21568(P1) × W3110] | This study |
| PR4240 | Like PR4237, but wzyE::gm [PR4119(P1) × PR4237] | This study |
| Salmonella enterica serovar Typhimurium | Wild type | Laboratory collection |
| Shigella dysenteriae | Wild type | ATCC |
| Shigella flexneri | Wild type | ATCC |
| Enterobacter cloacae | Wild type | ATCC |
| Klebsiella pneumoniae | Wild type | ATCC |
| Proteus vulgaris | Wild type | ATCC |
| Pseudomonas aeruginosa | Wild type | ATCC |
| Plasmids | ||
| pBR322 | Cloning vector | Promega |
| pBAD18 | Expression vector | 15 |
| pBAD24 | Expression vector | 15 |
| pBAD102/D-TOPOR | Expression vector | Invitrogen |
| pBAD102/D/lacZ | Expression vector | Invitrogen |
| pACYC184 | Cloning vector | 10 |
| pRL147 | Wild-type wecA under the control of the pBAD promoter in pBAD18 | 35 |
| pRL163 | Wild-type wecG under the control of the pBAD promoter in pBAD102/D-TOPOR | This study |
| pRL164 | Wild-type wzyE-wecG under the control of the pBAD promoter in pBAD102/D-TOPOR | This study |
| pRL165 | Wild-type wzxE under the control of the lac promoter cloned in pACYC184 | This study |
| pRL166 | Wild-type wzxC under the control of the lac promoter cloned in pACYC184 | This study |
| pRL169 | Wild-type wzxO16 under the control of the lac promoter cloned in pACYC184 | This study |
| pCA53 | Wild-type wecA-wzzE on a 2.54-kb ClaI fragment cloned in pBR322 | 26 |
CGSC, E. coli Genetic Stock Center and M. Berlyn, Biology Department, Yale University, New Haven, CT; ATCC, American Type Culture Collection, Manassas, VA.
Construction of plasmids.
Plasmid pRL147 containing the wild-type wecA allele under the control of the pBAD promoter of plasmid pBAD18 was constructed as previously described (35). Plasmid pRL163 containing the wild-type wecG gene under the control of the pBAD promoter was constructed as follows. The wild-type wecG allele was obtained by PCR amplification using genomic DNA obtained from strain AB1133 as the template. The polynucleotides 5′-CACCATGAATAACAACACCACGGC-3′ and 5′-AAAGAGAGGAAAATCATAGGTTGCC-3′ were used as forward and reverse primers, respectively. The amplified sequence (758 bp) was cloned into the TA cloning site of the pBAD102/D-TOPO vector (Invitrogen) according to the manufacture's instructions.
Plasmid pRL164 containing the 5′-wzyE-wecG-3′ genes on a single DNA fragment of 2.1 kb was obtained by PCR amplification of the wzyE-wecG sequence using the genomic DNA of strain AB1133 as the template and 5′-CAC CATGAAGTCTGCTGCAATTCAGTGGCCTG-3′ and 5′-TCTAGAGTGACC ACTCCGTCGC-3′ as forward and reverse primers, respectively. The amplified sequence was cloned into the TA cloning site of the pBAD102/D-TOPO (Invitrogen) according to the manufacturer's instructions, thus placing both genes under the control of the pBAD promoter.
Plasmid pRL165 containing the wild-type wzxE gene under the control of the lac promoter was obtained by PCR amplification of the genomic DNA of AB1133 using 5′-CTTTGTTGAATTCCTTTTCCTGA-3′ and 5′-CCCAGTACGTGGATCCGTACAGTC-3′ as forward and reverse primers, respectively. EcoRI and BamHI restriction sites were incorporated into the forward and reverse primers (underlined sequences), respectively. The amplified product (1,267 bp) was digested with EcoRI and BamHI, and the product was ligated into the corresponding sites of the plasmid vector, pBAD24 (15), immediately downstream from the vector-encoded Shine-Dalgarno sequence located in the multicloning site of the vector. The cloned fragment, together with the 5′ Shine-Dalgarno sequence, was obtained by PCR amplification with 5′-CTACTGTTTATCGATACCCG-3′ and 5′-AGCCAAGCTTGCATGCCTGC-3′ as forward and reverse primers, respectively (the ClaI restriction site is underlined). The PCR product was digested with ClaI and BamHI and ligated into the corresponding sites in the multicloning site of the expression plasmid pBluescript KS+ immediately downstream from the lac promoter. A fragment containing the 5′-lac promoter-Shine-Dalgarno sequence-wzxE gene-3′ was obtained by PCR amplification with 5′-GTGCTG CAAGGCGTTTAAATTG-3′ and 5′-CGATTCTTTAAAGCAGCTGGCA-3′ as forward and reverse primers, respectively. A DraI restriction site was incorporated into both primers (underlined). The PCR product was then digested with DraI, and the fragment was ligated into the corresponding site of the vector, pACYC184, to yield plasmid pRL165.
Plasmid pRL166 containing wild-type wzxC under the control of the lac promoter was obtained by PCR amplification using the genomic DNA of strain AB1133 as the template and 5′-GGTTTCGGTACCAAAGCGG-3′ and 5′-CTTATCAGGCCTGCAGGTCC-3′ as forward and reverse primers, respectively. KpnI and PstI restriction sites (underlined) were incorporated into the forward and reverse primers, respectively. The PCR product (1.6 kb) was digested with KpnI and PstI, and the resulting fragment was ligated into the corresponding sites located immediately downstream of the lac promoter in the multicloning site of the expression vector pBluescript KS+. A fragment including the lac promoter of the vector and the downstream wild-type wzxC gene was obtained from this construct by digestion with the restriction enzyme PvuII, and the fragment was ligated into the PvuII site of the vector pACYC184, to yield plasmid pRL166.
Plasmid pRL169 containing the wild-type wzxO16 gene under the control of the lac promoter was obtained by PCR amplification using the genomic DNA of strain AB1133 as the template and 5′-CTTATAAGCTTAGGAGGAATTGCA TGAATACG-3′ and 5′-CATTCGCACAAACTGCAGCA-3′ as forward and reverse primers, respectively. HindIII and PstI restriction sites were incorporated into the forward and reverse primers, respectively (underlined sequences). In addition, the forward primer also contained a Shine-Dalgarno sequence (bold sequence) located 6 base pairs upstream of the translational start codon (bold italic sequence). The product (1.3 kb) was digested with the restriction enzymes HindIII and PstI, and the resulting fragment was ligated into the corresponding sites located immediately downstream from the lac promoter in the multicloning site of the expression vector pBluescript KS+. A fragment (1.7 kb) including the wild-type wzxO16 gene and the upstream lac promoter of the vector was obtained from this construct by PCR amplification using 5′-GTGCTGCAAGGCGTT TAAATTG-3′ and 5′-CGATTCTTTAAAGCAGCTGGCA-3′ as forward and reverse primers, respectively. A DraI restriction site was incorporated into both of these primers (underlined). The product was digested with the restriction enzymes PvuII and SmaI, and the resulting 1.6-kb fragment containing the 5′-lac promoter-Shine-Dalgarno sequence-wzxO16 gene-3′ was ligated into the DraI site of the vector pACYC184, to yield plasmid pRL169.
