Abstract
Cardioception is the ability of the central nervous system to process signals from the heart. Methods for determining cardioception are still under discussion. In the present study, we considered metrics for cardiac interoceptive accuracy (CA) assessments in three behavioral cardioception tasks − (1) the heartbeat tapping (HTT), (2) the heartbeat discrimination (HDT), and (3) the heartbeat counting (HCT) - and heartbeat evoked potentials (HEP) recorded by an electroencephalography during resting state and the tasks. The study included forty-eight healthy volunteers (25 females, 36 ± 7 age). CA in the HTT assessed using various metrics, except for the metric based on the circular variation between heartbeat and pressing time, positively correlated both with each other and with the metric in the HCT. The HDT showed no correlation with the other tasks. However, none of the metrics showed a clear advantage over the others in their association with the neurophysiological marker of interoception, the mean HEP amplitudes, during task performance or at rest. During all three tasks, the HEP amplitudes (1) did not differ between individuals with high and low CA metrics, (2) was not different from the HEP amplitudes during the resting state, (3) was lower during the HDT compared to the HCT. Thus, our results contribute to the debate on the interaction between behavioral cardioception tasks and the HEP.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-08779-5.
Keywords: Cardioception, Heartbeat evoked potentials, Heartbeat tapping task, Heartbeat discrimination task, Heartbeat counting task
Subject terms: Perception, Human behaviour, Neurophysiology
Introduction
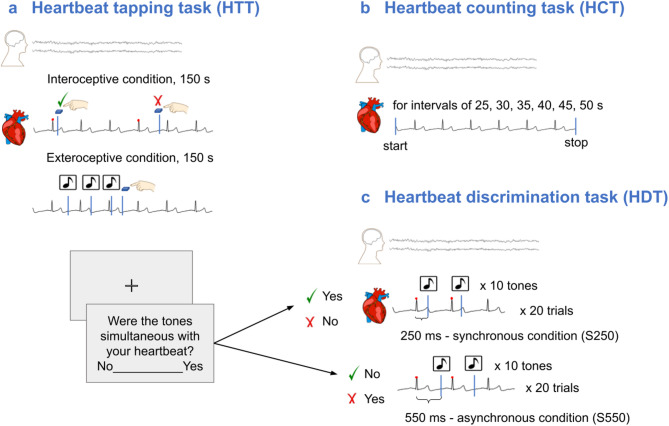
Interoception is a complex phenomenon conceptualized as perception, processing and integration of the internal bodily signals. Cardioception is gaining increasing attention in various research fields. In healthy individuals cardioception was shown to be associated with somatosensory perception and attention1, motor cortical excitability2, emotional processing and decision-making34,5. In clinical populations interoceptive processing is actively studied in patients with various psychoneurological and developmental disorders6–10, endocrine11,12, cardiological diseases13,14, and others15. Currently the most popular tasks for cardioception are: counting heartbeats during given time intervals (HCT)16, pressing a button at the moment of heartbeat sensation (HTT)17, determining whether the presented series of sound signals is synchronous or asynchronous to heartbeats (HDT)18,19 (Fig. 1).
Fig. 1.
The experimental procedure of the HTT, HCT and HDT. (a) HTT involved identifying the moment of a heartbeat sensation and reporting it via a button press. The task included two conditions lasting 150 s preceded by a 10-s training trial: (1) an exteroceptive condition in which participants pressed the “space” key each time they heard a sound signal (1 Hz, 500 ms), and (2) an interoceptive condition in which participants pressed the button when they felt a heartbeat. (b) HCT required participants to count their heartbeat sensations during six randomly presented time intervals. The main task was preceded by a 25-s training session. (c) HDT used biofeedback in the form of auditory signals delivered with specific delay after the online-registered R peak in ECG. Participants were instructed to determine whether the signals were simultaneous (synchronous) with their heartbeat. Two conditions were used in the task: S250 (250 ms delay) and S550 (550 ms delay). The conditions were presented randomly, with 20 trials of each condition. Note. HTT - heartbeat tapping task, HCT - heartbeat counting task, HDT - heartbeat discrimination task.
A major prerequisite for evaluating and comparing any tasks is to assess their reliability and validity. The reliability of the HCT was previously demonstrated in several studies20–24. CA metric, reflecting the pressings occurring within a specific delay after the heartbeat25in the HTT also showed stability from the beginning to the end of the task. For the HDT, reliability has been demonstrated in several studies: using split-half reliability for two repeated sessions run intermittently26, odd-even reliability23, test-retest reliability with a one-week interval, and by correlating conditions with 1-, 5-, and 10-tone sequences27. The review suggests a link between CA and interoceptive questionnaires on body awareness, which may implicitly confirm that they measure a single construct (convergent validity)28. At the same time, a short longitudinal study did not find this relationship29. Current research on the association between other questionnaires evaluating depression, anxiety, alexithymia and interoception presents conflicting results – negative30,31, positive32 or nonsignificant33 associations.
Noteworthy, all cardioception tasks face significant criticism for various reasons23,34. Thus, some researchers suggest that the existing tasks are unrelated to genuine cardioception35 and are prone to biased results36. For instance, some authors suggest that HCT and HTT results, when measured as the difference between estimated and recorded heartbeats, may be influenced by participants’ knowledge of their heart rate35,37. Others highlight the insensitivity of the HCT to heart rate changes induced by a pacemaker38 or to changes in posture37, indicating that participants do not rely on detecting actual heart rate to perform the task. Another criticism has been directed at the HTT and the HDT, suggesting that the task structure could interfere with cardioception due to competition for attentional resource23. Moreover, different CA metrics in the HTT4,9,22,36 and HDT40,41 have been proposed, each with its own distinctive features. Tables 1 and 2 provide a brief description of the most common CA metrics and their abbreviations. The delay-based and CAcmotor metrics require participants to tap within a set time limit but do not penalize extra taps, leading to inflated scores due to response frequency bias. The d_mod metric addresses this issue by penalizing false alarms, but it still relies on an arbitrarily defined response window, which does not account for individual differences in the time between the actual heartbeat and the conscious feeling of it. The resVec metric overcomes this limitation by assessing the phase consistency between heartbeats and motor responses without rewarding frequent tapping and selecting a specific window25. Similarly, the md metric mitigates response bias by comparing response and cardiac frequencies across overlapping time windows rather than single time spans, making it robust against subjective heart rate estimates and arbitrary response classifications while capturing dynamic behavioral adjustments39. Fittipaldi et al. demonstrated that for the HTT, md was more reliable than the other two mainstream CA metrics (mSI and d_mod) because md was explained by markers of interoception such as heartbeat evoked potentials (HEP), fMRI functional connectivity within interoceptive hubs, and socio-demographics characteristics39. Abrevaya et al. revealed that md may be a distinguishing feature between groups with cardiac or neurological disorders in terms of interoception, in contrast to other metrics in the HTT, such as mSI, d_mod, and delay-based9. However, no such analysis has been performed for recent CA metrics such as those from Körmendi et al.22.
Table 1.
Dictionary of the used metrics to assess CA in the HTT.
| Task | Abbreviation | Description of CA metrics | Reference |
|---|---|---|---|
| HTT | delay-based | Ratio of the total number of pressings that fell within a heart rate-dependent time window (delay) after the nearest preceding R peak to all recorded heartbeats in the task. Ranges from 0 to 1, with 1 indicating high cardioception. | Refs9,62,87–91. |
| mSI | Modified Schandry’s classic index, ratio of the total number of pressings to all recorded heartbeats in the task. Ranges from 0 to 1, with 1 indicating high cardioception. | Refs9,39. | |
| md | Mean distance, a measure of the mean synchrony between the pressing frequency and the heartbeat frequency in overlapping time windows starting from each R peak. Ranges from 0 to 1, with 1 indicating high cardioception. | Refs9,39. | |
| d_mod | Modified from the classic d-prime based on signal detection theory, a measure of sensitivity to feel a heartbeat in the heart rate-dependent time window (delay) after the nearest preceding R peak and not to feel outside the window. Had no defined limits, higher values indicated greater cardioception. | Refs9,39. | |
| resVec | Mean resultant vector, a measure of the variation of a time delay between the pressing and the nearest preceding R peak normalized by the corresponding inter-beat interval to that R peak. Ranges from 0 to 1, with 1 indicating high cardioception. | Refs4,22. | |
| CAcmotor | Ratio of the total number of pressings that fell within a 350–650 ms time window after the nearest preceding R peak to all recorded heartbeats in the task. Ranges from 0 to 1, with 1 indicating high cardioception. | Ref22. |
Note. CA - cardiac accuracy, HTT- heartbeat tapping task.
Table 2.
Dictionary of the used metrics to assess CA in the HDT and in the HCT.
| Task | Abbreviation | Description of CA metrics | Reference |
|---|---|---|---|
| HDT | ncorrect | Ratio of correct synchronicity judgments to total number of trials. Ranges from 0 to 1, with 1 indicating high cardioception. | Refs92,93. |
| d′ | d-prime based on signal detection theory, a measure of the sensitivity to synchronous and asynchronous auditory signals delivered after the R peak with small and large delays, respectively. Had no defined limits, higher values indicated greater cardioception. | Refs19,94. | |
| с value | Criterion, propency to answer “yes” or “no” in judgments about the synchronicity of the series of tones with the heartbeats. Had no defined limits, the higher (or more conservative) the criterion, the more tendency to answer “no”. | Refs87. | |
| HCT | SI | Schandry’s classic index, mean ratio of counted heartbeats to all recorded heartbeats in the time interval. Ranges from 0 to 1, with 1 indicating high cardioception. | Ref16. |
| corSI | Corrected Schandry’s classic index, taken into account when the number of counted heartbeats was much higher than the recorded heartbeats; mean ratio of counted heartbeats to half of the sum of all recorded heartbeats in the time interval and the counted heartbeats. Ranges from − 1 to 1, with 1 indicating high cardioception. | Ref95. |
Note. CA - cardiac accuracy, HCT- heartbeat counting task, HDT - heartbeat discrimination task.
