Abstract
The glucitol operon (gutAEBDMRQ) of Escherichia coli encodes a phosphoenolpyruvate:sugar phosphotransferase system that metabolizes the hexitol d-glucitol (sorbitol). The functions for all but the last gene, gutQ, have been previously assigned. The high sequence similarity between GutQ and KdsD, a d-arabinose 5-phosphate isomerase (API) from the 3-deoxy-d-manno-octulosonate (KDO)-lipopolysaccharide (LPS) biosynthetic pathway, suggested a putative activity, but its role within the context of the gut operon remained unclear. Accordingly, the enzyme was cloned, overexpressed, and characterized. Recombinant GutQ was shown to indeed be a second copy of API from the E. coli K-12 genome with biochemical properties similar to those of KdsD, catalyzing the reversible aldol-ketol isomerization between d-ribulose 5-phosphate (Ru5P) and d-arabinose 5-phosphate (A5P). Genomic disruptions of each API gene were constructed in E. coli K-12. TCM11[(ΔkdsD)] was capable of sustaining essential LPS synthesis at wild-type levels, indicating that GutQ functions as an API inside the cell. The gut operon remained inducible in TCM7[(ΔgutQ)], suggesting that GutQ is not directly involved in d-glucitol catabolism. The conditional mutant TCM15[(ΔgutQΔkdsD)] was dependent on exogenous A5P both for LPS synthesis/growth and for upregulation of the gut operon. The phenotype was suppressed by complementation in trans with a plasmid encoding a functional copy of GutQ or by increasing the amount of A5P in the medium. As there is no obvious obligatory role for GutQ in the metabolism of d-glucitol and there is no readily apparent link between d-glucitol metabolism and LPS biosynthesis, it is suggested that A5P is not only a building block for KDO biosynthesis but also may be a regulatory molecule involved in expression of the gut operon.
d-Arabinose 5-phosphate isomerase (API) is the first enzyme in the biosynthetic pathway of 3-deoxy-d-manno-octulosonate (KDO), a unique 8-carbon sugar component of lipopolysaccharides (LPSs). Lipopolysaccharides are amphiphilic macromolecules located in the outer leaflet of the outer membrane of gram-negative bacteria, forming an asymmetric lipid bilayer that surrounds the cell. The LPS layer is anchored in the outer membrane by the highly conserved lipid A domain and is followed by an oligosaccharide core region usually containing at least one KDO molecule that connects lipid A to an extended hypervariable repeating polysaccharide chain (O antigen) (25). In addition to providing a measure of intrinsic antibiotic resistance and defense against host responses, LPS (also referred to as endotoxin) is the main mediator of gram-negative bacterial pathogenesis (13, 33). The minimal LPS structure required for viability of Escherichia coli under laboratory conditions is KDO2-lipid A, also referred to as Re endotoxin (11, 25, 31).
API catalyzes the reversible 1,2-keto/aldol isomerization of the pentose pathway intermediate ribulose 5-phosphate (Ru5P) to d-arabinose 5-phosphate (A5P) and is the intracellular source of A5P used for the formation of KDO in gram-negative bacteria. API is encoded by the kdsD (formerly yrbH) gene in E. coli K-12, though it was speculated that other APIs may exist in E. coli based on homology searches (20). In particular, the last open reading frame of the glucitol operon GutQ has significant homology (45% identity) to KdsD. The glucitol (sorbitol) operon expresses a phosphoenolpyruvate:sugar phosphotransferase system that is responsible for the uptake and catabolism of d-glucitol from the environment (24). The operon was originally studied by Lengeler (16, 17) and subsequently by Yamada and Saier (37, 38) and is known to consist of seven genes, gut(srl)AEBDMRQ (Fig. 1). The EIIGut complex is formed by GutA (EIIC1 domain), GutE (EIIBC2 domains), and GutB (EIIA domain) and transports d-glucitol across the inner membrane and into the cell as d-glucitol 6-phosphate. d-Glucitol 6-phosphate is then further metabolized by GutD, an NADH-dependent dehydrogenase (22), to the glycolytic intermediate d-fructose 6-phosphate. Expression of the gut operon is tightly controlled by a complex multicomponent regulatory system, consisting of a transcriptional repressor (GutR) and a transcriptional activator (GutM) in addition to cyclic AMP (cAMP)-catabolite activator protein-mediated regulation (39). However, the function of GutQ remains unknown (40). As there is no obvious obligatory role for GutQ in the metabolism of d-glucitol and there is no readily apparent link between d-glucitol metabolism and KDO biosynthesis, the study of both the in vitro and in vivo function of GutQ was undertaken to address the effects of GutQ on LPS biosynthesis and d-glucitol metabolism.
FIG. 1.
The gut operon of E. coli K-12 MG1655. Genes that are highly conserved in both gram-negative and gram-positive bacterial gut operons are shaded dark gray while those that are specific to gram-negative bacteria are shaded light gray.
MATERIALS AND METHODS
Materials.
Genomic E. coli K-12 MG1655 DNA was purchased from the American Type Culture Collection (ATCC 700926D). The Promega Wizard DNA purification kit was used for plasmid purification. Chemically competent E. coli XL1-Blue (Stratagene) and E. coli BL21(DE3) (Novagen) were used for cloning and protein expression, respectively. Sugars and sugar phosphates were purchased from Sigma-Aldrich, except for d-glucitol 6-phosphate, which was prepared by the sodium tetraborohydride reduction of the d-glucose 6-phosphate (G6P) (3), purified by anion-exchange chromatography (AG MP-1; Bio-Rad), and desalted by gel filtration (Bio-Gel P-2; Bio-Rad). Protein concentrations were determined using the Bio-Rad protein assay reagent with bovine serum albumin as the standard. Strains, plasmids, and primers are listed in Table 1.