Mass spectrometry (MS) studies.
Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectra were obtained using an Applied Biosystems Voyager-DE STR biospectrometry workstation. Calibrations were conducted in the reflector mode using the peptide adrenocorticotropic hormone. Samples were analyzed using a laser intensity of 2,200. The matrix was 2,5-dihydroxybenzoic acid at a concentration of 10 mg/ml in 20% acetonitrile. Spectra were acquired in the negative mode.
Isolation of soluble periplasmic and cytoplasmic contents.
The soluble periplasmic fraction of cells was released by osmotic shock using a modification of the procedure described by Rech et al. (34). Briefly, cells were grown to stationary phase in LB broth at 37°C. The cells were harvested by centrifugation at 4°C and resuspended in osmotic shock buffer (0.5 M sucrose, 0.1 M Tris-HCl, pH 8.2, and 1 mM EDTA) at a ratio of 5 ml buffer to 1 g (wet weight) of cells. The cells were placed on ice for 10 min and then isolated by centrifugation at 4°C. The supernatant solution was discarded, and the cell pellet was resuspended in 5 mM MgSO4 and incubated on ice for 10 min. The supernatant solution containing the soluble periplasmic contents was isolated by centrifugation at 12,000 × g for 10 min at 4°C, and it was either analyzed immediately or stored at −20°C. The particulate fraction containing the osmotically shocked cells was resuspended in 5 mM MgSO4 in an ice bath, and the soluble cytoplasmic fraction was released by sonication. Cellular debris was removed by centrifugation at 100,000 × g for 1 h at 4°C, and the supernatant solution containing the soluble cytoplasmic contents was either analyzed immediately or stored at −20°C. This procedure resulted in the detection of β-galactosidase and β-lactamase activities only in the cytoplasmic and periplasmic fractions, respectively, when these fractions were obtained from strain PR4227 (W3110/pBR322) grown in the presence of 100 μM isopropylthiogalactoside. The concentrations of protein in cell extracts, cytoplasmic fractions, and periplasmic fractions were determined using the Bio-Rad protein assay reagent according to the procedure recommended by the manufacturer.
Identification and isolation of ECACYC molecules.
To identify ECACYC molecules in cell extracts, cells were grown in 25 ml of M9-glucose medium at 37°C for 12 h and harvested by centrifugation (10,000 × g, 4°C). The cells were washed with 5 mM MgSO4, resuspended in 10 ml of 5 mM MgSO4, and disrupted by sonication at 0°C. Cellular debris was removed by centrifugation (10,000 × g, 4°C) followed by centrifugation of the supernatant solution at 100,000 × g for 1 h at 4°C. Absolute ethanol (1.5 volumes) was added to the supernatant solution obtained from the high-speed centrifugation, and the mixture was incubated at 70°C for 5 min. The resulting precipitate was removed by centrifugation (10,000 × g, 4°C), and the supernatant solution was reduced to dryness under vacuum at 0 to 4°C. The residue was resuspended in distilled deionized water (ddH2O; 1 ml), and particulate matter was removed by filtration using a Millex-GV filter unit (Millipore) containing a 4-mm Durapore polyvinylidene difluoride membrane (0.22-μm pore size). Samples of the filtrate (100 μl) were analyzed by reverse-phase high-pressure liquid chromatography using an Agilent 1100 series instrument and a Nucleosil C18 column (4.6 by 250 mm, 5-μm particle size, 300-Å pore size; Thermo Electron Corp.). Chromatographic analyses were carried out at room temperature with a linear gradient of 0 to 30% acetonitrile in an acidic phosphate solution (50 mM NaOH adjusted to pH 2.0 with 85% phosphoric acid and containing sodium azide at a final concentration of 0.0005%) at a flow rate of 0.5 ml/min. Carbohydrate-containing fractions were detected by their absorbance at 206 nm.
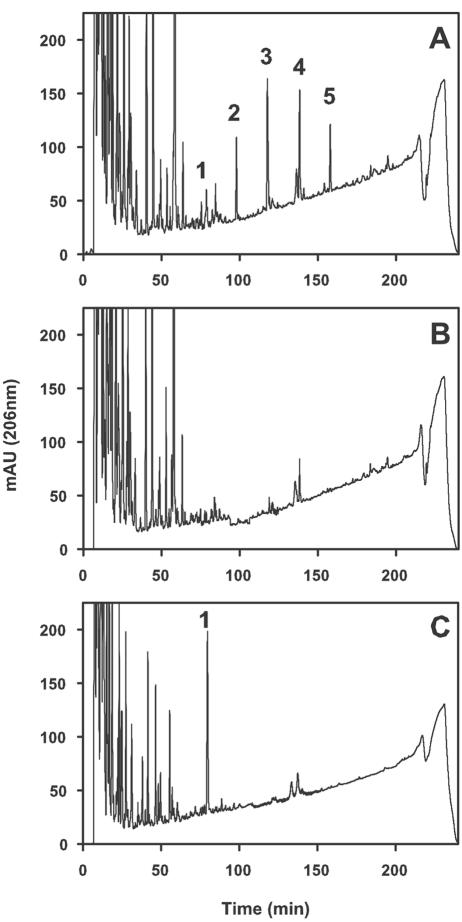
The ECACYC molecules that eluted in peaks 1 to 5 (Fig. 2A) were collected separately for analysis by MALDI-TOF. The material in these fractions was dried under vacuum and subsequently dissolved in 100 μl of ddH2O. The samples were desalted by passage through a ZipTipC18 pipette tip (Millipore Corp.) using a modification of the procedure recommended by the manufacturer. Briefly, the C18 bed in the pipette tips was wetted with 100% acetonitrile and then equilibrated with 0.1% trifluoroacetic acid (TFA) in ddH2O. The aqueous samples were adsorbed onto the C18 bed and eluted with 5 μl of 50% acetonitrile in ddH2O.
FIG. 2.
Detection of ECACYC molecules in whole-cell extracts as determined by reverse-phase HPLC. (A) HPLC analysis of a whole-cell extract obtained from strain PR4227 (wild-type). (B) HPLC analysis of a whole-cell extract obtained from strain PR4228 (wecA::Tn10). (C) HPLC analysis of a whole-cell extract obtained from strain PR4227 following treatment of the extract with mild alkali. Details regarding the preparation of whole-cell extracts and the conditions for treatment of the extract with mild alkali are provided in “Materials and Methods.” Peaks 1, 2, 3, 4, and 5 contain ECACYC molecules containing zero, one, two, three, and four O-acetyl substituents, respectively. mAU, milliabsorption units.