Therefore, in addition to evaluating the reliability and validity of tasks, the extent to which behavioral tasks measure cardioception should be assessed by examining their association with an objective neurophysiological proxy for cardioception, such as the HEP. Schandry et al. was the first group who reported the existence of the HEP and revealed that the latency of the cortical activity peak, occurring 200–300 ms after the R peak, may be influenced by the direction of attention towards internal or external stimuli42. Later, intracranial EEG studies confirmed the existence of genuine neural sources of HEP, proving that this is not an artifact due to volume conduction from ECG43,44. Besides physiological pathways and mechanisms underlying HEP discussed by Park et al.44 recent studies provided more information for our understanding of brain-heart communications45,46. Building upon Schandry et al. research42Pollatos and Schandry demonstrated significant correlation between the CA in the HCT and the average amplitude of HEP within the 250–300 ms period47. In their later work, they did not show this outcome48. A recent meta-analysis49 emphasized that the relationship between HEP and interoception remains unclear, and the few existing studies report controversial results of correlations between the HEP and CA in tasks.
To our knowledge, our study is the first to analyze cardioception in the same subjects using (1) the three most frequently used cardioception tasks, along with both commonly used and novel CA metrics, and (2) relating these measures to the objective neurophysiological marker of interoception, HEP. We emphasize the importance of conducting such task-based studies within the same group of participants, as clinical characteristics are known to influence CA33,50,51. If these characteristics are not accounted for, it becomes challenging to generalize findings from one group to another.
Results
Cardioception tasks comparison
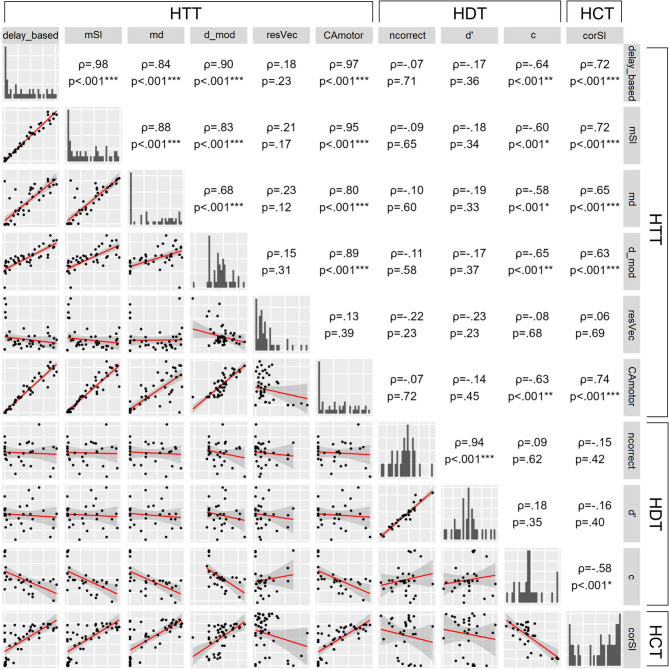
The order of tasks was fully randomised. Participants completed the HTT and HCT either before (HTT: n = 22, HCT: n = 26) or after (HTT: n = 26, HCT: n = 22) the HDT. There were no significant differences in metrics for the HTT and HCT tasks depending on whether they were performed before or after the HDT, indicating that task order did not influence performance. Most of the HTT metrics correlated with the number of pressings – delay-based (n = 48, ⍴ = 0.99, p <0.001), mSI (n = 48, ⍴ = 0.98, p <0.001), md (n = 48, ⍴ = 0.85, p <0.001), d_mod (n = 48, ⍴ = 0.88, p <0.001), CAmotor (n = 48, ⍴ = 0.96, p <0.001). Only the resVec in the HTT showed no correlation with number of pressings (n = 47, ⍴ = 0.19, p =0.21). We examined the correlations among the HTT metrics and between the HTT metrics and those in the HCT and HDT. The aim was to identify groups of metrics that potentially measure a similar construct or share the same biases, and to explore the relationship between three behavioral cardioception tasks. Figure 2 shows the results of a pairwise correlation analysis. Most metrics in the HTT were positively correlated with each other. Specifically, d_mod and md were moderately correlated (n = 48, ⍴ = 0.68, p <0.001, pB < 0.001), while other metrics showed strong correlations (⍴ ≥0.7, p <0.001, pB < 0.001). Metrics in the HTT were also correlated with corSI from the HCT (n = 48, ⍴ ≥0.7, p <0.001, pB < 0.001 for delay-based, mSI, and CAcmotor; ⍴ ≥0.6, p <0.001, pB < 0.001 for md and d_mod). The exception was resVec, which did not show a significant correlation either with other metrics in the HTT or with metrics in other tasks. (p = n.s.). For the HDT, ncorrect and d′ were strongly correlated (n = 30, ⍴ = 0.94, p <0.001, pB < 0.001). There were no correlations between metrics in the HTT and ncorrect and d′ in HDT, or between the HCT and ncorrect and d′ in HDT. A negative correlation was found between the c value in the HDT and several metrics in the HTT: delay-based (⍴ = − 0.64, p <0.001, pB = 0.007), mSI (⍴ = − 0.6, p <0.001, pB =0.02), md (⍴ = − 0.58, p <0.001, pB = 0.04), d_mod (⍴ = − 0.65, p <0.001, pB = 0.005), and CAcmotor (⍴ = − 0.63, p <0.001, pB = 0.009) (n = 30 for all correlations). Additionally, a negative correlation was observed between c value in the HDT and metric in the HCT (n = 30, ⍴ = − 0.58, p <0.001, pB = 0.03).
Fig. 2.
Association between CA metrics assessed for HTT, HDT and HCT (Spearman’s rank correlation). The 95% confidence bands are displayed. Note. CA - cardiac accuracy, HCT - heartbeat counting task, HDT - heartbeat discrimination task, HTT - heartbeat tapping task. *** pB < 0.001, ** pB < 0.01, * pB <0.05, where pB - p-values after Bonferroni correction.
CA was compared between detectors and non-detectors to identify metrics that differentiate between groups with different levels of cardioception (Table 3). Detectors (n = 31) had higher md and resVec in the HTT compared to non-detectors (n = 9). There were no significant differences between the groups regarding sex (χ² < 1, p = 1; non-detectors: 16 females, 15 males; detectors: 5 females, 4 males), age (p =.16; non-detectors: 36.61 ± 7.11 years; detectors: 32.89 ± 5.67 years), or BMI (p =.35; non-detectors: 23.94 ± 3.56; detectors: 24.87 ± 3.16).
Table 3.
CA in the HTT, HDT, and HCT in groups with uniform (non-detectors) and non-uniform (detectors) pressings circular distribution relative to R peak in ECG (Wilcoxon rank sum test with Bonferroni correction).
| Task | CA | Non-detectors (M ± SD (n)) | Detectors (M ± SD (n)) | W | p | p B |
|---|---|---|---|---|---|---|
| HTT | delay-based | 0.29 ± 0.23 (31) | 0.53 ± 0.26 (9) | 207 | 0.03* | 0.28 |
| mSI | 0.36 ± 0.28 (31) | 0.66 ± 0.32 (9) | 211 | 0.02* | 0.2 | |
| md | 0.4 ± 0.31 (31) | 0.74 ± 0.29 (9) | 233 | 0.002** | 0.02* | |
| d_mod | 0.87 ± 0.58 (31) | 1.11 ± 0.93 (9) | 198 | 0.06 | 0.59 | |
| resVec | 0.18 ± 0.19 (31) | 0.33 ± 0.22 (9) | 224 | 0.002** | 0.02* | |
| CAcmotor | 0.12 ± 0.1 (31) | 0.21 ± 0.1 (9) | 206 | 0.03* | 0.31 | |
| HDT | ncorrect | 0.45 ± 0.11 (21) | 0.47 ± 0.11 (3) | 38.5 | 0.57 | 1 |
| d′ | −0.35 ± 0.61 (21) | −0.07 ± 1.02 (3) | 37 | 0.68 | 1 | |
| с value | −0.28 ± 0.74 (21) | −0.9 ± 0.35 (3) | 10 | 0.07 | 0.66 | |
| HCT | corSI | 0.16 ± 0.67 (31) | 0.72 ± 0.28 (9) | 216 | 0.01* | 0.12 |
Note. CA - cardiac accuracy, HCT- heartbeat counting task, HDT - heartbeat discrimination task, HTT- heartbeat tapping task, n - number of participants, M - mean, SD - standard deviation. ** p <0.01, * p <0.05. pB - p-values after Bonferroni correction.
Three metrics in the HTT were selected for further analysis: md, resVec, and CAcmotor. md was selected due to its significant correlation with other metrics in both the HTT and HCT. resVec was selected because it showed no correlation with the other metrics; moreover, both md and resVec differed significantly between detectors and non-detectors. CAcmotor was selected because, like resVec, it is not a mainstream metric. d′ in the HDT was selected due to its correlation with ncorrect. An additional characteristic c value was selected due to its negative correlation with several metrics in HTT and HCT. Finally, corSI was selected in the HCT.
The purpose of the measuring internal consistency was to determine how similar the metrics are in the assessment of the level of cardioception. Cronbach’s alphas for the normalized versions of the selected metrics in three tasks (n = 30) were as follows: α = 0.63 for the combination of md, Pc2IFC, and SI; α = 0.14 for the combination of resVec, Pc2IFC, and SI; α = 0.48 for the combination of CAcmotor, Pc2IFC, and SI.