TABLE 1.
Strains, plasmids, and primers
| Strain, plasmid, or primer | Description | Source |
|---|---|---|
| BW30270 | E. coli K-12 MG1655; rph+fnr+ | CGSCc |
| TCM3 | BW30270(pGQ1) | This study |
| TCM7 | BW30270(ΔgutQ) | This study |
| TCM11 | BW30270(ΔkdsD) | This study |
| TCM15 | TCM11(ΔkdsDΔgutQ) | This study |
| TCM18 | TCM15(pGQ1) | This study |
| pGQ1 | E. coli K-12 gutQ inserted into NdeI/BamHI sites of pT7-7; Ampr | This study |
| F | GGTGCTAGAATTCATATGAGTGAAGCACTACTGAACGa | Invitrogen |
| R | GAATTCGGATCCAAGTTAAATAATCCCGGCCTGATAGAAATCCTGCb | Invitrogen |
| GQF | GATCGATGTGATCATAACCGGAGAGAGCAATGAGTGAAGCGTGTAGGCTGGAGCTGCTTC | Invitrogen |
| GQR | CGGCTGGCGAAACGTCTGGGATTGAAGGATTAAATAATCCATTCCGGGGATCCGTCGACC | Invitrogen |
| KDF | GCGATGTTGTACTGGTTATCGCCAATACTCGTTGAATAACTGGAAACGCATTGTGTAGGCTGGAGCTGCTTCG | Invitrogen |
| KDR | GCGACGCACCTGCTTTGCTCATTGTTGTTTATCCTTGAATCTTTACACTACGGATATGAATATCCTCCTTAG | Invitrogen |
| GDF | ATGAATCAGGTTGCCGTTGTC | Invitrogen |
| GDR | CACCAGATTCACCTGTAGCG | Invitrogen |
NdeI site underlined.
BamHI site underlined.
CGSC, E. coli Genetic Stock Center (CGSC no. 7925).
Cloning, overexpression, and purification of GutQ.
The gutQ gene was amplified using standard PCR methodology with the F-R primer pair (Table 1), digested with NdeI and BamHI, and directly ligated into similarly restricted linearized pT7-7 expression vector that had been treated with calf alkaline phosphatase. The ligation mixture was used to transform chemically competent E. coli XL1-Blue cells, and transformants harboring the pGQ1 plasmid were identified by restriction analysis and sequenced. E. coli BL21(DE3) cells were transformed with plasmid pGQ1 for protein expression.
E. coli BL21(DE3)/pGQ1 cells were grown in 2× YT medium containing ampicillin (100 mg/liter) at 37°C with shaking (250 rpm). Once the culture reached the mid-log growth phase (optical density at 600 nm of ∼0.7 to 0.9), the culture was allowed to cool to 18°C before being induced with isopropyl-β-d-thiogalactoside at a final concentration of 0.4 mM. After 16 h of growth at 18°C, the cells were harvested by centrifugation (6,500 × g, 15 min, 4°C). The cell pellet was suspended in 20 ml of buffer A (20 mM Tris-HCl, 1 mM dithiothreitol; pH 8.0) and sonicated on ice (five times for 30 seconds; 2-min pauses between pulses). Cellular debris was removed by centrifugation (29,000 × g, 40 min, 4°C), and the supernatant was filtered through a 0.22-μm Millex filter. The solution was loaded onto a Hi Load (16/10) Q-Sepharose fast flow column that had been preequilibrated with buffer A. Protein was eluted using an 0 to 900 mM gradient of NaCl in buffer A over 120 min. Fractions containing primarily recombinant protein (∼33 kDa) as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were pooled. A saturated solution of ammonium sulfate was slowly added with stirring at room temperature until 15% saturation was reached. The solution was clarified by centrifugation (29,000 × g, 30 min, 22°C), and the supernatant was brought to 30% saturation. The protein pellet was collected by centrifugation (29,000 × g, 30 min, 22°C), resuspended in buffer A, and dialyzed against 2 liters of buffer A overnight at 4°C. Preparations were greater than ∼95% homogeneous as judged by SDS-PAGE with a yield of 180 mg recombinant GutQ/liter of cell culture.
Gel electrophoresis.
SDS-PAGE was performed on protein samples (∼5 to 10 μg) under reducing conditions on a 12% polyacrylamide gel, and gels were stained with 0.25% Coomassie brilliant blue R-250 solutions. LPS samples were analyzed by Tricine SDS-PAGE (stacking, 4% T, 3% C; separating, 16.5% T, 6% C) (19) and visualized by silver staining (14).
Enzyme assays.
API activity was determined by the discontinuous cysteine-carbazole colorimetric assay adapted to 96-well microplates as previously described (20). All plates contained internal Ru5P standards and appropriate A5P controls in triplicate.
A second, more sensitive coupled assay was developed to determine API activity in crude cell extracts that utilized 3-deoxy-d-manno-octulosonate 8-phosphate synthase (KdsA) from Arabidopsis thaliana (34). This enzyme catalyzes the irreversible stereospecific condensation of A5P and phosphoenolpyruvate to form 3-deoxy-d-manno-octulosonate 8-phosphate (KDO8P) and inorganic phosphate. Reaction mixtures containing 5 μl of a purified KdsA solution (3 mg/ml; 10 U/mg), 10 mM Ru5P, 6 mM phosphoenolpyruvate, and 1 mM EDTA in 40 μl of 100 mM Tris-HCl (pH 8.25) were incubated for 3 min at 37°C. The reaction was initiated by the addition of 10 μl of cell extract. After 5 min, reactions were quenched by adding 50 μl of 10% (wt/vol) trichloroacetic acid. The amount of KDO8P produced was determined by the Aminoff periodate-thiobarbituric acid assay (29). Under these conditions, KdsA was not rate limiting in the formation of KDO8P.
d-Glucitol 6-phosphate dehydrogenase (GutD) activity was measured using a continuous spectrophotometric assay by monitoring the formation of NADH at 340 nm (22). Enzyme solutions (100 mM Tris-HCl, pH 8.7, 5 mM NAD+) were preincubated at 25°C for 2 min before the reactions were initiated by the addition of d-glucitol 6-phosphate at a final concentration of 20 mM.