Purified ECACYC molecules in peak 1 (Fig. 2A) were isolated on a preparative scale using reverse-phase HPLC. Strain PR4227 was grown with vigorous aeration in 1 liter of LB broth at 37°C for 12 h, and the cells were harvested by centrifugation (10,000 × g, 4°C). Subsequent steps were the same as those described for the identification of ECACYC in cell extracts, with the exception that the volume of the samples injected onto the Nucleosil C18 column was 300 μl, and the column was developed with a linear gradient of 0 to 15% acetonitrile in the previously described acidic (pH 2.0) phosphate solution. The material in peak 1 (Fig. 2A) was collected and then lyophilized. The residue was dissolved in 500 μl ddH2O and desalted by reverse-phase HPLC as described above. Chromatography was carried out at room temperature at a flow rate of l ml/min using a linear gradient 0 to 100% acetonitrile in ddH2O. Following sample injection, the column was washed with ddH2O for 15 min and then developed with a linear gradient of acetonitrile over a period of 30 min. Column fractions were monitored at 206 nm, and a single component was detected. The fractions containing this component were collected and then dried under vacuum. The dried material from three such preparations was dissolved in 500 μl ddH2O and stored at −20°C.
Treatment of ECACYC with alkali.
Strain PR4227 was grown in 25 ml of M9-glucose medium at 37°C for 12 h and harvested by centrifugation (10,000 × g, 4°C). The cells were washed with 5 mM MgSO4, resuspended in 10 ml of MgSO4, and then disrupted by sonication at 0°C. Cellular debris was removed by centrifugation (10,000 × g, 4°C), followed by centrifugation of the supernatant solution at 100,000 × g for 1 h at 4°C. Absolute ethanol (1.5 volumes) was added to the supernatant solution, and the mixture was incubated at 70°C for 5 min. The resulting precipitate was removed by centrifugation (10,000 × g, 4°C), and the supernatant solution was reduced to dryness under vacuum at 0 to 4°C. The residue was dissolved in ddH2O (500 μl), to which was then added 40 μl of 4 N NaOH. The mixture was incubated for 15 min at 37°C and then acidified by the addition of 200 μl of 4 N HCl. The acidified reaction mixture was next filtered using a Millex-GV filter unit (Millipore) containing a 4-mm Durapore polyvinylidene difluoride membrane (0.22-μm pore size). Aliquots of the filtered reaction mixture were then analyzed by reverse-phase HPLC using the methods described above for the identification of ECACYC in whole-cell extracts.
Quantification of ECACYC.
The amount of ECACYC in extracts was determined by establishing the relationship between a given chemical quantity of ECACYC and the corresponding area of the peak generated by its absorbance at 206 nm when analyzed by reverse-phase HPLC. ECACYC (peak 1) (Fig. 2A) was purified on a preparative scale as described above. The chemical quantity of the ECACYC in these preparations was determined by quantifying the amount of glucosamine released from aliquots of these preparations following total acid hydrolysis. Samples containing various amounts of ECACYC in a total volume of 10 μl were mixed with an equal volume of 8 M TFA and incubated for 7 h at 100°C in a sealed tube. The hydrolysate was then dried under vacuum, washed with isopropanol (40 μl), and then dried under vacuum. The free glucosamine in hydrolysates was analyzed by HPLC as the corresponding p-aminobenzoic acid ethyl ester (ABEE) monosaccharide derivative using an ABEE labeling kit (Honen Corp., Tokyo, Japan) according to the manufacturer's instructions. Briefly, samples were dissolved in ddH2O (10 μl), to which was added ABEE labeling reagent (40 μl). The reaction mixtures were incubated for 1 h at 80°C and then cooled to room temperature. Chloroform (200 μl) and ddH2O (200 μl) were added to reaction mixtures with mixing, the upper aqueous layer was removed, and aliquots were analyzed for the ABEE-glucosamine derivative by reverse-phase HPLC using a Nucleosil C18 column (250 by 4.7 mm, 300-Å pore size; Thermo Electron Corp.) and a linear gradient of 10% to 50% acetonitrile in 0.02% TFA at a flow rate of 1 ml/min at 45°C. The ABEE derivative of glucosamine was detected by fluorescence monitoring (excitation at 305 nm and emission at 360 nm). A standard curve was constructed with known amounts of N-acetyl-d-glucosamine that were then subjected to total acid hydrolysis and converted to the ABEE derivative using the procedures described above. This assay was linear over the entire range of GlcNAc concentrations examined (0.08 to 1.28 nmol). The amount of ECACYC in samples was calculated based on the observation that single ECACYC molecules in E. coli K-12 uniformly contain four trisaccharide repeat units (12) and that each repeat unit contains one GlcNAc residue. Accordingly, a standard curve was constructed relating known amounts of ECACYC to the areas of their corresponding peaks generated by their absorbance at 206 nm when analyzed by reverse-phase HPLC. This assay was linear over the entire range of ECACYC concentrations examined (110 to 710 pmol).
Passive hemagglutination.
The presence of ECA in cell extracts was determined by passive hemagglutination assay with monospecific polyclonal rabbit anti-ECA as previously described (37).
RESULTS
Characterization of ECACYC molecules in cell extracts.
Erbel et al. (12) reported the occurrence of water-soluble ECACYC molecules in cell extracts of E. coli B and E. coli K-12. These cyclic polysaccharides were found to contain four ECA trisaccharide repeat units. Earlier studies established that position 6 of GlcNAc residues in linear ECAPG polymers are nonstoichiometrically substituted with O-acetyl groups (11, 22). Analyses of the ECACYC preparations by mass spectrometry indicated that they also contained O-acetyl groups, and five molecular species were identified owing to the nonstoichiometric substitution of molecules with up to four O-acetyl residues (12). However, the mass spectrometry data obtained in these studies were obtained from unfractionated ECACYC preparations. The separation of individual molecular species of ECACYC has not been reported. Thus, we employed reversed-phase high-performance liquid chromatography and MALDI-TOF MS) to separate and identify the five ECACYC molecular species in extracts of E. coli K-12.
As shown in Fig. 2A, five prominent molecular species were detected when extracts of E. coli strain PR4227 (wild type, W3110/pBR322) were analyzed by reverse-phase HPLC. Under the conditions employed, these species eluted at 79 min (peak 1), 99 min (peak 2), 119 min (peak 3), 143 min (peak 4), and 160 min (peak 5) in a ratio of approximately 1.0:2.1:4.6:3.5:1.8, respectively. The molecules in these peaks were tentatively identified as ECACYC molecules since they were water soluble and they were not detected in extracts of strain PR4228, a derivative of strain PR4227 that is unable to synthesize ECACYC due to a null mutation in the wecA gene (Fig. 2B). The relative amounts of the individual species were very similar to those reported by Erbel et al. (12). MALDI-TOF MS analyses of the molecules in peaks 1, 2, 3, 4, and 5 revealed molecular ions ([M—H]−) that were in agreement with those predicted to be derived from ECACYC parent molecules containing four trisaccharide repeat units substituted with zero, one, two, three, and four O-acetyl groups (Table 2). The calculated mass of a linear ECA polysaccharide containing four trisaccharide repeat units and lacking O-acetyl substituents is 2,448 Da.
TABLE 2.