HEP amplitudes comparison within conditions in the whole sample and in groups with different levels of cardioception
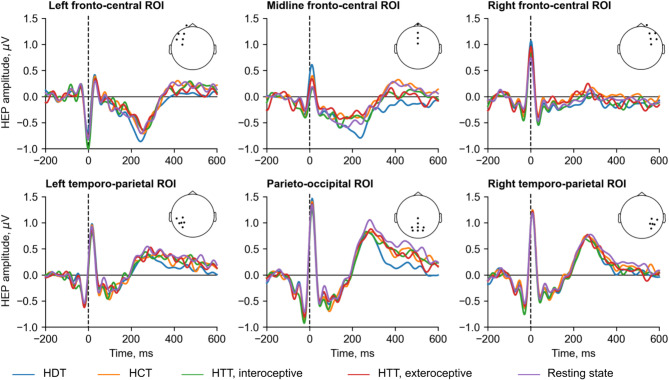
Figure 3 illustrates the HEP amplitudes in channel groups. The comparison aimed to assess (1) whether different cardioception tasks influence interoception level expressed as a pattern of HEP amplitudes, (2) whether patterns differ from the off-task condition, and (3) whether patterns differ in task within people with good and poor cardioceptive abilities (divided by the level of metric in the corresponding task into high and low CA metric groups) and based on the pressings circular distribution in the HTT (detector and non-detector groups).
Fig. 3.
Grand averages of HEP amplitudes during the interoceptive and exteroceptive conditions in the HTT, HDT, HCT and resting state, averaged over six ROIs. Channels positions corresponding to each ROI are indicated by dots on the head map. Note. HCT - heartbeat counting task, HDT - heartbeat discrimination task, HEP - heartbeat evoked potentials, HTT - heartbeat tapping task, ROIs - regions of interest.
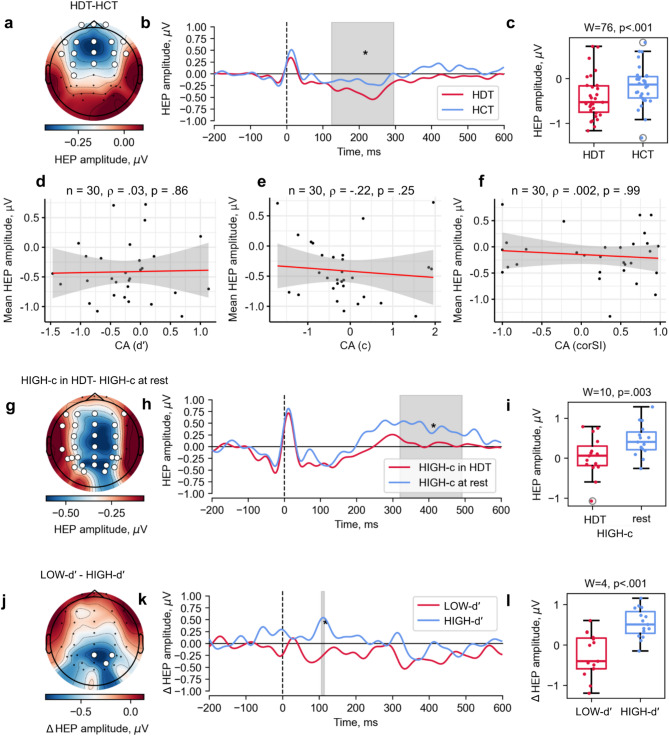
A significant effect of condition in the whole sample was observed when comparing the HEP amplitudes recorded during the HDT and HCT (Monte Carlo p =0.046). A significant cluster, spanning from 124 to 296 ms, included the following channels: Fp1, Fpz, Fp2, F7, F3, Fz, F4, F8, FT7, FC3, FCz, FC4, FT8, C3, Cz, C4, and CP3 (Fig. 4a-c). Cluster-averaged HEP amplitudes for the HDT (n = 30, M = −0.41, SD = 0.48) were significantly less (W = 76, p <0.001) than for HCT (n = 30, M = −0.16, SD = 0.45). After restricting the time window from 200 to 600 ms, this result became nonsignificant. An effect of condition was also found in a group of participants with high c value for the HDT and the resting state comparison (Monte Carlo p =0.03). A significant cluster, occurring between 320 and 492 ms, included channels F7, F3, Fz, F8, FT7, FC3, FCz, FC4, C3, Cz, C4, TP7, CP3, CPz, CP4, T5, P3, Pz, P4, P5, PO3, POz, PO4, P6, PO7, O1, Oz, O2, and PO8 (Fig. 4g-i). Cluster-averaged HEP amplitudes for the group with high c value during HDT (n = 15, M = 0.04, SD = 0.48) were significantly less (W = 10, p =0.003) than during resting state (n = 15, M = 0.45, SD = 0.39). After restricting the time window from 200 to 600 ms, this result remained significant. There was no significant difference between the HEP amplitudes during other behavioral cardioception tasks within the whole sample, between the HEP amplitudes during tasks and resting state within the whole sample, within groups with high and low CA metric (see Supplementary Fig. S1-S2) and within detectors and non-detectors groups (see Supplementary Fig. S3). A summary of the results is presented in Supplementary Figure S4.
Fig. 4.
Comparison of HEP amplitudes between conditions (nonparametric permutation paired t-test). HEP were averaged over a window of − 200 to 600 ms relative to the onset of the R-peak in the ECG. Significant clusters were defined as combinations of channels and time intervals where HEP amplitude differed between conditions. (a) Topographic representation of the difference in HEP amplitudes between the HDT and HCT, shown for each electrode across the scalp. The map displays average HEP amplitude within the significant time range of 124–296 ms (n = 40, Monte Carlo p =0.046); significant cluster channels are highlighted in white. (b) Grand averages of HEP amplitudes during the HDT and HCT within the significant cluster (white channels in panel a); the significant time range is highlighted in gray. (c) Distribution of HEP amplitude averaged over the cluster channels in the significant time range during the HDT and HCT. Wilcoxon signed-rank test results are shown. (d-f) Spearman’s rank correlation between HEP amplitudes (averaged over the cluster channels in the significant time range) and CA metric (d) d′ in the HDT, (e) c value in the HDT, (f) corSI in the HCT. (g) Topographic representation of the difference in HEP amplitudes between the HDT and the resting state in participants in the HIGH-c group, shown for each electrode across the scalp. The map displays average HEP amplitude within the significant time range of 320–492 ms (n = 15, Monte Carlo p =0.03; significant cluster channels are highlighted in white). (h) Grand averages of HEP amplitudes during the HDT and resting state in the HIGH-c group within the significant cluster (white channels in panel g); the significant time range is highlighted in gray. (i) Distribution of HEP amplitudes averaged over the cluster channels in the significant time range during the HDT and resting state. Wilcoxon signed-rank test results are shown. (j) Topographic representation of the difference in HEP amplitudes modulation (Δ) between groups with high (HIGH-d′) and low (LOW-d′) d′ value in the HDT, shown for each electrode across the scalp. Participants were divided into high and low groups based on a median split of CA d′ scores. Within each group, HEP modulation was calculated for each participant as the difference in amplitude between the HDT and the resting state. The map displays the average HEP amplitudes modulation within the significant time range of 106–114 ms (n = 15, Monte Carlo p =0.02; significant cluster channels are highlighted in white). (k) Grand averages of HEP amplitudes modulation during the HDT for the two groups, shown within the significant cluster (white channels in panel j); the significant time range is highlighted in gray. (l) Distribution of HEP amplitudes modulation, averaged over the cluster channels in the significant time range, for the two groups. Wilcoxon signed-rank test results are shown. Note. CA - cardiac accuracy, HCT - heartbeat counting task, HDT - heartbeat discrimination task, HEP - heartbeat evoked potentials, HIGH-c - HEP amplitudes in participants with high c value in HDT, HTT - heartbeat tapping task, ΔHIGH, ΔLOW-d′ - HEP amplitudes modulation in participants with high and low d′ in HDT respectively.
HEP amplitudes modulation comparison between groups with different levels of cardioception
The purpose of the comparison was to assess whether task modifications produce different levels of interoception modulation expressed as a pattern of task-rest difference of HEP amplitudes. Comparisons were performed between people with good and poor cardioceptive abilities divided by the level of CA metric in the corresponding task (high and low CA metric groups) and by the pressings circular distribution in the HTT (detector and non-detector groups). A group effect was found for comparison between groups with high and low d′ value for the HDT (Monte Carlo p = 0.02). A significant cluster, occurring between 106 and 114 ms, included channels Pz, P4 and PO4 (Fig. 4j-l). Cluster-averaged HEP amplitudes modulation for the group with low d′ value (n = 15, M = −0.25, SD = 0.51) were significantly less (W = 4, p <0.001) than for the group with high d′ value (n = 15, M = 0.54, SD = 0.35). There was no significant result after restricting the time window from 200 to 600 ms. There were no significant clusters when comparing the HEP amplitude modulation between the others high and low CA metric groups (see Supplementary Fig. S1-S2), or between the detector and non-detector groups (see Supplementary Fig. S3).
Association between mean HEP amplitudes and CA within each cardioception task
Mean HEP amplitudes in channels, ROIs and CA
There were no correlations between the HEP amplitudes averaged over channels belonging to significant cluster within the 124–296 ms time range and the corresponding metric in HDT and HCT (Fig. 4d-f). There were several significant correlations between metrics and mean HEP amplitudes both during resting state (Fig. 5a) and the task (Fig. 5b). However, results did not survive after correction for multiple comparisons using PCA. Multivariate analyses revealed resVec was predicted by HEP amplitudes in parieto-occipital ROI in HTT (n = 47, β = 0.2, SE = 0.08, p = 0.01, pBH = 0.3) and during resting state (n = 47, β = 0.24, SE = 0.09, p = 0.009, pBH = 0.3) but after BH correction results became a nonsignificant. There were no associations between mean HEP amplitudes in ROIs during other tasks and resting state and CA in tasks.
Fig. 5.