Characterization of GutQ.
The characterization of GutQ was similarly performed according to methods reported for KdsD (20). Briefly, for substrate specificity enzyme samples were diluted in 100 mM Trizma-HCl buffer (pH 8.25) and assayed by initiating the reaction with substrate (15 nM GutQ, 10 mM sugar, 1 mM EDTA). After 10 min at 37°C, reactions of mixtures containing the potential substrates d-arabinose, d-ribose 5-phosphate, G6P, d-glucose 1-phosphate, d-glucosamine 6-phosphate, or d-mannose 6-phosphate were quenched, and the presence of ketose was determined. Product appearance for d/l-glyceraldehyde 3-phosphate, d-erythrose 4-phosphate, and d-fructose 6-phosphate was assayed by 31P nuclear magnetic resonance (NMR) (20). Kinetic constants were determined at 37°C using the discontinuous microplate assay and were initiated by the addition of substrate. Concentrations typically ranged from 0.2 Km to 10 Km. After 2 min, the reactions (50 mM Tris-HCl at pH 8.25, 5 nM GutQ, 1 mM EDTA) were quenched, at which point approximately less than 10% of substrate had been consumed. Initial rates were determined in triplicate and fitted to the standard Michaelis-Menten equation using nonlinear least-squares regression to determine Km and kcat values for both the formation and disappearance of Ru5P. The equilibrium constant (Keq) was determined using 31P NMR as described for KdsD (20). The pH optimum of GutQ was determined by diluting the enzyme in Bis-Tris propane buffer solutions of various pH values (pH 6.25 to 10, adjusted at 37°C). Activity was measured as outlined above in triplicate with a reaction time of 3 min (100 mM Bis-Tris propane, 15 nM GutQ, 10 mM A5P, 1 mM EDTA). Enzyme samples of GutQ as isolated were diluted in 100 mM Trizma-HCl buffer (pH 8.25) and incubated with various divalent metals or EDTA for 30 min at 4°C. Remaining activity was then assayed at 37°C under saturating substrate conditions in triplicate with a 3-min reaction time (15 nM GutQ, 10 mM A5P, 10 μM metal or EDTA).
Strain construction and growth conditions.
E. coli strain BW30270 was used as the host for chromosomal gutQ and kdsD gene disruptions using a phage λ Red recombinase system according to the procedure of Datsenko and Wanner (7). Kanamycin and chloramphenicol were used at 50 μg/ml. Primer pairs GQF-GQR and KDF-KDR with either pKD13(kan) or pKD3(cat) as template, respectively, were used to construct insert cassettes targeting gutQ and kdsD. The resistance markers were then excised using the FLP recombinase system as described previously (7), and the deletion mutants are listed in Table 1. TCM15, containing deletions in both API genes, was similarly constructed from TCM11 using the GQF-GQR PCR product insert, except that medium and plates were supplemented at all times with G6P (10 μM) and A5P (15 μM) for subsequent manipulations performed after electrotransformation. All strains used were colony purified and tested for loss of all antibiotic resistances, and the relevant locus was sequenced to confirm the expected deletion site.
Cultures were grown in either M9 minimal medium (27) or morpholinepropanesulfonic acid (MOPS) minimal medium (21) supplemented with thiamine (1 μg/ml) and the indicated carbon source(s) at 37°C with shaking (250 rpm). TCM15 cultures were additionally supplemented with G6P (10 μM) and A5P (5 to 50 μM). Ampicillin (100 μg/ml) was added to those strains carrying the pT7-7 (Ampr) plasmid.
Preparation of cellular extracts for enzymatic assays, LPS analysis, and RT-PCR.
Overnight cultures were grown in minimal medium with glycerol (0.2%) as the sole carbon source and the indicated supplements. Cultures were diluted (1:20, vol/vol) into fresh minimal medium and shaken for 2 hours at 37°C to allow the bacteria to return to exponential growth. Cultures of TCM15 were preinduced during this period to upregulate the hexose phosphate transport system (uhp) by adding A5P (5 μM) and G6P (10 μM). Cells were pelleted by centrifugation to remove traces of G6P (6,500 × g, 5 min, 22°C) and then inoculated into fresh medium. Where indicated, 10 mM d-glucitol was added to the cultures, and growth was continued for an additional 4 to 6 hours to allow upregulation of the gut operon, at which point all cultures were in early to mid-log growth. Cells were harvested by centrifugation (6,500 × g, 5 min, 4°C). Fractions to be assayed for API and GutD activity were twice washed with a chilled 1% NaCl solution and then resuspended in buffer (20 mM Tris-HCl, 1 mM dithiothreitol, pH 8.0). Cells were disrupted by sonication and clarified by centrifugation (8,000 × g, 20 min, 4°C), and the supernatant fractions were aliquoted and stored at −20°C. Culture samples for LPS analysis were washed twice with Dulbecco phosphate-buffered saline, and the cell pellets were resuspended in lysis buffer (200 mM Tris, pH 6.8, 2% SDS, 4% 2-mercaptoethanol, 10% glycerol). Equal numbers of cells based on the optical density at 600 nm were processed according to the method of Hitchcock and Brown (14). Cell pellets to be analyzed for RNA were rapidly resuspended in Max Bacterial Enhancement reagent (Invitrogen) and extracted using Trizol (Invitrogen) according to the manufacturer's protocol. RNA samples were further purified by digestion with RNase-free DNase and isolated using the RNeasy minikit (QIAGEN). The quality of the RNA was inspected by agarose gel electrophoresis and quantified by UV absorbance at 260 nm. Qualitative reverse transcription-PCR (RT-PCR) was performed using the Superscript II One-Step RT-PCR system (Invitrogen) as directed by the manufacturer with 1 pg of purified total RNA as template and GDF-GDR primers (0.2 μM) to amplify the first 342 bp of the gutD gene, and aliquots were removed for analysis after 35 cycles.