MALDI-TOF MS analysis of ECACYC molecules purified by reverse-phase HPLC
| Peak | Retention time (min) | Molecular ion [M—H]− (Da)
|
Proposed structure of parent moleculea | |
|---|---|---|---|---|
| Observedb | Predicted | |||
| 1 | 79 | 2,428.9 ± 1 | 2,429 | [ECACYC]4 |
| 2 | 99 | 2,471.3 ± 1 | 2,471 | [ECACYC]4 + 1 O-acetyl substituent |
| 3 | 119 | 2,515.2 ± 1 | 2,513 | [ECACYC]4 + 2 O-acetyl substituents |
| 4 | 143 | 2,555.2 ± 1 | 2,555 | [ECACYC]4 + 3 O-acetyl substituents |
| 5 | 160 | 2,597.5 ± 1 | 2,597 | [ECACYC]4 + 4 O-acetyl substituents |
[ECACYC]4 refers to a molecule of cyclic ECA containing four trisaccharide repeat units.
Values are means±standard deviations.
Further support for the conclusion that ECACYC molecules in peaks 2, 3, 4, and 5 are O acetylated was provided by treatment of the ECACYC preparation described above with mild alkali. This treatment resulted in the loss of these peaks and was accompanied by a pronounced increase in the amount of ECACYC molecules devoid of O-acetyl groups in peak 1 (Fig. 2C).
Subcellular localization and the cellular content of ECACYC molecules.
Previous estimates of the amount of ECACYC in cells of E. coli B using indirect methods indicated that cells grown overnight in broth contain approximately 2 μg ECACYC per milligram of dried cells (12). Assuming that ECACYC molecules have an average molecular mass of 2,513 Da, this amount corresponds to approximately 795 pmol of ECACYC per milligram (dry weight) of cells. The ECACYC content in cell extracts prepared from E. coli K-12 cells (strain PR4227) that were grown overnight in broth was determined by direct measurement of the amount of de-O-acetylated ECACYC present in extracts following treatment with mild alkali. These results were in excellent agreement with the data obtained from earlier estimates; cell extracts contained approximately 2 μg of ECACYC per milligram (dry weight) of cells (Table 3). Examination of subcellular fractions revealed that approximately 80% of the ECACYC was released from the cells by osmotic shock, and essentially no ECACYC was found in the cytoplasm. The inability to quantitatively recover ECACYC in the soluble material released by osmotic shock is most likely due to losses incurred during the preparation of this fraction. In addition, no ECACYC was detected in cell-free culture filtrates (data not shown). These observations support the conclusion that ECACYC resides exclusively in the periplasm.
TABLE 3.
Amount of ECACYC in cell fractions
Fractions were obtained from strain PR4227 grown in LB broth for 12 h at 37°C. The material contained in the fractions was treated with mild alkali and subsequently analyzed by reverse-phase HPLC. Accordingly, the stated values represent the amount of de-O-acetylated ECACYC in each of the fractions. The quantification of ECACYC is described in Materials and Methods.
Cell extracts were prepared as described in Materials and Methods.
Periplasmic and cytoplasmic fractions were obtained as described in Materials and Methods.
The synthesis of ECACYC was not restricted to exponentially growing cells. Significant synthesis occurred in cells in the late exponential phase of growth and continued for a prolonged period after the cells had entered stationary phase (Fig. 3). Similar results were obtained with cells grown in M9-glucose minimal medium and proteose peptone beef extract broth (data not shown).
FIG. 3.
Synthesis and accumulation of ECACYC in strain PR4227 (wild type, W3110/pBR322) as a function of growth stage. ▪, absorbance at 600 nm; ○, nanomoles of ECACYC per 100 ml of culture. Additional details are provided in “Materials and Methods.”
The assembly of ECACYC requires the wzyE gene.
Lipid III is a precursor for the synthesis of both ECACYC and the linear polysaccharide chains of ECAPG (12). The available evidence supports the conclusion that lipid III is synthesized on the inner face of the cytoplasmic membrane and subsequently translocated en bloc across the membrane to the periplasmic face, where it is utilized for the assembly of the linear polysaccharide chains of ECAPG. Recent experiments have provided support for the conclusion that the transbilayer movement of lipid III across the cytoplasmic membrane is mediated by a translocase or “flippase” encoded by the wzxE gene (35). Subsequent assembly of ECA polysaccharide chains occurs by the “block polymerization” mechanism described for the assembly of wzy-dependent O-antigen polysaccharides (17), and this reaction is catalyzed by a polymerase encoded by the wzyE gene.
The specific steps involved in the utilization of lipid III for the assembly of ECACYC molecules remain to be established. Thus, initial experiments were conducted to determine if the synthesis of ECACYC was dependent on the polymerase WzyE. Previous attempts to address this question have been hampered by the loss of cell viability that accompanies the insertional inactivation of wzyE; this phenotype is believed to be due to the accumulation of lipid III in the cytoplasmic membrane (unpublished results). However, an experimental approach to this problem was made possible by the construction of strains that possessed null mutations in both wecG and wzyE but that were unaltered in cell growth when they were grown under specific conditions. Null mutations in wecG preclude the synthesis of ECACYC and ECAPG due to the inability of these mutants to synthesize lipid III (Fig. 1). However, experiments with strain PR4214 (wecG::Tn10/pRL163 [with wild-type wecG under the control of the PBAD promoter]) revealed that the “leaky” or basal-level expression of wild-type wecG from the PBAD promoter in the absence of exogenously supplied arabinose is sufficient to rescue the ability of this strain to synthesize both ECACYC and ECAPG (Table 4). Normal growth accompanied the basal level expression of wild-type wecG from the PBAD promoter in strain PR4216, a derivative of strain PR4214 that also possesses a null mutation in wzyE; however, synthesis of both ECACYC and ECAPG were abolished. Presumably, the basal level of wecG expression results in the synthesis of sufficient amounts of lipid III to support the synthesis of ECACYC and ECAPG in strains that possess a wild-type wzyE allele. However, this level of wecG expression does not appear to result in the accumulation of deleterious amounts of lipid III in mutants lacking a functional wzyE gene product. Experiments using strain PR4217 (wecG::Tn10 wzyE::gm/pRL164) revealed that the inability to synthesize ECACYC and ECAPG was complemented by the basal-level expression of the wild-type wzyE and wecG alleles from a common PBAD promoter on plasmid pRL164. The amount of ECACYC synthesized by strain PR4217 was approximately fourfold greater than that observed in strain PR4214, and this most likely reflects a gene dosage-related increase in WzyE activity due to the elevated copy number of the pBAD102/D-TOPO vector. These data support the conclusion that a functional polymerase (WzyE) is required for the assembly of ECACYC.
TABLE 4.