Spearman’s rank correlation between CA in the HTT, HDT, and HCT and mean HEP amplitudes in the 200–600 ms time range for channels recorded (a) during tasks and (b) resting state. Only results with significant p-values prior to correction for multiple comparisons based on PCA are shown. The 95% confidence bands are displayed. Note. CA - cardiac accuracy, HCT - heartbeat counting task, HDT - heartbeat discrimination task, HEP - heartbeat evoked potentials, HTT - heartbeat tapping task, p_adj - p-values after the correction for multiple comparisons based on PCA.
Mean HEP amplitudes in spatio-temporal clusters and CA
There were no clusters with significant correlations based on the spatio-temporal permutation cluster test on correlation.
Discussion
The current work provides, for the first time, an evaluation of the three most commonly used behavioral tasks for cardioception and their comparison with the electrophysiological marker of interoception - HEP - in a group of healthy volunteers.
Our results demonstrate that not all CA metrics from three cardioception tasks correlate with each other. In the HTT, we found that five metrics were significantly correlated: based on (1) the heart rate-dependent time delay (delay-based), (2) the synchrony of response frequency and heartbeat frequency (md), (3) the modified Schandry index (mSI), (4) the sensitivity to feel a heartbeat within a heart rate-dependent time window (d_mod), and (5) the CAcmotor metric recently suggested by Körmendi et al.22. The strong correlations observed among delay-based, mSI, md, d_mod, and CAcmotor suggest that these metrics may share common biases rather than measuring the same underlying construct. Of particular concern is the high correlation of d_mod and md with these metrics, as this challenges the assumption that they are resistant to response frequency bias. Furthermore, the association of delay-based, md, mSI, d_mod, and CAcmotor with the metrics of HCT, which are biased by guessing strategies, raises additional concerns. On the contrary, the sixth metric, resVec (based on the circular variation between heartbeat and pressing time), did not significantly correlate with any of the other metrics in HTT and HCT. It was also the only one not associated with the number of pressings. It can be assumed that it is less susceptible to response biases, guessing strategies and predefined time windows. In a previous study, resVec was associated with mSI and CAcmotor, showing a negative correlation25. We suggest that metrics need further refinement and validation and caution should be exercised when using any of the five metrics in the HTT (delay-based, md, mSI, d_mod, or CAcmotor), while resVec is a promising measure of CA.
In addition, these metrics were compared between individuals with good and poor cardioceptive abilities. In previous studies such division was performed mainly for the HCT (summarized by Coll et al.49) and the HDT27,52. We followed the approach proposed by Körmendi et al.22in which participants were divided into detectors and non-detectors based on the uniformity of the time between heartbeats and button pressings. We found that 22.5% of participants fell into the detector group, which is higher than the 12% reported by Kormendi and colleagues. Detectors showed a higher resVec metric, which can be attributed to the fact that both the detector classification and resVec are based on variations in pressing time. Among the remaining metrics, only the md metric in the HTT showed a significant difference between the detector and non-detector groups, independent of sex, age, and BMI. These results align with Fittipaldi et al.39who suggested that md also may be a better proxy for cardioception in the HTT. Our findings are consistent with previous studies showing that only a minority of healthy participants are good heartbeat perceivers in other tasks (have an CA above the median). In the HCT, 35% of participants had an CA above 0.8553, while in the HDT, 30% of participants were able to perceive their heartbeat54.
We performed a correlation analysis to explore the relationship between three behavioral cardioception tasks. Although a recent meta-analysis55 identified a weak relationship between CA in the HCT and HDT (R² = 0.044), results from various studies differ, ranging from moderate to small correlations40,56 to no correlation57–59which is consistent with the findings of the present study. Our results align with those of Körmendi et al.25 who found a correlation between CA in the HTT and HCT. We expand on their work by showing a correlation not only between one metric (CAcmotor) but between five HTT metrics and the HCT. Studies comparing CA between the HTT and HDT are limited. One early study by Pennebaker and Hoover60 reported no correlation as in the present study. Furthermore, we observed a negative correlation between the c value in the HDT and CA in the other two tasks except resVec, suggesting that the c value in the HDT and CA in other tasks reflect participants’ tendency to guess. In the HDT, participants often respond “yes” to tone-heartbeat synchrony despite uncertainty, while in other tasks, they may guess their heart rate, potentially inflating performance.
The variability of performance from task to task may also be an important characteristic of the stability of a person’s attention and conscientiousness when completing a task. If we assume that three behavioral tasks evaluate the same phenomenon, cardioception, the CA across tasks should reflect a relatively stable characteristic of the individual. However, the Cronbach’s alpha for three tasks was less than 0.7 and did not show internal consistency, which may indicate that these tasks probe different aspects of cardioceptive ability. Indeed, there is ongoing debate regarding the utility of different approaches to measuring cardioception (for details see Körmendi et al.61).
A growing body of evidence suggests a relationship between interoceptive ability and the HEP (for review, see Coll et al.49), making HEP amplitude a potential electrophysiological marker of interoception44. Most studies examining the associations between behavioral task interoception and HEP parameters implemented a maximum of two behavioral tasks9,62–65. The current study aimed to investigate the relationship between CA in three tasks and HEP in the same sample of participants. This represents a key aspect of the novelty of our research, as comparing results across studies that use different behavioral tasks is often challenging due to methodological heterogeneity (as discussed by Coll et al.49) and the multimodal nature of interoceptive ability, which is influenced by various factors such as sex and body composition12, age33,66 and cardiac hemodynamics67.
Fittipaldi et al.39 found that the higher the cardioception, as measured by md (but not by mSI or d_mod), the more negative was the amplitude of the HEP in HTT (but not for mSI or d_mod). However, our study did not reveal any such correlation for these CA. One possible explanation could be the difference in the age range of participants. Fittipaldi et al.‘s study included participants aged 17 to 84 years, whereas our study focused on those aged 20 to 50 years. The authors found that the md metric did not correlate with age in a univariate analysis. However, in a multivariate model that included electrophysiological, hemodynamic, and socio-emotional features, md could be predicted by age. It is possible that a wider age range might contribute to the relationship between CA in the HTT and the HEP amplitudes, as age is one of the factors that may influence interoceptive abilities33,66.
In contrast to our study, an earlier study showed a significant negative correlation between CA in the HDT and the amplitude of the evoked potential (the authors called it ‘N1’) locked to the heartbeat68. However, this correlation was demonstrated for another CA metric (the standard deviation of the mean time delay that participants preferred to judge as synchronous with the heartbeat). Banellis and Cruse found that HEP amplitudes differed between stimulus anticipation of synchronous and asynchronous trials, but reported lack of correlation between d′ and the HEP amplitudes69. The authors provided three arguments explaining this result: the misperception of both conditions as mostly asynchronous by poor perceiving participants, individual differences in time perception and the greater latency of the HEP. Our data support the idea of misperception as indicated by high c value, while CA was low. Another study70 also reinforces our findings. The authors found a marginally significant suppression of N1 auditory evoked potentials in central-frontal electrodes during the presentation of synchronous tones with different latencies relative to the exteroceptive condition. Authors also noted that there was no association between suppression and percentage of correct answers in the task, which was at chance level across participants. Thus, CA in the HDT does not reflect sensory suppression in the processing of heartbeat-related information.
Although a positive correlation between CA in the HCT and HEP amplitudes during the task was previously demonstrated47,71, other studies have found no correlation between behavioral and neurophysiological data for this task, which aligns with our results48,63–65,72,73. The lack of association observed in our study contributes to the ongoing discussion regarding the ability of the HCT to reliably indicate interoception. A recent large-scale study74 demonstrated that CA in the HCT has a low correlation with the actual number of heartbeats and exhibits non-linearity across CA quantiles. One potential approach to increasing the utility of the HEP amplitudes during behavioral tasks may involve accounting for the breathing cycle. Zaccaro et al.73 demonstrated that the HEP amplitudes vary between inhalation and exhalation during interoceptive tasks. Another factor complicating the interpretation of results from different studies using the HCT is the variation in instructions given to participants. In earlier studies, participants were asked to count heartbeats even if they did not feel them, relying on intuition4,47. More recent studies, however, instruct participants to report only perceived heartbeats, which helps to reduce the influence of estimation and better capture interoceptive ability73,75,76. We used the latter approach. Interestingly, Desmedt et al.76 compared both instructions and demonstrated that the second approach (report only perceived, not estimated heartbeats number) reduced CA.
During tasks, the HEP amplitudes did not differ from the resting state. This contradicts the expectation that HEP is modulated as the participant entered an interoceptive state during the task. Couto et al.4 demonstrated, that the HEP amplitudes differed between conditions when participants were (1) instructed to “think freely and pay attention to nothing in particular” (in the current study the same instruction was given for the resting EEG data acquisition), (2) to pay attention to heartbeats (which is analogous to the performance in the behavioral tasks in our experimental procedure), and (3) to count sounds (which corresponds to the exteroceptive condition of the HTT in our study). Yoris et al.13 demonstrated decreased HEP amplitudes modulation between exteroceptive and interoceptive conditions in hypertensive patients compared to controls. Similarly, Schulz et al.65 found higher HEP amplitudes during the HCT compared to the resting state in control individuals, but not in patients with depersonalization disorders.
HEP amplitudes did not differ either between individuals with high and low CA (except for c value and d′) or between detectors and non-detectors. The presence of HEP amplitudes modulation was observed in the group with high c value. As previously mentioned, a higher c value indicates a bias towards negative responses regarding the synchronicity of stimuli in this task. Participants who responded with a tendency to answer “no” had low CA (there was a negative correlation between metrics and c value). One of the assumptions might be that it indicated their shift of attention to external stimuli and an inability to perceive interoceptive signals. In the HDT, they did not hear the heart (corSI was low – they claimed few contractions) and listened only to sounds, i.e. exteroception was predominant. Performing a predominantly exteroceptive task disguises the HEP relative to the resting state over the head77,78. An alternative assumption could be that people with poor cardioceptive abilities made more effort to perform the task, which was reflected in the negative deflection of HEP. Between-group analyses of HEP modulation showed a significant difference between groups with low and high CA d′, with more negative HEP observed in the group with low d′. However, the significant time window did not fall within the range of 200–600 ms. Some studies restricted the analysis to time windows beginning at least 200 ms after the R-peak to avoid contamination of the HEP by cardiac-related artifacts13,65,77. In contrast, other studies did not impose this constraint, relying on the removal of cardiac field artifacts4,90. These studies demonstrated HEP differences close to 200 ms and beyond which limits the interpretation of the results as directly related to interoceptive processing.