RESULTS
Purification and characterization of recombinant GutQ.
Purification to homogeneity of recombinant GutQ was achieved in two steps using Q-Sepharose anion-exchange chromatography followed by ammonium sulfate precipitation. The protein appeared as a single band by SDS-PAGE (∼33 kDa), and the specific activity was 329 U/mg with A5P as substrate. Monosaccharides that share common functionalities with A5P were tested as potential alternative substrates for GutQ. In the cysteine-carbazole colorimetric assay, 2-ketohexoses and 2-ketopentoses form purple-red chromophores which absorb light at 540 nm (8). The conversion of aldose to ketose can be observed by measuring the increase in the ratio of absorbance at 540 nm of sample to control. None of the tested sugars (d-arabinose, d-ribose 5-phosphate, G6P, d-glucose 1-phosphate, d-glucosamine 6-phosphate, and d-mannose 6-phosphate) were converted by GutQ to their respective ketose forms (data not shown). Further, the short-chain phosphorylated aldoses d/l-glyceraldehyde 3-phosphate and d-erythrose 4-phosphate as well as d-fructose 6-phosphate did not serve as alternate substrates as determined by 31P NMR (data not shown). The biochemical properties of GutQ were determined to be quite similar to those of KdsD and are summarized in Table 2. The kinetic parameters, pH optima, lack of cofactor requirement, and quaternary structure were all comparable. Within the limits of detection, GutQ is a specific phosphosugar aldol-ketol isomerase for A5P and Ru5P.
TABLE 2.
Biochemical properties of E. coli APIs
| Property | KdsDa | GutQ |
|---|---|---|
| Km (A5P) | 0.61 ± 0.06 mM | 1.2 ± 0.1 mM |
| Km (Ru5P) | 0.35 ± 0.08 mM | 0.64 ± 0.08 mM |
| Kcat (A5P to Ru5P) | 157 ± 4 s−1 | 218 ± 4 s−1 |
| Kcat (Ru5P to A5P) | 255 ± 16 s−1 | 242 ± 11 s−1 |
| Keq (calc.)b | 0.47 (0.35) | 0.47 (0.48) |
| Optimum pH | 8.4 | 8.25 |
| Equivalent of Zn2+/subunitc | 1.0 ± 0.1 | 1.4 ± 0.2 |
| Inhibition by 10 μM Zn2+d | Yes | Yes |
| Activation by EDTAe | Yes | Yes |
| Subunit mol. mass (calc.)f | 35,104 Da (35,196 Da) | 33,909 Da (34,031 Da) |
| Native mol. massg | 122 ± 5 kDa (tetramer) | 133 ± 4 kDa (tetramer) |
Data from reference 20.
Measured by 31P NMR (calculated [calce] from Haldane relationship [Ru5P]/[A5P].
Equivalents of Zn2+ per monomer as determined by high-resolution inductively coupled plasma-mass spectrometry.
Less than 5% activity remaining.
As isolated enzyme with 10 μM EDTA.
Molecular mass determined by electrospray ionization mass spectrometry, calculated from protein sequence.
Molecular mass determined by gel filtration.
GutQ is capable of sustaining lipopolysaccharide biosynthesis.
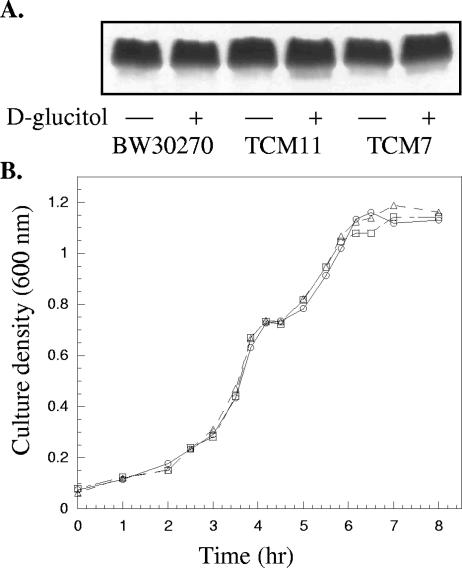
To assess the ability of GutQ to function as an API in vivo, TCM7 and TCM11 were constructed using a λ Red (γ, β, exo) homologous recombination system (7). Neither mutation was lethal, indicating the likely presence of multiple API-encoding genes that can provide sufficient quantities of A5P for LPS biosynthesis. LPS gel analysis indicated nearly equal amounts of the wild-type K-12 LPS core regardless of whether the gut operon was induced (Fig. 2A), indicating that A5P synthesis was not rate limiting in any of the strains under these growth conditions. Basal levels of GutQ (2 nmol/min/mg in comparison with 14 nmol/min/mg; see Table 3) in TCM11 were adequate to supply enough A5P to sustain viability and elaborate a functional LPS layer.
FIG. 2.