ECACYC synthesis in E. coli K-12 strains containing null mutations in the wzyE, wzzE, and wzxE genes
| Enzyme | Strain | Relevant genotype | Synthesis of ECAPGa | Concn of ECACYCb (nmol/mg protein) |
|---|---|---|---|---|
| Polymerase (WzyE) | W3110 | Wild type | + | 3.69 |
| PR4237 | Like W3110, but wecG::Tn10 | − | ND | |
| PR4214 | wecG::Tn10/pRL163 (wild-type wecG under the control of the pBAD promoter) | + | 5.14 | |
| PR4216 | wecG::Tn10 wzyE::gm/pRL163 (wild-type wecG under the control of the pBAD promoter) | − | ND | |
| PR4217 | wecG::Tn10 wzyE::gm/pRL164 (wild-type wzyE and wecG under the control of the same pBAD promoter) | + | 19.7 | |
| PR4224 | wecG::Tn10/pBAD102/D/lacZ | − | ND | |
| Chain length regulator | PR4220 | Like W3110, but wzzE::km | + | ND |
| (WzzE) | PR4219 | wecG::Tn10 wzzE::km/pRL163 (wild-type wecG under the control of the pBAD promoter) | + | ND |
| PR4221 | wzzE::km/pCA53 (wild-type wzzE) | + | 2.97 | |
| PR4226 | wzzE::km/pBR322 | + | ND | |
| Translocase (WzxE) | Sθ874 | Δ(wzxO16wzxC) | + | 2.92 |
| PR4210 | Like Sθ874, but wecA::km wzxE::cm/pRL147 (wild-type wecA under the control of the pBAD promoter), pRL165 (wild-type wzxE under the control of the lac promoter) | + | 2.84 | |
| PR4236 | Like Sθ874, but wecA::km wzxE::cm/pRL147 (wild-type wecA under the control of the pBAD promoter), pRL166 (wild-type wzxC under the control of the lac promoter) | + | 1.51 | |
| PR4233 | Like Sθ874, but wecA::km wzxE::cm/pRL147 (wild-type wecA under the control of the pBAD promoter), pRL169 (wild-type wzxO16 under the control of the lac promoter) | + | 1.92 | |
| PR4225 | Like Sθ874, but wecA::km wzxE::cm/pACYC184 | − | ND | |
| PR4223 | Like W3110, but wecG::Tn10 wzxE::cm/pRL163 (wild-type wecG under the control of the pBAD promoter) | + | 3.9 |
Determined by passive hemagglutination assay as described in Materials and Methods.
Amount in cell extracts. The preparation of cell extracts and the quantification of ECACYC are described in detail in Materials and Methods. ND, not detected.
The assembly of ECACYC requires the wzzE gene.
WzyE-mediated polymerization of the linear chains of ECAPG occurs by a mechanism that involves the modulation of polysaccharide chain length to yield a modal population of polymers, and this modulation is mediated by the wzzE gene product (2). Null mutations in wzzE do not block the synthesis of ECAPG molecules, but they result in the synthesis of ECAPG molecules having a random nonmodal distribution of polysaccharide chain lengths (2). In contrast to the synthesis of ECAPG, the synthesis of ECACYC was abolished in strains PR4219 and PR4220, both of which possess an insertion of a nonpolar cassette carrying the aphA-3 kanamycin resistance determinant of Enterococcus faecalis (28) in the wzzE gene (Table 4). As shown for strain PR4221 (wzzE::km/pCA53) (Table 3), the defect in ECACYC synthesis due to the null mutation in wzzE was rescued by expression of the wild-type wzzE allele in plasmid pCA53. These results indicate that wzyE-mediated polymerization of ECA trisaccharide repeat units must be accompanied by wzzE-mediated chain length regulation as a prerequisite for the assembly of ECACYC molecules.
The assembly of ECACYC requires the wzxE gene.
The dependence of ECACYC assembly on functional wzyE and wzzE gene products supports the conclusion that the assembly of ECACYC molecules from lipid III occurs on the periplasmic face of the cytoplasmic membrane. Therefore, this assembly process should be dependent on the transbilayer movement of lipid III. Accordingly, attempts were made to examine the role of wzxE in the assembly of ECACYC. Previous such attempts have been severely hampered by the lethal phenotype of wzxE null mutants. Thus, the arabinose-induced expression of wild-type wecA in E. coli PR4180 [Δ(wzxO16 wzxC) wecA::Tn10 wzxE::cm/pRL147, wild-type wecA under the control of the PBAD promoter] resulted in a pronounced loss of cell viability, and this was accompanied by the accumulation of lipid III (35). However, normal cell growth, synthesis of ECAPG, and synthesis of wild-type levels of ECACYC occurred following expression of both the wild-type wecA and wzxE genes under the control of the PBAD and lac promoters, respectively, in a similar strain, strain PR4210 [Δ(wzxO16 wzxC) wecA::Tn10 wzxE::km/pRL147 pRL165 (Table 4)]. This observation is in agreement with the conclusion that WzxE functions as a translocase for the transbilayer movement of lipid III across the cytoplasmic membrane, where it is subsequently utilized for the synthesis of ECACYC.
It was somewhat surprising to observe that null mutations in wzxE did not affect the cell viability or the synthesis of ECAPG and ECACYC in strain PR4223 (wecG::Tn10 wzxE::cm/wild-type wecG under the control of the PBAD promoter) following the expression of wecG (Table 4). However, unlike strain PR4180, which was derived from parental strain Sθ874 [Δ(wzxO16 wzxC)] (Table 1), strain PR4223 was derived from parental strain W3110, which possesses wild-type wzxO16 and wzxC genes; these genes encode the putative translocases involved in the assembly of the O16 O antigen and colanic acid, respectively. These observations suggested the possibility that either WzxO16 or WzxC or perhaps both of these putative translocases are able to mediate the transbilayer movement of lipid III in the absence of functional WzxE. Indeed, as shown by the data obtained with strain PR4236, expression of the wild-type wzxC gene was able to complement the defects in cell viability, synthesis of ECAPG, and synthesis of ECACYC due to the null mutation in wzxE (Table 4). In addition, similar data were obtained with strain PR4233 as a result of the expression of the wild-type wzxO16 gene in the same genetic background. In both cases, the synthesis of ECACYC was not fully restored to wild-type levels; however, these data nevertheless suggest that both WzxC and WzxO16 are able to mediate the translocation of lipid III across the cytoplasmic membrane.
Occurrence of ECACYC in other gram-negative enteric organisms.
The lack of a convenient method for the detection of ECACYC is largely responsible for the fact that ECACYC has been demonstrated only in the cell extracts of a few gram-negative organisms (6, 11, 22, 42, 43). Accordingly, the possibility that it is present in all members of the Enterobacteriaceae has remained open. The recent demonstration of ECACYC in cell extracts of E. coli B and E. coli K-12 (12) suggested the possibility of its occurrence among other members of the Enterobacteriaceae. A limited survey of other gram-negative enteric bacteria using the assay described here revealed the occurrence of ECACYC in all of the organisms examined (Table 5). However, ECACYC was not detected in the nonenteric gram-negative organism Pseudomonas aeruginosa.
TABLE 5.
Presence of ECACYC in Gammaproteobacteriaa
| Organism | ECACYC |
|---|---|
| Salmonella enterica serovar Typhimurium | + |
| Shigella dysenteriae | + |
| Shigella flexneri | + |
| Enterobacter cloacae | + |
| Proteus vulgaris | + |
| Klebsiella pneumoniae | + |
| Pseudomonas aeruginosa | − |
The presence or absence of ECACYC in cell extracts of organisms was determined by reverse-phase HPLC as described in Materials and Methods.