We also observed a difference in HEP amplitudes during the HDT and HCT in the frontal and central channels while this was not accompanied by a correlation between CA and HEP in these channels in the tasks (Fig. 4e-f). This result aligns with the discussion by Schulz et al.65suggesting that HEP during the HDT may be influenced by auditory-evoked potentials. Also Baess79 showed N1 auditory evoked potential suppression in center-frontal regions for self-initiated sounds triggered by a participant’s button pressing. In recent years, new methods have been developed for measuring cardioception. In a narrative review by Körmendi et al., methods that involve a combination of tracking, detection, and discrimination are referred to as mixed methods (for details, see the review61). Although we applied the traditional HDT methodology, rather than one of the new mixed methods, our data confirm that this task, compared to HCT and HTT, demands more attentional effort from the participant.
The study’s limitations may include an insufficient sample size to detect weak to moderate effect sizes, the inclusion of only HEP without psychological questionnaire data or other markers of interoception, working in the sensor domain rather than the source domain, relying primarily on correlation analyses instead of multivariate analyses, and using a specific montage scheme (for details see Supplementary Fig. S5). Although the order of tasks was fully randomized across participants, it is possible that completing the HDT before the HTT or HCT may have influenced subsequent performance by increasing participants’ knowledge of their actual heart rate. While our analyses did not reveal any significant order effects, we acknowledge this as a potential limitation. The resVec metric does not adequately reflect actual cardioceptive accuracy in cases where only a single response is made during the HTT. Therefore, this metric should be interpreted with caution, and its use necessitates establishing a minimum number of responses. Concerns about the sensitivity of this metric to a small number of responses are refuted by the presence of a participant with seven responses who showed a low resVec (0.2). At the same time, a participant in the high resVec (0.88) group also made seven responses, which occurred within closely aligned time intervals. This confirms that high resVec is associated with temporal consistency rather than the number of responses. In our analysis, we set this minimum at two responses, although we acknowledge that more stringent inclusion criteria may be warranted in future research.
Conclusion
In conclusion, this study aimed to add evidence to the debate on task selection for the assessment of cardioception. We compared a set of different CA metrics (1) within tasks, (2) between tasks and (3) with the electrophysiological marker of interoception - HEP amplitudes during resting state and task conditions. Although most metrics in the HTT were highly correlated, only resVec remained independent, suggesting it was not influenced by the same biases as the other metrics. Additionally, md effectively differentiated individuals with high and low cardioceptive ability, highlighting its potential for assessing CA. HEP amplitudes were lower in the HDT in comparison with resting state and HCT, which may indicate the predominance of the exteroceptive state over the interoceptive and complexity of HDT in comparison with two other tasks. The lack of association between CA and HEP amplitudes contributes to the growing body of literature suggesting that HEP parameters may reflect multiple processes related to cardioception, attention, respiration, etc., and thus may not necessarily show a simple association with the accuracy of heartbeat perception. Although the association between HEP amplitudes and CA did not survive correction, it is worth discussing this result as potentially significant for task selection, but requiring further investigation of our highlighted metrics. Our findings could drive future theoretical developments and clinical progress in interoception research.
Methods
Participants
The study included 48 healthy volunteers (25 females; Median [Q25, Q75] = 35.5 [29.75, 43.25] years old; Median [Q25, Q75] = 24.25 [21.98, 26.90] body mass index (BMI)). After checking the biofeedback quality, as outlined in the Experimental Procedure section of the Methods, 30 participants were included in the HDT analysis.
Healthy participants were recruited through a local mailing list. We used G*Power 3.1.9.7 to calculate the sample sizes needed to achieve 80% power with a two-tailed alpha level of 0.05 for detecting correlations. Correlations from weak (0.28) to strong (0.8) were previously reported between tasks, and between HEP and tasks (see Supplementary Information for details). We therefore concluded that, when comparing all three conditions, a sample size of 48 participants was adequate. See Supplementary Information for the exclusion criteria and medical examinations undergone by the participants. The study followed the ethical norms outlined in the 1964 Declaration of Helsinki. Participation in the study was voluntary. Informed consent was obtained from all participants. The consent written by the participants was approved by the local Ethics Committee at the National Medical Research Center for Therapy and Preventive Medicine in accordance with the Declaration of Helsinki. The experimental protocol (No. 02–02/21 of 25.02.2021) was approved by the local Ethics Committee at the National Medical Research Center for Therapy and Preventive Medicine in accordance with the Declaration of Helsinki.
Experimental procedure
Experiment was implemented in an open source software package PsychoPy (v2022.2.4, https://www.psychopy.org)80. The participants were sitting quietly with their eyes open. At the beginning, five minutes of resting EEG data were recorded, during which participants were instructed to focus on the fixation cross in the middle of the screen in front of them and to avoid moving and excessive blinking. Before each of the tasks, presented in random order, participants had a training session and were instructed to focus on their internal sensations. Instructions were shown on a computer, and participants’ responses were recorded using a keyboard and mouse. Figure 1 shows the task procedures. During all three tasks, participants were asked to focus solely on actual heartbeats sensations, without palpating their pulse or guessing. They were, however, permitted to consider any weak heartbeat sensations they experienced. Participants were allowed not to pressing the button and report zero counts if they did not perceive any heartbeats.
The HDT consisted of 43 trials, with the first three serving as training trials and were not included in the analysis81. S250 condition should be perceived as synchronous with the heartbeat according to previous work82 where delay between 200 and 300 ms got more synchronous judgements compared to 500 ms. S550 condition should be perceived as asynchronous to the heartbeats. Each trial consisted of 10 auditory signals (440 Hz, 100 ms)27. Technical details of the task implementation can be found in the Supplementary Information. We excluded trials with poor quality of the biofeedback (see Supplementary Information for quality criteria). As a result, participants had an average of 17.6 ± 3.42 synchronous and 17.17 ± 2.44 asynchronous trials.
EEG, EMG, ECG, EOG recording
Electrophysiological data were recorded using the NVX-52 EEG amplifier (Medical Computer Systems, Ltd. (MCS)) with 36 Ag/AgCl electrodes placed on elastic EEG caps according to the international 10–20 system. Channels T3 and T4 were used as online ipsilateral references. The T3 reference was used for electrodes on the left side, the T4 reference for the right side, and the average of (T3 + T4)/2 for electrodes on the central sagittal line. The impedance across all channels during recording was generally around 10 kΩ and did not exceed 20 kΩ. Surface EMG was recorded from the first dorsal interosseous muscle using a belly-tendon montage. ECG was recorded using three pairs of electrodes in a bipolar montage: the first pair on the anterior forearm, the second pair 2 cm below the clavicles in the infraclavicular fossa, and the third pair on the right and left sides of the neck as it was used previously67. Two EOG electrodes were placed on the lateral sides of the eyes. The sampling frequency was set at 500 Hz, and the data were filtered at 0.1–70 Hz with a 50 Hz notch filter.
Data analysis
ECG and EEG data were processed and analyzed using custom-made scripts in Python (v3.11, https://www.python.org) and the MNE-Python toolbox (v1.6.0, https://mne.tools/stable/index.html)83. Both ECG and EEG data were notch-filtered at 50 Hz and then bandpass filtered from 0.5 to 45 Hz using a zero-phase FIR filter with a Hamming window using the raw.filter function.
EMG, ECG data analysis
EMG data were used to assess the precise timing of the button pressing (see Supplementary Information for details). R peaks were detected from the ECG lead with the fewest artifacts (chest lead below the clavicles for most participants) using the MNE-Python. ECG data were always visually inspected by cardiologists to verify the accuracy of R peak detection and to identify any extrasystoles (premature ventricular and supraventricular contractions). Both sinus rhythm R peaks and extrasystoles were considered when analyzing CA during the tasks. However, epochs time-locked to extrasystoles were removed from further analysis of HEP (see the EEG analysis section below).
EEG data analysis
We removed eye-movements and cardiac-field artifacts using independent component analysis (ICA) (fastica algorithm) to find components explaining 99% of the variance. We selected and deleted two EOG and one ECG components. See Supplementary Information for components’ selection protocol. These components were then projected from the EEG data. Then EEG data were filtered with a high-pass at 0.5 Hz and a low-pass at 20 Hz71. Bad channels (two at max) were interpolated. EEG data were segmented into epochs time-locked to R peaks, ranging from − 200 to 600 ms, with baseline correction from − 200 to −100 ms. We dropped epochs time-locked to the extrasystoles, two epochs before and one after the extrasystoles and epochs corresponding to the interval between R peaks less than 600 ms. We used the AutoReject algorithm84 to delete and/or interpolate bad epochs. We visually inspected the EEG epochs and excluded any containing excessive noise, limiting the total exclusion to no more than 10% of the data. The final average number of epochs was 157 ± 20 in the exteroceptive condition in the HTT, 152 ± 22 in the interoceptive condition in HTT, 374 ± 51 in the HDT, 228 ± 34 in the HCT, and 301 ± 46 in the resting state.