The effect of GutQ on LPS biosynthesis and d-glucitol utilization. (A) Silver-stained Tricine SDS-PAGE LPS gels of proteinase K-treated whole-cell lysates from BW30270, TCM7, and TCM11. Equal amounts of bacterial cells growing in minimal medium (0.2% glycerol) with (+) or without (−) d-glucitol (10 mM) were harvested in early log phase and processed as described in Materials and Methods. (B) Diauxic growth curves for BW30270 (□), TCM7 (○), and TCM3 (▵). Overnight cultures grown in M9 minimal medium supplemented with 1 μg/ml thiamine and 10 mM d-glucose were diluted into fresh medium with 2 mM d-glucose and 2 mM d-glucitol as dual carbon sources. Cell growth was monitored by measuring the turbidity at 600 nm.
TABLE 3.
Specific activity of GutD and API
| E. coli straina | d-Glucitolb | GutD activityc | API activityc,d |
|---|---|---|---|
| BW30270 | − | <1 | 14 ± 3 |
| + | 242 ± 14 | 48 ± 5 | |
| TCM7 | − | <1 | 13 ± 3 |
| + | 374 ± 13 | 15 ± 2 | |
| TCM11 | − | <1 | 2 ± 1 |
| + | 581 ± 48 | 46 ± 5 | |
| TCM3 | − | <1 | 2,573 ± 78 |
| + | 323 ± 28 | 2,457 ± 117 |
Strains were grown in M9 minimal medium with 0.2% glycerol as carbon source.
d-Glucitol was added at 10 mM to the cultures where indicated (+) 4 hours before harvesting.
Specific activity reported in nmoles/min/mg.
Values include KdsD and/or GutQ.
LPS biosynthesis in ΔAPI strain TCM15.
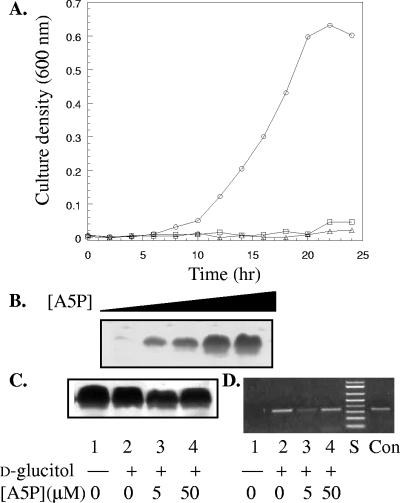
Both gutQ and kdsD genes were able to be disrupted by using the G6P-inducible hexose phosphate transporter to supply exogenous A5P. A5P is a high-affinity, though noninducing, substrate of the hexose phosphate transport system (uhp) (10). MOPS minimal medium, which has a low concentration of inorganic phosphate (1.3 mM), was used to prevent inhibition of uhp-mediated transport by inorganic phosphate (28). The natural substrate of the uhp system G6P was required for efficient induction and transport of A5P into the cells. A5P or G6P alone was unable to restore growth, as there was no detectable growth unless both A5P and G6P were included in the medium (Fig. 3A). GutQ and KdsD are thus the sole intracellular sources of A5P for KDO synthesis. Further, d-arabinose could not substitute for A5P (data not shown). Overnight cultures from which A5P had been exhausted from the medium were used to inoculate fresh cultures supplemented with various concentrations of A5P in order to enable lipopolysaccharide biosynthesis. After an extended 6-hour incubation period, the LPS levels corresponded to the concentration of A5P in the medium (Fig. 3B).
FIG. 3.
Growth and LPS synthesis in the ΔAPI strain TCM15. (A) Growth curve of E. coli TCM15 in MOPS minimal medium with thiamine (1 μg/ml) and glycerol (0.1%) as sole carbon source. Sugar phosphates were supplemented in the medium with either 10 μM G6P (▵), 15 μM A5P (□), or both (○). (B) Correlation of LPS with concentration of A5P. A stationary-phase culture grown in MOPS minimal medium (0.2% glycerol, 5 μM A5P, 10 μM G6P) that had ceased dividing was diluted into fresh medium containing G6P (10 μM) and various concentrations of A5P (0.1, 1, 10, 50, and 100 μM) and shaken for 6 h. LPS samples were prepared from the same number of cells based on optical density at 600 nm and analyzed by Tricine SDS-PAGE and silver staining. (C and D) LPS Tricine SDS-PAGE (C) and qualitative RT-PCR transcript analysis (D) of gutD in samples prepared from wild-type BW30270 (lanes 1 and 2) and TCM15 (lanes 3 and 4). TCM15 was preinduced with 10 μM G6P and 5 μM A5P, pelleted, resuspended in fresh MOPS minimal medium (0.2% glycerol) with only A5P and 10 mM d-glucitol as indicated, and shaken for an additional 4 h before harvesting for analyses. Lane S, 0.1- to 1-kb DNA molecular size markers; lane Con, genomic DNA as template.
Expression of the gut operon.
BW30270, TCM7, and TCM3 were grown in M9 minimal medium containing dual carbon sources, d-glucose and d-glucitol. All three strains grew at nearly identical rates (Fig. 2B) and exhibited the characteristic unusually long diauxic lag time of approximately 40 min after d-glucose had been exhausted from the medium (18). Under these conditions, induction does not appear to be influenced by the levels of GutQ. Strains BW30270, TCM7, and TCM11 were grown in M9 minimal medium with glycerol as the carbon source. Total API (KdsD and/or GutQ) and GutD specific activities were measured in all three strains (Table 3). The gut operon in both TCM7 and TCM11 remained inducible, with only an approximately two- to threefold difference in degree of induction as estimated by GutD activity compared to the isogenic wild-type BW30270 strain. API activity levels increased in both BW30270 and TCM11 when d-glucitol was added to the medium, indicating that GutQ was upregulated along with GutD. As expected, there was no change in observed API levels in TCM7 upon the addition of d-glucitol, though the strain remained capable of upregulating GutD. The majority of API activity (∼90%) is attributable to KdsD in medium lacking d-glucitol, confirming the identification of KdsD as the constitutively expressed LPS pathway enzyme. TCM3 was used to investigate the effect of elevated API levels on the gut operon (Table 3). API specific activity was increased ∼250-fold, but no appreciable difference was observed in GutD levels, as the operon remained repressed unless d-glucitol was provided in the medium.