DISCUSSION
ECACYC has been reported to occur in only a few gram-negative enteric organisms owing to the lack of a convenient assay for its detection. The limited survey described here revealed the presence of ECACYC in all gram-negative enteric bacteria examined. In addition, inspection of complete bacterial genome sequences contained in the National Center for Biotechnology Information database revealed that the wec gene cluster is present in all members of the Enterobacteriaceae; however, it is not present in other Gammaproteobacteria. Thus, there exists the potential for synthesis of ECACYC in many, if not all, gram-negative enteric bacteria, and it appears likely that it is unique to these organisms.
It is interesting to note that the degree of polymerization of ECACYC molecules obtained from various gram-negative enteric organisms does not appear to be uniform. Thus, ECACYC molecules obtained from Shigella sonnei phase I have been reported to contain four to six trisaccharide repeat units (11), whereas those of E. coli B and E. coli K-12 uniformly contain four repeat units (12; this study). However, the basis for this variation is not understood.
It has been previously established that the GlcNAc residues in the linear polysaccharide chains of ECAPG are nonstoichiometrically O acetylated at position 6 (11, 22). Structural analyses of ECACYC molecules obtained from E. coli B and E. coli K-12 revealed that they are also O acetylated to various degrees (12). Accordingly, when these organisms were grown in broth cultures, they synthesized ECACYC molecules that are substituted with from zero to four O-acetyl groups. Although the location of these O-acetyl groups has not been determined, it seems probable that they are also located at position 6 of GlcNAc residues. In this regard, both the degree to which the ECACYC molecules of E. coli K-12 are O acetylated and the total amount of ECACYC synthesized by cells appear to be highly dependent on the composition of the culture medium in which the cells are grown. The synthesis of molecules containing one to four O-acetyl groups occurs to a much greater degree when cells are grown in M9-glucose minimal medium than when cells are grown in rich broth (unpublished results). In contrast, the synthesis of all ECACYC species is significantly reduced when cells are grown in MOPS (morpholinepropanesulfonic acid)-glucose minimal medium (13). The basis for the relationship between ECACYC synthesis and the composition of the growth medium remains to be established.
Previous studies established that lipid III is used for the synthesis of both ECAPG and ECACYC (12). The assembly of ECAPG involves translocation of lipid III across the cytoplasmic membrane by the flippase WzxE and its subsequent utilization as a substrate for WzyE-mediated “block polymerization” of trisaccharide repeat units (Fig. 1). In addition, the polymerization process is accompanied by the modulation of polysaccharide chain lengths by WzzE to yield a modal population of linear polymers comprised of approximately 1 to 14 repeat units with an apparent modal value of 5 to 7 (2). The linear polysaccharide chains are then transferred from the lipid carrier to an as-yet-unidentified diacylgycerol-containing acceptor to yield ECAPG; however, the mechanism of this reaction remains to be established. Data obtained in the current study demonstrate that ECACYC is also assembled by a mechanism that requires WzxE- and WzyE-mediated transmembrane translocation and polymerization, respectively. Figure 4 depicts a proposed model for the assembly of ECACYC that is based on the currently available data. Accordingly, following the WzxE-mediated translocation of lipid III across the cytoplasmic membrane, subsequent reactions involve the WzyE-mediated polymerization of trisaccharide repeat units and cyclization. Nothing is known concerning the mechanism of the cyclization reaction or the enzyme that catalyzes this reaction. It is possible that cyclization involves the formation of an O-glycosidic linkage as a result of the transfer of the potential reducing terminal GlcNAc residue from an undecaprenylpyrophosphate-linked linear polysaccharide chain to the nonreducing terminal Fuc4NAc residue of the same chain. As previously stated, the ECACYC molecules synthesized by E. coli B and E. coli K-12 uniformly contain four trisaccharide repeat units (12). Thus, in the case of the mechanism proposed above, close coordination between the cyclization and polymerization reactions would be necessary in order to maintain stringent control of the degree of polymerization. Although this model predicts that lipid III is a precursor of ECACYC, a direct precursor-product relationship has not yet been demonstrated.
FIG. 4.
Proposed model for the assembly of ECACYC in Escherichia coli K-12. It is proposed that WzxE mediates the translocation of lipid III to the periplasmic face of the cytoplasmic membrane. The transmembrane translocation of lipid III is followed by the WzyE catalyzed “block polymerization” of ECA trisaccharide repeat units and cyclization to yield ECACYC molecules containing four repeat units. The assembly of ECACYC from lipid III also appears to be dependent on a functional polysaccharide chain length regulator (WzzE); however, the role of WzzE in the assembly process has not been established. The cyclization reaction presumably involves synthesis of an intramolecular glycosidic linkage between the potential reducing terminal GlcNAc residue of Fuc4NAc-ManNAcA-GlcNAc-(Fuc4NAc-ManNAcA-GlcNAc)2-Fuc4NAc-ManNAcA-GlcNAc-P-P-Und and the hydroxyl group in position 3 of the nonreducing terminal Fuc4NAc residue, thus resulting in the release of undecaprenylpyrophosphate. Neither the putative enzyme that catalyzes this reaction nor its structural gene has been identified. It is possible that the cyclization reaction is dependent on the formation of a complex between the putative “cyclase,” WzyE and WzzE. Indeed, this complex may also include WzxE. Alternatively, it is possible that WzyE or WzzE is bifunctional and is able to catalyze the cyclization reaction as well as polymerization or chain length regulation, respectively. In either case, the assembly of ECACYC from lipid III may be dependent on the formation of a membrane-bound complex that includes some or all of these proteins.
Quite surprisingly, our studies revealed that synthesis of ECACYC is dependent on WzzE, the modulator of ECA polysaccharide chain length. Although two models have been proposed for how Wzz proteins function to regulate the chain length of lipopolysaccharide O antigens (5, 30), essentially nothing is known concerning the mechanism of this process. Similarly, the mechanism involved in the WzzE-mediated modulation of the chain lengths of ECAPG polysaccharides remains to be established. Null mutations in wzzE result in a random nonmodal distribution of ECAPG polysaccharide chains in which the amount of polymer of a given chain length appears to be inversely related to chain length (2). Thus, although ECAPG molecules containing polysaccharides comprised of four repeat units are relatively more abundant than ECAPG molecules with polysaccharides having greater degrees of polymerization, the abundance of ECAPG molecules, regardless of their polysaccharide chain length, appears to be dramatically reduced in wzzE null mutants. Accordingly, it is possible that the cyclization reaction involved in ECACYC synthesis is essentially abolished in wzzE null mutants as a result of a decreased availability of undecaprenylpyrophosphate-linked polysaccharide substrate. Alternatively, the activity of the enzyme responsible for the cyclization reaction may be dependent on the formation of a complex between WzyE and WzzE. Indeed, this complex may also include WzxE. As previously stated, neither the enzyme that catalyzes the cyclization reaction nor its structural gene has been identified. The available data do not exclude the possibility that either WzyE or WzzE is bifunctional and is able to catalyze the cyclization reaction as well as polymerization or modulation of polysaccharide chain length, respectively.