We divided channels into spatial ROIs as it was done previously47,64: left fronto-central (Fp1, F3, FC3, C3, F7, FT7), midline fronto-central (Fpz, Fz, FCz, Cz), right fronto-central (Fp2, F4, FC4, C4, F8, FT8), left temporo-parietal (TP7, CP3, P3, T5, P5, PO7), parieto-occipital (CPz, Pz, POz, Oz, PO3, O1, PO4, O2), and right temporo-parietal (TP8, CP4, P4, T6, P6, PO8). HEP amplitudes among the channels and ROIs were obtained from the within-channel and within-ROI epoch-wise averaging of the HEP amplitudes respectively. Mean HEP amplitudes over channels and ROIs were obtained in the time range 200–600 ms after R peak. Additionally, our analysis incorporated not only HEP amplitudes during the tasks but also HEP modulation, which was calculated by subtracting the resting state HEP amplitudes across channels from the HEP amplitudes recorded during the tasks.
Behavioral cardioception tasks analysis
We used metrics summarized in Tables 1 and 2. Eight participants who did not press in the HTT were given the zero score of md and resVec. One participant was excluded from the calculation of the circular variance–based resVec metric because they pressed only once. Circular variance is undefined or yields a trivial value in such cases, leading to a resultant vector length of one. This would misleadingly indicate perfect consistency, which is not meaningful with only one observation. We used the normalized versions of the metrics for the comparison of CA between tasks: Pc2IFC for d, and SI for corSI. See Supplementary Information to explore for full details of the calculations.
For further analyses we divided the sample into groups with different levels of cardioception using two approaches. First, individuals were categorized depending on whether their metric was higher or lower than the median for this metric (high and low CA metric groups)85. This process was repeated separately for each metric, resulting in a number of high and low CA metric groups equal to the number of metrics used. Table 4 shows CA in groups with high and low CA metrics in tasks. Second, participants who pressed in the HTT were categorised into the detector and non-detector groups based on the non-uniform or uniform pressings circular distribution in the HTT, respectively. For the estimation of the uniformity we applied the Rayleigh uniformity test22. See Supplementary Information for the calculation of the pressings circular distribution.
Table 4.
CA in the HTT, HDT, and HCT in LOW and HIGH.
| Task | CA | median | LOW group (M ± SD) (n) | HIGH group (M ± SD) (n) |
|---|---|---|---|---|
| HTT | md | 0.32 | 0.08 ± 0.12 (24) | 0.72 ± 0.17 (24) |
| resVec | 0.1 | 0.05 ± 0.04 (24) | 0.31 ± 0.22 (23) | |
| CAcmotor | 0.1 | 0.03 ± 0.04 (24) | 0.21 ± 0.07 (24) | |
| HDT | d′ | −0.2 | −0.69 ± 0.39 (15) | 0.22 ± 0.43 (15) |
| с value | −0.2 | −0.75 ± 0.47 (15) | 0.52 ± 0.85 (15) | |
| HCT | corSI | 0.41 | − 0.37 ± 0.54 (24) | 0.76 ± 0.17 (24) |
Note. HCT- heartbeat counting task, HDT - heartbeat discrimination task, HIGH group - group with CA above the median of the given CA, HTT- heartbeat tapping task, CA - cardiac accuracy, LOW group - group with CA below the median of the given CA, n - number of participants, M - mean, SD - standard deviation.
Statistical analysis
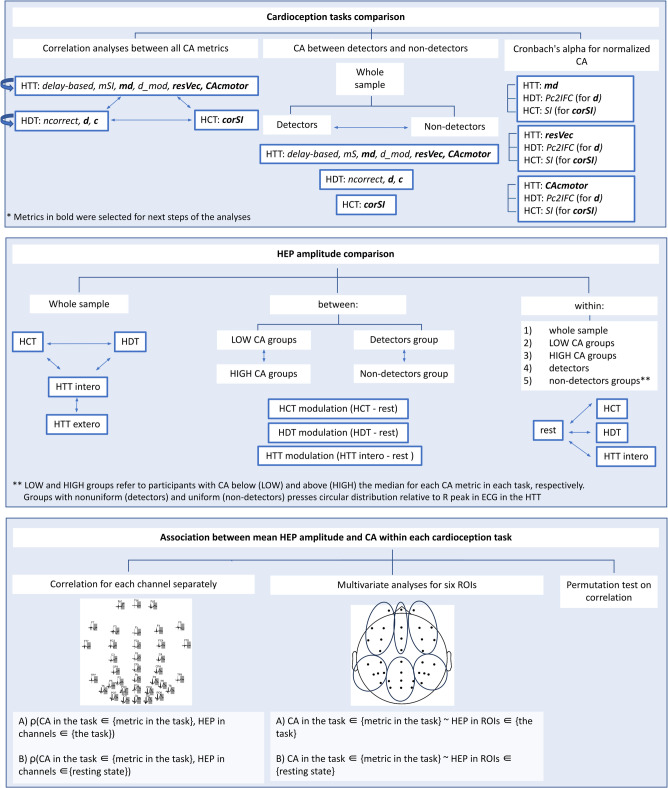
Figure 6. Scheme of statistical analysis. Note. CA - cardiac accuracy, HCT - heartbeat counting task, HDT - heartbeat discrimination task, HEP - heartbeat evoked potentials, HTT - heartbeat tapping task, ROIs - region of interest.
Fig. 6.
Presents a scheme of statistical analysis.
Cardioception tasks comparison
The analysis was performed using the open-source RStudio environment (v4.3.1, https://posit.co/download/rstudio-desktop/). The Shapiro-Wilk test was used to assess the deviation of the distribution from normal. The association between the number of pressings and the metrics in the HTT was examined using Spearman’s rank correlation. We conducted a pairwise correlation analysis between metrics across the tasks using Spearman’s rank correlation with Bonferroni correction. The comparison of metrics between detectors and non-detectors was performed using a Wilcoxon rank sum test with Bonferroni correction. The internal consistency of the three tasks was tested by Cronbach’s alphas.
HEP amplitudes comparison within conditions in the whole sample and in groups with different levels of cardioception
HEP amplitudes were compared between three behavioral cardioception tasks (HTT (interoceptive condition) vs. HDT, HTT (interoceptive condition) vs. HCT, HDT vs. HCT); between two conditions in the HTT (HTT (interoceptive condition) vs. HTT (exteroceptive condition)) within the whole sample. We also compared HEP amplitudes between tasks and resting state (HTT (interoceptive condition) vs. resting state, HDT vs. resting state, HCT vs. resting state) within the whole sample, within groups with high and low CA metrics, within detectors and non-detectors groups. A nonparametric spatio-temporal permutation test of the MNE-Python was used to compare HEP amplitudes. Test implemented nonparametric analysis86 to compare dependent data (using a two-tailed paired t-test) with temporal and spatial dimensions. See Supplementary Information for details on how this test worked.
HEP amplitudes modulation comparison between groups with different levels of cardioception
We used a nonparametric spatio-temporal permutation test from MNE-Python (see above) for independent (with one-way ANOVA) data to compare HEP amplitudes modulation between groups with high and low CA metrics in tasks, between detectors and non-detectors.
Association between mean HEP amplitudes and CA within each cardioception task
We analysed an association of HEP amplitudes during task (HTT (interoceptive condition), HDT, HCT) and each CA metric in the corresponding task: (1) for each channel separately; (2) for the mean HEP amplitudes for the six ROIs; (3) using a spatiotemporal cluster permutation test for a correlation. The cluster test was additionally used to address the problem of multiple comparisons that arises when dealing with channels and regions of interest. The cluster test allowed us to broaden or narrow the group of channels and the time period when it was significantly associated with CA.
Mean HEP amplitudes in channels, ROIs and CA.
The analysis was performed using the RStudio. Spearman’s rank correlation with correction for multiple comparisons was performed between (1) mean HEP amplitudes in channels during task and CA within each cardioception task, (2) mean HEP amplitudes in channels during resting state and CA within each cardioception task. The multiple comparison correction relied on the assumption that CA within tasks had high association and HEP amplitudes within channels also had high association. Therefore, the calculation of the number of genuine independent comparisons made in the correlation analysis was based on the number of principal components explaining 95% of the variance in the principal component analysis (PCA). PCA was computed on the matrix where participants were represented as rows and mean HEP amplitudes in channels during the task as columns. The number of components explaining 95% of the variance was 17 for the HTT, 15 for the HDT, 15 for the HCT and 17 for the resting state and used for Bonferroni correction.
We used a multivariate analysis to explore how HEP amplitudes among ROIs during this task and during resting state predicted each CA metric in the corresponding task. The combination of ROI-based predictors with the largest number and a variance inflation factor (VIF) below 3 was selected separately for each task and resting state condition to minimize multicollinearity. Left and right fronto-central, right temporo-parietal, and parieto-occipital were used in A1-A3, left and right fronto-central, and left temporo-parietal in A4-A6, midline and right fronto-central, right temporo-parietal, and parieto-occipital in B1-B3 and B6, left and right fronto-central, and left temporo-parietal in B4-B5. Generalized linear models were then fitted using these predictors.
A1-A3. CA in HTT ∈ {md, resVec, CAmotor} ~ HEP in ROIs ∈ {HTT}.
A4-A5. CA in HDT ∈ {d′, c value} ~ HEP in ROIs ∈ {HDT}.
A6. CA in HCT ∈ {corSI} ~ HEP in ROIs ∈ {HCT}.
B1-B3. CA in HTT ∈ {md, resVec, CAmotor} ~ HEP in ROIs ∈ {resting state}.
B4-B5. CA in HDT ∈ {d′, c value} ~ HEP in ROIs ∈ {resting state}.
B6. CA in HCT ∈ {corSI} ~ HEP in ROIs ∈ {resting state}.
We used a Benjamini-Hochberg correction across all ROI-predictor p-values from all models.
Mean HEP amplitudes in spatio-temporal clusters and CA.