A5P may be necessary for upregulation of the gut operon.
Since no difference was observed in the regulation of the gut operon when a single API gene was disrupted, failure to directly observe the effect may have been due to suppression by the second copy of API. The inducibility of the gut operon was investigated in TCM15. Overnight cultures were grown in MOPS minimal medium (0.2% glycerol, 15 μM A5P, 10 μM G6P) and diluted into fresh medium (0.2% glycerol, 5 μM A5P, 10 μM G6P) to return the cells to exponential growth. After 2 h of shaking, the cells were harvested and used to inoculate medium containing only glycerol and A5P. Since the cells were preinduced for the uhp transporter genes, no G6P was added. Two concentrations of A5P (5 and 50 μM) were chosen so that differences in the level of LPS and growth rates were minimal during the time course of the experiment. At 50 μM A5P, GutD remained inducible to near-wild-type levels (Table 4). The GutQ protein itself is not necessary for expression of the operon. When the A5P concentration was decreased to 5 μM, there was a marked and reproducible decrease in GutD activity in d-glucitol-grown cells. The level of LPS, however, was only slightly decreased in comparison (Fig. 3C). This suggests a correlation between A5P levels and the amount of GutD and suggests that the difference is not due to the consequence of pleiotropic effects stemming from a depleted LPS layer. Analysis of the expression level of the GutD gene indicated that the decrease in measured specific activity of GutD was correlated with the amount of amplifiable mRNA (Fig. 3D). The gut operon was inducible under the same growth conditions with 5 μM A5P when complemented by a plasmid encoding GutQ.
TABLE 4.
Specific activity of GutD in ΔAPI strains
| E. coli straina | d-Glucitolb | A5P (μM) | GutD activityc | API activityc |
|---|---|---|---|---|
| TCM15 | − | 50 | <1 | NDd |
| + | 50 | 278 ± 33 | ND | |
| − | 5 | <1 | ND | |
| + | 5 | 9.8 ± 1 | ND | |
| TCM18 | − | 5 | <1 | 1,366 ± 180 |
| + | 5 | 356 ± 27 | 976 ± 101 |
Strains were grown in MOPS minimal medium with 0.2% glycerol and preinduced with 10 μM G6P-5 μM A5P.
d-Glucitol was added at 10 mM to the cultures where indicated (+) 4 hours before harvesting.
Specific activity reported in nmoles/min/mg.
ND, no activity detected.
DISCUSSION
The essential nature of LPS in most gram-negative bacteria has attracted much attention towards developing specific antibiotics targeting enzymes involved in its synthesis. Substantial progress in elucidating the genes of the LPS biosynthetic pathway has been achieved, particularly with KDO, for which each gene has been assigned (25, 32, 35). The minimal lipopolysaccharide structure required for viability in E. coli is lipid A-KDO2 (25, 31). However, the construction of the viable mutant TCM11 from this pathway prompted further investigation of the function of other API homologues and their potential relationship to LPS biosynthesis in E. coli.
Homology searches identified GutQ as a prime candidate based on the high degree of sequence similarity to KdsD (20, 32). Further, GutQ shares the same domain architecture with KdsD, namely, an N-terminal catalytic isomerase domain followed by a pair of CBS (cystathionine β-synthase) domains (2). Indeed, GutQ is the namesake of the “GUTQ” isomerase domain listed in the NCBI Conserved Domain Databases that is common to all APIs characterized to date. This homology extends into their similar biochemical properties as well (Table 2). To avoid confusion in nomenclature between these two paralogues, it is suggested that GutQ be referred to as G-API (G = glucitol) and KdsD as L-API (L = lipopolysaccharide).
The rate of LPS biosynthesis has been correlated with the rate of KDO production, which in turn is believed to be limited by CMP-KDO synthetase (∼2 nmol KDO/min/mg) (26). The API levels in TCM11 are approximately the same, indicating that basal levels of G-API alone may be sufficient to maintain wild-type levels of flux through the KDO pathway. In agreement, no discernible differences were seen in the amounts of LPS in either TCM11 or TCM7 (Fig. 2A). LPS levels do, however, become dependent upon the concentration of A5P in cultures of TCM15 that had been preinduced for uhp-mediated transport. Cultures not induced for A5P uptake ceased dividing within a few generations, proving that the ΔAPI genotype is conditionally lethal in E. coli. The titratable nature of the K-12 LPS core in TCM15 provides the unique ability to control the ratio of mature wild-type LPS to LPS precursors by varying the amount of A5P in the medium. Many LPS precursors, in particular lipid IVa (6), are known to be antagonists of endotoxin-induced septic shock, and conditional mutants in LPS biosynthesis are potentially useful resources in potential vaccine/adjuvant development (23, 36). The potency of any developed or future KDO pathway competitive inhibitors (4, 9, 12, 15), long considered a drug target in E. coli, may become subject to A5P levels inside the cell due to induction of G-API.