Previous studies determined that the flippases involved in the assembly of Wzy-dependent O antigens appear to have relaxed specificities for the structure of the oligosaccharide moiety of their substrates (14). This conclusion is supported by the data presented in this study that demonstrate that the WzxC and WzxO16 translocators involved in the assembly of colanic acid and the O16 O antigen of E. coli, respectively, also appear able to translocate lipid III across the cytoplasmic membrane in vivo. However, the extent to which these translocators function in vivo for lipid III transbilayer movement is not clear. In contrast, the available data suggest that the substrate specificity of WzxE is rather stringent. Thus, WzxE appears to function only in vivo for the translocation of lipid III.
The dependence of ECACYC synthesis on WzxE, WzyE, and WzzE clearly support the conclusion that this polymer is assembled in the periplasm. The data presented here also support the conclusion that ECACYC resides in the periplasm; it was not detected in either in the cytoplasm or in culture filtrates. It is not known at what stage in the assembly of ECACYC that the O acetylation of GlcNAc residues occurs. It seems likely that acetyl-coenzyme A is the donor of acetyl substituents, and it is possible that these groups are transferred to lipid III molecules prior to their WzxE-mediated translocation across the cytoplasmic membrane. Alternatively, O acetylation of nascent or completed ECACYC molecules on the periplasmic side of the membrane might occur in a manner similar to that proposed for the transfer of succinyl residues by MdoC (8). In either case, the enzyme responsible for catalyzing O acetylation remains to be identified.
It has recently been reported that the resistance of Salmonella enterica serovar Typhimurium to bile salts is dependent on the ability of cells to synthesize ECA (33). However, it was not determined if this resistance was related to the occurrence of ECAPG or ECACYC or to the presence of both forms of ECA. Data obtained in the present study revealed that the synthesis of ECACYC is abolished in null mutants that lack the chain length regulator WzzE. In contrast, the synthesis of ECAPG is not abolished in these mutants, but its assembly in these mutants occurs without chain length modulation (2). These mutants possess wild-type levels of resistance to bile salts (unpublished results). Therefore, it seems likely that the resistance of gram-negative enteric bacteria to bile salts is unrelated to the occurrence of ECACYC in the periplasm, but it appears to be due to the presence of ECAPG in the outer membrane.
The function of ECACYC in gram-negative enteric bacteria is not known. In some respects ECACYC appears to be similar to the osmoregulated periplasmic glucans (OPGs) synthesized by many gram-negative Proteobacteria (8). Although there are considerable differences in the structures of the OPGs synthesized by various species of Proteobacteria, all OPGs have the following characteristics: (i) glucose is the only carbohydrate component, (ii) all are oligosaccharides containing from 5 to 24 glucose residues that are primarily linked through β-glycosidic linkages, and (iii) they are localized exclusively in the periplasm. In addition, the OPGs of many Proteobacteria are cyclic glucans; however, several species synthesize linear glucans. Perhaps the best known of the linear OPGs are the membrane-derived oligosaccharides that were first discovered in E. coli (18). A general characteristic of the OPGs synthesized by the vast majority of Proteobacteria, regardless of their structure, is that their concentrations within the periplasm increase in response to decreases in the osmolarity of the environment (8). In this regard, the cyclic 1,2-β-glucan synthesized by Brucella abortis appears to be an exception (16). The function of OPGs has not been clearly defined, and mutants unable to synthesize OPGs display widely varied phenotypes (8). Nevertheless, the periplasmic location and cyclic structures of ECACYC and many OPGs make it tempting to speculate that they have similar functions. However, this appears unlikely since the composition and other structural features of ECACYC and cyclic OPGs differ markedly, and the concentration of ECACYC within the periplasm is considerably less than is the case for MDO and other OPGs. Assuming that E. coli cells growing in a medium of low osmolarity have a periplasmic volume of 0.31 μl per milligram (dry weight) (9), the concentration of ECACYC in the periplasm is about 2.5 mM. In contrast, the periplasmic concentration of membrane-derived oligosaccharides in cells grown in medium of low osmolarity is about 50 mM (18). Furthermore, the concentration of ECACYC within the periplasm does not appear to be affected by the osmolarity of the environment (unpublished results). Finally, the available data suggest that ECAPG and ECACYC are synthesized only in gram-negative enteric bacteria. Therefore, unlike OPGs, which are found in a wide variety of gram-negative bacteria, it appears that ECACYC serves an as-yet-unknown function that is unique to members of the Enterobacteriaceae.
Acknowledgments
This research was supported by NIGMS grant GM52882.
We acknowledge the generous gift of Escherichia coli strain 14.5 (wzxE::cm) from Paul N. Danese. We also thank Colleen Rogers, Kathy Barr, James Hsu, and Kenneth Gable for their helpful suggestions and support. Finally, we acknowledge Michael Flora for his excellent assistance in obtaining the MALDI-TOF data for this study.
REFERENCES
- 1.Acker, G., D. Bitter-Suermann, U. Meier-Dieter, H. Peters, and H. Mayer. 1986. Immunocytochemical localization of enterobacterial common antigen in Escherichia coli and Yersinia enterocolitica cells. J. Bacteriol. 168:348-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr, K., J. Klena, and P. D. Rick. 1999. The modality of enterobacterial common antigen polysaccharide chain lengths is regulated by o349 of the wec gene cluster of Escherichia coli K-12. J. Bacteriol. 181:6564-6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr, K., P. Nunes-Edwards, and P. D. Rick. 1989. In vitro synthesis of a lipid-linked trisaccharide involved in synthesis of enterobacterial common antigen. J. Bacteriol. 171:1326-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr, K., and P. D. Rick. 1987. Biosynthesis of enterobacterial common antigen in Escherichia coli. In vitro synthesis of lipid-linked intermediates. J. Biol. Chem. 262:7142-7150. [PubMed] [Google Scholar]
- 5.Bastin, D. A., G. Stevenson, P. K. Brown, A. Haase, and P. R. Reeves. 1993. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol. Microbiol. 7:725-734. [DOI] [PubMed] [Google Scholar]
- 6.Basu, S., H.-M. Kuhn, A. Neszmelyi, K. Himmelspach, and H. Mayer. 1987. Chemical characterization of enterobacterial common antigen isolated from Plesiomonas shigelloides ATCC 14029. Eur. J. Biochem. 162:75-81. [DOI] [PubMed] [Google Scholar]
- 7.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 8.Bohin, J.-P. 2000. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiol. Lett. 186:11-19. [DOI] [PubMed] [Google Scholar]
- 9.Cayley, S., B. A. Lewis, H. J. Guttman, and M. T. Record, Jr. 1991. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J. Mol. Biol. 222:281-300. [DOI] [PubMed] [Google Scholar]
- 10.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dell, A., J. Oates, C. Lugowski, E. Romanowska, L. Kenne, and B. Lindberg. 1984. The enterobacterial common antigen, a cyclic polysaccharide. Carbohydr. Res. 133:95-104. [DOI] [PubMed] [Google Scholar]
- 12.Erbel, P. J. A., K. Barr, N. Gao, G. J. Gerwig, P. D. Rick, and K. H. Gardner. 2003. Identification and biosynthesis of cyclic enterobacterial common antigen in Escherichia coli. J. Bacteriol. 185:1995-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erbel, P. J. A., R. Seidel, S. E. Macintosh, L. N. Gentile, J. C. Amor, R. A. Kahn, J. H. Prestegard, L. P. McIntosh, and K. H. Gardner. 2004. Cyclic enterobacterial common antigen: potential contaminant of bacterially expressed protein preparations. J. Biomol. NMR 29:199-204. [DOI] [PubMed] [Google Scholar]
- 14.Feldman, M. F., C. L. Marolda, M. A. Monteiro, M. B. Perry, A. J. Parodi, and M. Valvano. 1999. The activity of a putative polyisoprenol-linked sugar translocase (Wzx) involved in Escherichia coli O-antigen assembly is independent of the chemical structure of the O repeat. J. Biol. Chem. 274:35129-35138. [DOI] [PubMed] [Google Scholar]
- 15.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iannino, N. I., G. Briones, F. Iannino, and R. A. Ugalde. 2000. Osmotic regulation of cyclic 1,2-β-glucan synthesis. Microbiology 146:1735-1742. [DOI] [PubMed] [Google Scholar]
- 17.Keenleyside, W. J., and C. Whitfield. 1999. Genetics and biosynthesis of lipopolysaccharide O-antigens, p. 331-358. In H. Brade et al. (ed.), Endotoxin in health and disease. Marcel Dekker, New York, N.Y.