The spatio-temporal permutation cluster test on correlation between CA and HEP amplitudes during tasks and resting state was performed using the MNE-Python with Monte-Carlo statistics. See Supplementary Information for parameters we set. The analysis was the same as in Maris and Oostenveld86 but Spearman’s rank correlation between CA and HEP amplitudes over samples in channels was converted to t-statistic.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Nikulin V.V. for counseling during the study conduction and manuscript preparation and to Huseynova K.A for assistance in the participants’ selection and recruitment and visual ECG inspection.
Author contributions
I.M. conducted data acquisition, formal analysis, developed the experiment software, and wrote the manuscript. A.L. contributed to conceptualization, funding acquisition, design, data acquisition, participant recruitment and selection, EEG data analysis, and writing. A.S. contributed to data acquisition and EEG data analysis. V.K. and I.M. performed the statistical analysis. M.N. contributed to conceptualization, funding acquisition, and manuscript revision. A.E. contributed to conceptualization, funding acquisition, design, participant recruitment and selection, study supervision, and manuscript revision. O.D. contributed to conceptualization and study supervision. All authors reviewed and approved the submitted version.
Funding
The authors received no specific funding for this work.
Data availability
The datasets generated and analysed during the current study and code we used are available in the Open Science Framework webpage. Please see https://osf.io/c3qws/?view_only=ea629fa512d34097976def3331354fe4.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Irina Minenko, Email: iaminenko@yandex.ru.
Alena Limonova, Email: limonova-alena@yandex.ru.
References
- 1., E. Al et al. Heart–brain interactions shape somatosensory perception and evoked potentials. Proc. Natl. Acad. Sci.117, 10575–10584 (2020). [DOI] [PMC free article] [PubMed]
- 2.Al, E. et al. Cardiac activity impacts cortical motor excitability. PLoS Biol.21, 1–23 (2023). [DOI] [PMC free article] [PubMed]
- 3.Zaki, J., Davis, J. I. & Ochsner, K. N. Overlapping activity in anterior Insula during interoception and emotional experience. Neuroimage62, 493–499 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couto, B. et al. The man who feels two hearts: the different pathways of interoception. Soc. Cogn. Affect. Neurosci. 9, 1253–60 (2014). [DOI] [PMC free article] [PubMed]
- 5.Herman, A. M., Esposito, G. & Tsakiris, M. Body in the face of uncertainty: the role of autonomic arousal and interoception in decision-making under risk and ambiguity. Psychophysiology58, e13840 (2021). [DOI] [PubMed]
- 6.Garfinkel, S. N. et al. Discrepancies between dimensions of interoception in autism: implications for emotion and anxiety. Biol. Psychol.114, 117–126 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Murphy, J., Brewer, R., Catmur, C. & Bird, G. Developmental cognitive neuroscience interoception and psychopathology : A developmental neuroscience perspective. Accid. Anal. Prev.23, 45–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palser, E. R., Fotopoulou, A., Pellicano, E. & Kilner, J. M. The link between interoceptive processing and anxiety in children diagnosed with autism spectrum disorder: extending adult findings into a developmental sample. Biol. Psychol.136, 13–21 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Abrevaya, S. et al. At the heart of neurological dimensionality: Cross-Nosological and multimodal cardiac interoceptive deficits. Psychosom. Med.82, 850–861 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer, R., Murphy, J. & Bird, G. Atypical interoception as a common risk factor for psychopathology: A review. Neurosci. Biobehav Rev.130, 470–508 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leopold, C. & Schandry, R. The heartbeat-evoked brain potential in patients suffering from diabetic neuropathy and in healthy control persons. Clin. Neurophysiol.112, 674–682 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Robinson, E., Foote, G., Smith, J., Higgs, S. & Jones, A. Interoception and obesity: a systematic review and meta-analysis of the relationship between interoception and BMI. Int. J. Obes.45, 2515–2526 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoris, A. et al. Multilevel convergence of interoceptive impairments in hypertension: new evidence of disrupted body–brain interactions. Hum. Brain Mapp.39, 1563–1581 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumral, D. et al. Attenuation of the Heartbeat-Evoked potential in patients with atrial fibrillation. JACC Clin. Electrophysiol. 8, 1219-1230 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Bonaz, B. et al. Diseases, Disorders, and Comorbidities of Interoception. Trends in Neurosciences44, 39–51 (2021). [DOI] [PubMed]
- 16.Schandry, R. Heart beat perception and emotional experience. Psychophysiology18, 483–488 (1981). [DOI] [PubMed] [Google Scholar]
- 17.McFarland, R. A. Heart Rate Perception and Heart Rate Control. Psychophysiology12, 402–405 (1975). [DOI] [PubMed]
- 18.Brener, J. & Jones, M. Interoceptive discrimination in intact humans: detection of cardiac activity. Physiol. Behav.13, 763–767 (1974). [DOI] [PubMed] [Google Scholar]
- 19.Whitehead, W. E., Drescher, V. M., Heiman, P. & Blackwell, B. Relation of heart rate control to heartbeat perception. Biofeedback Self Regul.2, 371–392 (1977). [PubMed] [Google Scholar]
- 20.Forrest, L. N. & Smith, A. R. A multi-measure examination of interoception in people with recent nonsuicidal self-injury. Suicide Life Threat Behav.51, 492–503 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Santos, L. E. R. et al. Reliability of the heartbeat tracking task to assess interoception. Appl. Psychophysiol. Biofeedback. 48, 171–178 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Körmendi, J., Ferentzi, E. & Köteles, F. A heartbeat away from a valid tracking task. An empirical comparison of the mental and the motor tracking task. Biol Psychol171, 108328 (2022). [DOI] [PubMed]
- 23.Brener, J. & Ring, C. Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philosophical Trans. Royal Soc. B: Biol. Sciences371, 20160015 (2016). [DOI] [PMC free article] [PubMed]
- 24.Schulz, A., Back, S. N., Schaan, V. K., Bertsch, K. & Vögele, C. On the construct validity of interoceptive accuracy based on heartbeat counting: cardiovascular determinants of absolute and tilt-induced change scores. Biol. Psychol.164, 108168 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Körmendi, J., Ferentzi, E., Petzke, T., Gál, V. & Köteles, F. Do we need to accurately perceive our heartbeats? Cardioceptive accuracy and sensibility are independent from indicators of negative affectivity, body awareness, body image dissatisfaction, and alexithymia. PLoS One18, e0287898 (2023). [DOI] [PMC free article] [PubMed]
- 26.Brener, J., Liu, X. & Ring, C. A method of constant stimuli for examining heartbeat detection: comparison with the Brener-Kluvitse and Whitehead methods. Psychophysiology30, 657–665 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Brener, J., Ring, C. & Liu, X. Effects of data limitations on heartbeat detection in the method of constant stimuli. Psychophysiology31, 309–312 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Badoud, D. & Tsakiris, M. From the body’s viscera to the body’s image: is there a link between interoception and body image concerns? Neurosci. Biobehav Rev.77, 237–246 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Drew, R. E., Ferentzi, E., Tihanyi, B. T. & Köteles, F. There are no short-term longitudinal associations among interoceptive accuracy, external body orientation, and body image dissatisfaction. Clinical Psychol. Europe2, e2701 (2020). [DOI] [PMC free article] [PubMed]
- 30.Pollatos, O., Traut-Mattausch, E. & Schandry, R. Differential effects of anxiety and depression on interoceptive accuracy. Depress. Anxiety. 26, 167–173 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Shah, P., Hall, R., Catmur, C. & Bird, G. Alexithymia, not autism, is associated with impaired interoception. Cortex81, 215–220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domschke, K., Stevens, S., Pfleiderer, B. & Gerlach, A. L. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of Neurobiological findings. Clin. Psychol. Rev.30, 1–11 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Desmedt, O. et al. How does heartbeat counting task performance relate to Theoretically-Relevant mental health outcomes? A Meta-Analysis. Collabra Psychol8, 33271 (2022).