The identification of GutQ as an API, both in vitro and in vivo, suggests that a pentose phosphate isomerase is a specifically regulated constituent of a hexitol phosphate catabolizing operon. Neither d-arabitol 5-phosphate dehydrogenase activity nor an increase in the culture density of cells grown in the presence of d-arabinose after being preinduced with d-glucitol was observed (data not shown), arguing against G-API being involved in the catabolism of potentially cotransported pentose substrates. Instead, it appears that G-API functions to synthesize A5P to modulate the expression level of the operon. Levels of GutD were lower after the addition of d-glucitol when A5P was included at 5 μM in comparison to 50 μM. It therefore appears that the physiological function of G-API may be to synthesize the regulatory molecule A5P, which in turn participates in the induction of the gut operon through an unknown mechanism. It is unclear why no effect was observed in either single API knockout strain. Suppression due to the second chromosomal copy of API is a likely cause, particularly considering the dynamic nature of the equilibrium between A5P and Ru5P, a substrate of multiple primary metabolism pathway enzymes.
G-API is the last gene product of the seven-gene gutAEBDMRQ operon (Fig. 1), of which six have been assigned a role or putative function. Genome analysis reveals a similar organization in other bacteria, including several gram-positive organisms (5, 30), with homologues of the transport/metabolic genes (gutAEBD) and the transcriptional activator gene (gutM) being highly conserved. However, GutQ has a narrow phylogenetic distribution confined to a subset of enterobacteria thus far limited to the sequenced gut operons in Escherichia, Shigella, and Salmonella species and Erwinia amylovora (1). Homologues of the repressor GutR located within the gut operon, like G-API, are also confined to gram-negative bacterial genomes. It seems likely that in an ancestor common to E. coli and other closely related gram-negative bacteria the kdsD gene was duplicated and then ultimately selected to serve as a regulatory enzyme of the gut operon.
Details concerning the molecular mechanism by which A5P affects expression of the gut operon remain unclear. A5P may bind to an unidentified protein that in turn attenuates transcription by either increasing the levels of cAMP or derepressing the operon, or A5P itself may directly influence the metabolic flux within the cell. It is clear, however, that the presence of d-glucitol results in increased API levels within the cell. The need for elevated levels of A5P produced by G-API during induction appears to be conditional and independent of LPS-related biosynthesis. There are multiple layers of control for the gut operon, and induction may become subject to the concentration of A5P only under certain growth conditions. Further work is needed to define the complex regulatory system of the gut operon.
Acknowledgments
This research was supported by the Pfizer Fellowship in Medicinal Chemistry (T.C.M.) and the Chemical Biology Interface (CBI) training grant (T.C.M.).
We thank Ted Houston of the University of Michigan Department of Geology at the W. M. Keck Elemental Geochemistry Laboratory for measuring metal content. We also thank other members of the Woodard group for helpful discussions.
REFERENCES
- 1.Aldridge, P., M. Metzger, and K. Geider. 1997. Genetics of sorbitol metabolism in Erwinia amylovora and its influence on bacterial virulence. Mol. Gen. Genet. 256:611-619. [DOI] [PubMed] [Google Scholar]
- 2.Bateman, A. 1997. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem. Sci. 22:12-13. [DOI] [PubMed] [Google Scholar]
- 3.Bigham, E. C., C. E. Gragg, W. R. Hall, J. E. Kelsey, W. R. Mallory, D. C. Richardson, C. Benedict, and P. H. Ray. 1984. Inhibition of arabinose 5-phosphate isomerase. An approach to the inhibition of bacterial lipopolysaccharide biosynthesis. J. Med. Chem. 27:717-726. [DOI] [PubMed] [Google Scholar]
- 4.Birck, M. R., T. P. Holler, and R. W. Woodard. 2000. Identification of a slow tight-binding inhibitor of 3-deoxy-dmanno-octulosonic acid 8-phosphate synthase. J. Am. Chem. Soc. 122:9334-9335. [Google Scholar]
- 5.Boyd, D. A., T. Thevenot, M. Gumbmann, A. L. Honeyman, and I. R. Hamilton. 2000. Identification of the operon for the sorbitol (glucitol) phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans. Infect. Immun. 68:925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandenburg, K., and A. Wiese. 2004. Endotoxins: relationships between structure, function, and activity. Curr. Top. Med. Chem. 4:1127-1146. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dische, Z., and E. Borenfreund. 1951. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J. Biol. Chem. 192:583-587. [PubMed] [Google Scholar]
- 9.Du, S. C., H. Tsipori, and T. Baasov. 1997. Synthesis and evaluation of putative oxocarbenium intermediate mimic in the Kdo8P synthase-catalyzed reaction as a tool for the design of potent inhibitors for lipopolysaccharide biosynthesis. Bioorg. Med. Chem. Lett. 7:2469-2472. [Google Scholar]
- 10.Eidels, L., P. D. Rick, N. P. Stimler, and M. J. Osborn. 1974. Transport of d-arabinose-5-phosphate and d-sedoheptulose-7-phosphate by the hexose phosphate transport system of Salmonella typhimurium. J. Bacteriol. 119:138-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gronow, S., and H. Brade. 2001. Lipopolysaccharide biosynthesis: which steps do bacteria need to survive? J. Endotoxin Res. 7:3-23. [PubMed] [Google Scholar]