- 18.Kennedy, E. P. 1996. Membrane-derived oligosaccharides (periplasmic beta-d-glucans) of Escherichia coli, p. 1064-1071. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 19.Kuhn, H.-M., U. Meier-Dieter, and H. Mayer. 1988. ECA, the enterobacterial common antigen. FEMS Microbiol. Rev. 54:195-222. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn, H.-M., E. Neter, and H. Mayer. 1983. Modification of the lipid moiety of the enterobacterial common antigen by the “Pseudomonas factor.” Infect. Immun. 40:696-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lugowski, C., and E. Romanowska. 1978. Enterobacterial common antigen: isolation from Shigella sonnei, purification and immunochemical characterization. Eur. J. Biochem. 91:89-97. [DOI] [PubMed] [Google Scholar]
- 22.Lugowski, C., E. Romanowska, L. Kenne, and B. Lindberg. 1983. Identification of a trisaccharide repeating-unit in the enterobacterial common antigen. Carbohydr. Res. 118:173-181. [DOI] [PubMed] [Google Scholar]
- 23.Mäkela, P. H., and H. Mayer. 1976. Enterobacterial common antigen. Bacteriol. Rev. 40:591-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannel, D., and H. Mayer. 1978. Isolation and chemical characterization of the enterobacterial common antigen. Eur. J. Biochem. 86:361-370. [DOI] [PubMed] [Google Scholar]
- 25.Mayer, H., and G. Schmidt. 1979. Chemistry and biology of the enterobacterial common antigen (ECA). Curr. Top. Microbiol. Immunol. 85:99-153. [DOI] [PubMed] [Google Scholar]
- 26.Meier-Dieter, U., K. Barr, R. Starman, L. Hatch, and P. D. Rick. 1992. Nucleotide sequence of the Escherichia coli rfe gene involved in the synthesis of enterobacterial common antigen. Molecular cloning of the rfe-rff gene cluster. J. Biol. Chem. 267:746-753. [PubMed] [Google Scholar]
- 27.Meier-Dieter, U., R. Starman, K. Barr, H. Mayer, and P. D. Rick. 1990. Biosynthesis of enterobacterial common antigen in Escherichia coli. Biochemical characterization of Tn10 insertion mutants defective in enterobacterial antigen synthesis. J. Biol. Chem. 265:13490-13497. [PubMed] [Google Scholar]
- 28.Ménard, R., P. J. Sansonettie, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Morona, R., L. Van Den Bosch, and P. A. Manning. 1995. Molecular, genetic, and topological characterization of O-antigen chain length regulation in Shigella flexneri. J. Bacteriol. 177:1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuhard, J., and E. Thomassen. 1976. Altered deoxyribonucleotide pools in P2 eductants of Escherichia coli K-12 due to deletion of the dcd gene. J. Bacteriol. 126:999-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman, A., K. Barr, and P. D. Rick. 2001. Identification of the structural gene for the TDP-Fuc4NAc:lipid II Fuc4NAc transferase involved in synthesis of enterobacterial common antigen in Escherichia coli K-12. J. Bacteriol. 183:6509-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos-Morales, F., A. I. Prieto, C. R. Beuzón, D. W. Holden, and J. Casadesdús. 2003. Role for Salmonella enterica Enterobacterial common antigen in bile resistance and virulence. J. Bacteriol. 185:5328-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rech, S., C. Wolin, and R. P. Gunsalus. 1996. Properties of the periplasmic ModA molybdate-binding protein of Escherichia coli. J. Biol. Chem. 271:2557-2562. [DOI] [PubMed] [Google Scholar]
- 35.Rick, P. D., K. Barr, K. Sankaran, J. Kajimura, J. S. Rush, and C. J. Waechter. 2003. Evidence that the wzxE gene of Escherichia coli K-12 encodes a protein involved in the transbilayer movement of a trisaccharide-lipid intermediate in the assembly of enterobacterial common antigen. J. Biol. Chem. 278:16534-16542. [DOI] [PubMed] [Google Scholar]
- 36.Rick, P. D., G. L. Hubbard, M. Kitaoka, H. Nagaki, T. Kinoshita, S. Dowd, V. Simplaceanu, and C. Ho. 1998. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology 8:557-567. [DOI] [PubMed] [Google Scholar]
- 37.Rick, P. D., H. Mayer, B. A. Neumeyer, S. Wolski, and D. Bitter-Suermann. 1985. Biosynthesis of enterobacterial common antigen. J. Bacteriol. 162: 494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rick, P. D., and R. P. Silver. 1996. Enterobacterial common antigen and capsular polysaccharides, p. 104-122. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 39.Rinno, J., J. R. Golecki, and H. Mayer. 1980. Localization of enterobacterial common antigen: immunogenic and nonimmunogenic enterobacterial common antigen-containing Escherichia coli. J. Bacteriol. 141:814-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothfield, L., M. J. Osborn, and B. L. Horecker. 1964. Biosynthesis of bacterial lipopolysaccharide. II. Incorporation of glucose and galactose catalyzed by particulate and soluble enzymes in Salmonella. J. Biol. Chem. 239:2788-2795. [PubMed] [Google Scholar]
- 41.Silhavy, T. J., M. L. Burman, and L. W. Enquist. 1984. Experiments with gene fusions, p. 107. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Staaf, M., C. Hoog, B. Stevensson, A. Maliniak, and G. Widmalm. 2001. Conformational investigation of a cyclic enterobacterial common antigen employing NMR spectroscopy and molecular dynamics simulations. Biochemistry 40:3623-3628. [DOI] [PubMed] [Google Scholar]
- 43.Vinogradov, E. V., Y. A. Knirel, J. E. Thomas-Oates, A. S. Shashkov, and L. L'Vov. 1994. The structure of the cyclic enterobacterial common antigen (ECA) from Yersinia pestis. Carbohydr. Res. 258:223-232. [DOI] [PubMed] [Google Scholar]