- 34.Desmedt, O., Luminet, O., Walentynowicz, M. & Corneille, O. The new measures of interoceptive accuracy: A systematic review and assessment. Neurosci. Biobehav Rev.153, 105388 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Ring, C., Brener, J., Knapp, K. & Mailloux, J. Effects of heartbeat feedback on beliefs about heart rate and heartbeat counting: A cautionary Tale about interoceptive awareness. Biol. Psychol.104, 193–198 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Canales-Johnson, A. et al. Auditory feedback differentially modulates behavioral and neural markers of objective and subjective performance when tapping to your heartbeat. Cereb. Cortex. 25, 4490–4503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ring, C. & Brener, J. Influence of beliefs about heart rate and actual heart rate on heartbeat counting. Psychophysiology33, 541–546 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Windmann, S., Schonecke, O. W., Fröhlig, G. & Maldener, G. Dissociating beliefs about heart rates and actual heart rates in patients with cardiac pacemakers. Psychophysiology36, 339–342 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Fittipaldi, S. et al. A multidimensional and multi-feature framework for cardiac interoception. Neuroimage212, 116677 (2020). [DOI] [PMC free article] [PubMed]
- 40.Knoll, J. F. & Hodapp, V. A. Comparison between two methods for assessing heartbeat perception. Psychophysiology29, 218–222 (1992). [DOI] [PubMed] [Google Scholar]
- 41.Legrand, N. et al. The heart rate discrimination task: A psychophysical method to estimate the accuracy and precision of interoceptive beliefs. Biol. Psychol.168, 108239 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Schandry, R., Sparrer, B. & Weitkunat, R. From the heart to the brain: A study of heartbeat contingent scalp potentials. Int. J. Neurosci.30, 261–275 (1986). [DOI] [PubMed] [Google Scholar]
- 43.Kern, M., Aertsen, A., Schulze-Bonhage, A. & Ball, T. Heart cycle-related effects on event-related potentials, spectral power changes, and connectivity patterns in the human ECoG. Neuroimage81, 178–190 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Park, H. D. et al. Neural sources and underlying mechanisms of neural responses to heartbeats, and their role in bodily Self-consciousness: an intracranial EEG study. Cereb. Cortex. 28, 2351–2364 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Huynh, K. Heartbeat-induced pressure pulsations in cerebral arteries modulate neuronal activity. Nature Reviews Cardiology. 21, 218-218 (2024) [DOI] [PubMed]
- 46.Jammal Salameh, L., Bitzenhofer, S. H., Hanganu-Opatz, I. L., Dutschmann, M. & Egger, V. Blood pressure pulsations modulate central neuronal activity via mechanosensitive ion channels. Science383, eadk8511 (2024). [DOI] [PubMed]
- 47.Pollatos, O. & Schandry, R. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology41, 476–482 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Pollatos, O., Herbert, B. M., Mai, S. & Kammer, T. Changes in interoceptive processes following brain stimulation. Philosophical Trans. Royal Soc. B: Biol. Sci.371, 20160016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coll, M. P., Hobson, H., Bird, G. & Murphy, J. Systematic review and meta-analysis of the relationship between the heartbeat-evoked potential and interoception. Neurosci. Biobehav Rev.122, 190–200 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Rouse, C. H., Jones, G. E. & Jones, K. R. The effect of body composition and gender on cardiac awareness. Psychophysiology25, 400–407 (1988). [DOI] [PubMed] [Google Scholar]
- 51.Grabauskaitė, A., Baranauskas, M. & Griškova-Bulanova, I. Interoception and gender: what aspects should we pay attention to? Conscious. Cogn.48, 129–137 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Wiens, S., Mezzacappa, E. S. & Katkin, E. S. Heartbeat detection and the experience of emotions. Cogn. Emot.14, 417–427 (2000). [Google Scholar]
- 53.Herbert, B. M., Ulbrich, P. & Schandry, R. Interoceptive sensitivity and physical effort: implications for the self-control of physical load in everyday life. Psychophysiology44, 194–202 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Schneider, T. R., Ring, C. & Katkin, E. S. A test of the validity of the method of constant stimuli as an index of heartbeat detection. Psychophysiology35, 86–89 (1998). [PubMed] [Google Scholar]
- 55.Hickman, L., Seyedsalehi, A., Cook, J. L., Bird, G. & Murphy, J. The relationship between heartbeat counting and heartbeat discrimination: A meta-analysis. Biol. Psychol.156, 107949 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Hart, N., McGowan, J., Minati, L. & Critchley, H. D. Emotional regulation and bodily sensation: interoceptive awareness is intact in borderline personality disorder. J. Pers. Disord. 27, 506–518 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Forkmann, T. et al. Making sense of what you sense: disentangling interoceptive awareness, sensibility and accuracy. Int. J. Psychophysiol.109, 71–80 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Schulz, A., Lass-Hennemann, J., Sütterlin, S., Schächinger, H. & Vögele, C. Cold pressor stress induces opposite effects on cardioceptive accuracy dependent on assessment paradigm. Biol. Psychol.93, 167–174 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Ring, C. & Brener, J. Heartbeat counting is unrelated to heartbeat detection : A comparison of methods to quantify interoception. Psychophysiology55, e13084 (2018). [DOI] [PubMed]
- 60.Pennebaker, J. W. & Hoover, C. W. Visceral perception versus visceral detection: disentangling methods and assumptions. Biofeedback Self Regul.9, 339–352 (1984). [DOI] [PubMed] [Google Scholar]
- 61.Körmendi, J. & Ferentzi, E. Heart activity perception: narrative review on the measures of the cardiac perceptual ability. Biol. Futur. 75, 3-15 (2023). [DOI] [PubMed] [Google Scholar]
- 62.Yoris, A. et al. Multicentric evidence of emotional impairments in hypertensive heart disease. Sci. Rep.10, 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marshall, A. C., Gentsch, A., Schröder, L. & Schütz-Bosbach, S. Cardiac interoceptive learning is modulated by emotional Valence perceived from facial expressions. Soc. Cogn. Affect. Neurosci.13, 677–686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lutz, A. P. C. et al. Enhanced cortical processing of cardio-afferent signals in anorexia nervosa. Clin. Neurophysiol.130, 1620–1627 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Schulz, A. et al. Altered patterns of heartbeat-evoked potentials in depersonalization/derealization disorder: neurophysiological evidence for impaired cortical representation of bodily signals. Psychosom. Med.77, 506–516 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Khalsa, S. S., Rudrauf, D. & Tranel, D. Interoceptive awareness declines with age. Psychophysiology46, 1130–1136 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gray, M. A. et al. A cortical potential reflecting cardiac function. Proc. Natl. Acad. Sci. U S A. 104, 6818–6823 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katkin, E. S., Cestaro, V. L. & Weitkunat, R. Individual differences in cortical evoked potentials as a function of heartbeat detection ability. Int. J. Neurosci.61, 269–276 (1991). [DOI] [PubMed] [Google Scholar]
- 69.Banellis, L. & Cruse, D. Skipping a beat: Heartbeat-Evoked potentials reflect predictions during Interoceptive-Exteroceptive integration. Cereb Cortex Commun1, tgaa060 (2020). [DOI] [PMC free article] [PubMed]
- 70.Van Elk, M., Lenggenhager, B., Heydrich, L. & Blanke, O. Suppression of the auditory N1-component for heartbeat-related sounds reflects interoceptive predictive coding. Biol. Psychol.99, 172–182 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Mai, S., Wong, C. K., Georgiou, E. & Pollatos, O. Interoception is associated with heartbeat-evoked brain potentials (HEPs) in adolescents. Biol. Psychol.137, 24–33 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Terhaar, J., Viola, F. C., Bär, K. J. & Debener, S. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin. Neurophysiol.123, 1950–1957 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Zaccaro, A. et al. Attention to cardiac sensations enhances the heartbeat-evoked potential during exhalation. iScience27, 109586 (2024). [DOI] [PMC free article] [PubMed]
- 74.Zamariola, G., Maurage, P., Luminet, O. & Corneille, O. Interoceptive accuracy scores from the heartbeat counting task are problematic: evidence from simple bivariate correlations. Biol. Psychol.137, 12–17 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Marshall, A. C., Gentsch, A., Jelinčić, V. & Schütz-Bosbach, S. Exteroceptive expectations modulate interoceptive processing: repetition-suppression effects for visual and heartbeat evoked potentials. Sci. Rep.7, 16525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Desmedt, O., Luminet, O. & Corneille, O. The heartbeat counting task largely involves non-interoceptive processes: evidence from both the original and an adapted counting task. Biol. Psychol.138, 185–188 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Petzschner, F. H. et al. Focus of attention modulates the heartbeat evoked potential. Neuroimage186, 595–606 (2019). [DOI] [PubMed] [Google Scholar]
- 78.García-Cordero, I. et al. Attention, in and out: Scalp-Level and intracranial EEG correlates of interoception and exteroception. Front Neurosci11, 411 (2017). [DOI] [PMC free article] [PubMed]
- 79.Baess, P., Horváth, J., Jacobsen, T. & Schröger, E. Selective suppression of self-initiated sounds in an auditory stream: an ERP study. Psychophysiology48, 1276–1283 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Peirce, J. et al. PsychoPy2: experiments in behavior made easy. Behav. Res. Methods. 51, 195–203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleckner, I. R., Wormwood, J. B., Simmons, W. K., Barrett, L. F. & Quigley, K. S. Methodological recommendations for a heartbeat Detection-Based measure of interoceptive sensitivity. Psychophysiology52, 1432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiens, S. & Palmer, S. N. Quadratic trend analysis and heartbeat detection. Biol. Psychol.58, 159–175 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Gramfort, A. MEG and EEG data analysis with MNE-Python. Front Neurosci7, 267 (2013). [DOI] [PMC free article] [PubMed]
- 84.Jas, M. et al. Automated artifact rejection for MEG and EEG data. Neuroimage159, 417–429 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lenggenhager, B., Azevedo, R. T., Mancini, A. & Aglioti, S. M. Listening to your heart and feeling yourself: effects of exposure to interoceptive signals during the ultimatum game. Exp. Brain Res.230, 233–241 (2013). [DOI] [PubMed] [Google Scholar]
- 86.Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 164, 177–190 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Melloni, M. et al. Preliminary evidence about the effects of meditation on interoceptive sensitivity and social cognition. Behav. Brain Funct.9, 47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sedeño, L. et al. How do you feel when you can’t feel your body? Interoception, functional connectivity and emotional processing in Depersonalization-Derealization disorder. PLoS One. 9, e98769 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoris, A. et al. The roles of interoceptive sensitivity and metacognitive interoception in panic. Behav. Brain Funct.11, 14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoris, A. et al. The inner world of overactive monitoring: neural markers of interoception in obsessive–compulsive disorder. Psychol. Med.47, 1957–1970 (2017). [DOI] [PubMed] [Google Scholar]
- 91.García-Cordero, I. et al. Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philosophical Trans. Royal Soc. B: Biol. Sciences371, 20160006 (2016). [DOI] [PMC free article] [PubMed]
- 92.Herman, A. M., Rae, C. L., Critchley, H. D. & Duka, T. Interoceptive accuracy predicts nonplanning trait impulsivity. Psychophysiology56, e13339 (2019). [DOI] [PubMed]
- 93.Hina, F. & Aspell, J. E. Altered interoceptive processing in smokers: evidence from the heartbeat tracking task. Int. J. Psychophysiol.142, 10–16 (2019). [DOI] [PubMed] [Google Scholar]
- 94.Ewing, D. L. et al. Sleep and the heart: interoceptive differences linked to poor experiential sleep quality in anxiety and depression. Biol. Psychol.127, 163–172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garfinkel, S. N., Seth, A. K., Barrett, A. B., Suzuki, K. & Critchley, H. D. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol.104, 65–74 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study and code we used are available in the Open Science Framework webpage. Please see https://osf.io/c3qws/?view_only=ea629fa512d34097976def3331354fe4.