- 12.Hammond, S. M., A. Claesson, A. M. Jansson, L. G. Larsson, B. G. Pring, C. M. Town, and B. Ekstrom. 1987. A new class of synthetic antibacterials acting on lipopolysaccharide biosynthesis. Nature 327:730-732. [DOI] [PubMed] [Google Scholar]
- 13.Heine, H., E. T. Rietschel, and A. J. Ulmer. 2001. The biology of endotoxin. Mol. Biotechnol. 19:279-296. [DOI] [PubMed] [Google Scholar]
- 14.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohen, A., R. Berkovich, V. Belakhov, and T. Baasov. 1993. Stereochemistry of the Kdo8p synthase—an efficient synthesis of the 3-fluoro analogs of Kdo8p. Bioorg. Med. Chem. Lett. 3:1577-1582. [Google Scholar]
- 16.Lengeler, J. 1975. Mutations affecting transport of the hexitols d-mannitol, d-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J. Bacteriol. 124:26-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lengeler, J. 1975. Nature and properties of hexitol transport systems in Escherichia coli. J. Bacteriol. 124:39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lengeler, J., and E. C. C. Lin. 1972. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J. Bacteriol. 112:840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesse, A. J., A. A. Campagnari, W. E. Bittner, and M. A. Apicella. 1990. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Immunol. Methods 126:109-117. [DOI] [PubMed] [Google Scholar]
- 20.Meredith, T. C., and R. W. Woodard. 2003. Escherichia coli YrbH is a d-arabinose 5-phosphate isomerase. J. Biol. Chem. 278:32771-32777. [DOI] [PubMed] [Google Scholar]
- 21.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novotny, M. J., J. Reizer, F. Esch, and M. H. Saier, Jr. 1984. Purification and properties of d-mannitol-1-phosphate dehydrogenase and d-glucitol-6-phosphate dehydrogenase from Escherichia coli. J. Bacteriol. 159:986-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persing, D. H., R. N. Coler, M. J. Lacy, D. A. Johnson, J. R. Baldridge, R. M. Hershberg, and S. G. Reed. 2002. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10:S32-S37. [DOI] [PubMed] [Google Scholar]
- 24.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raetz, C. R., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray, P. H., J. E. Kelsey, E. C. Bigham, C. D. Benedict, and T. A. Miller. 1983. Synthesis and use of 3-deoxy-d-manno-2-octulosonate (KDO) in Escherichia coli: potential sites of inhibition in bacterial lipopolysaccharides. ACS Symp. Ser. 231:141-169. [Google Scholar]
- 27.Sambrook, J., and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. III. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Shattuck-Eidens, D. M., and R. J. Kadner. 1981. Exogenous induction of the Escherichia coli hexose phosphate transport system defined by uhp-lac operon fusions. J. Bacteriol. 148:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheflyan, G. Y., D. L. Howe, T. L. Wilson, and R. W. Woodard. 1998. Enzymatic synthesis of 3-deoxy-d-manno-octulosonate 8-phosphate, 3-deoxy-d-altro-octulosonate 8-phosphate, 3,5-dideoxy-d-gluco(manno)-octulosonate 8-phosphate by 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase. J. Am. Chem. Soc. 120:11027-11032. [Google Scholar]
- 30.Tangney, M., J. K. Brehm, N. P. Minton, and W. J. Mitchell. 1998. A gene system for glucitol transport and metabolism in Clostridium beijerinckii NCIMB 8052. Appl. Environ. Microbiol. 64:1612-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trent, M. S. 2004. Biosynthesis, transport, and modification of lipid A. Biochem. Cell Biol. 82:71-86. [DOI] [PubMed] [Google Scholar]
- 32.Tzeng, Y. L., A. Datta, C. Strole, V. S. Kolli, M. R. Birck, W. P. Taylor, R. W. Carlson, R. W. Woodard, and D. S. Stephens. 2002. KpsF is the arabinose-5-phosphate isomerase required for 3-deoxy-d-manno-octulosonic acid biosynthesis and for both lipooligosaccharide assembly and capsular polysaccharide expression in Neisseria meningitidis. J. Biol. Chem. 277:24103-24113. [DOI] [PubMed] [Google Scholar]
- 33.Wiese, A., K. Brandenburg, A. J. Ulmer, U. Seydel, and S. Muller-Loennies. 1999. The dual role of lipopolysaccharide as effector and target molecule. Biol. Chem. 380:767-784. [DOI] [PubMed] [Google Scholar]
- 34.Wu, J., M. A. Patel, A. K. Sundaram, and R. W. Woodard. 2004. Functional and biochemical characterization of a recombinant Arabidopsis thaliana 3-deoxy-d-manno-octulosonic 8-phosphate synthase. Biochem. J. 381:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu, J., and R. W. Woodard. 2003. Escherichia coli YrbI is 3-deoxy-d-manno-octulosonate 8-phosphate phosphatase. J. Biol. Chem. 278:18117-18123. [DOI] [PubMed] [Google Scholar]
- 36.Wyckoff, T. J., C. R. Raetz, and J. E. Jackman. 1998. Antibacterial and anti-inflammatory agents that target endotoxin. Trends Microbiol. 6:154-159. [DOI] [PubMed] [Google Scholar]
- 37.Yamada, M., and M. H. Saier, Jr. 1987. Glucitol-specific enzymes of the phosphotransferase system in Escherichia coli. Nucleotide sequence of the gut operon. J. Biol. Chem. 262:5455-5463. [PubMed] [Google Scholar]
- 38.Yamada, M., and M. H. Saier, Jr. 1987. Physical and genetic characterization of the glucitol operon in Escherichia coli. J. Bacteriol. 169:2990-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada, M., and M. H. Saier, Jr. 1988. Positive and negative regulators for glucitol (gut) operon expression in Escherichia coli. J. Mol. Biol. 203:569-583. [DOI] [PubMed] [Google Scholar]
- 40.Yamada, M., Y. Yamada, and M. H. Saier, Jr. 1990. Nucleotide sequence and expression of the gutQ gene within the glucitol operon of Escherichia coli. DNA Seq. 1:141-145. [DOI] [PubMed] [Google Scholar]