Abstract
Raf-1 protein kinase has been identified as an integral component of the Ras/Raf/MEK/ERK signalling pathway in mammals. Activation of Raf-1 is achieved by Ras.GTP binding and other events at the plasma membrane including tyrosine phosphorylation at residues 340/341. We have used gene targeting to generate a ‘knockout’ of the raf-1 gene in mice as well as a rafFF mutant version of endogenous Raf-1 with Y340FY341F mutations. Raf-1–/– mice die in embryogenesis and show vascular defects in the yolk sac and placenta as well as increased apoptosis of embryonic tissues. Cell proliferation is not affected. Raf-1 from cells derived from raf-1FF/FF mice has no detectable activity towards MEK in vitro, and yet raf-1FF/FF mice survive to adulthood, are fertile and have an apparently normal phenotype. In cells derived from both the raf-1–/– and raf-1FF/FF mice, ERK activation is normal. These results strongly argue that MEK kinase activity of Raf-1 is not essential for normal mouse development and that Raf-1 plays a key role in preventing apoptosis.
Keywords: apoptosis/knockout/MEK kinase/Raf-1/tyrosine phosphorylation
Introduction
The mammalian raf-1 gene was first identified as the cellular homologue of v-raf, the oncogene responsible for the induction of sarcomas in mice by the MSV3611 virus. Two other genes highly homologous to raf-1 have been cloned: A-raf and B-raf. All three raf genes code for serine/threonine protein kinases and they share a high degree of sequence similarity. The N-terminal region encodes the regulatory domain and binds essential cofactors including Ras while the C-terminal region contains the catalytic kinase domain. Deletion of the N-terminal regulatory regions of all three kinases gives rise to proteins that are constitutively active and are oncogenic in a wide variety of cell types. The kinase domain of B-raf appears to be the most potent of the three in these assays (Pritchard et al., 1995). In the mouse, transcripts for all three raf genes are detectable in all cells (Storm et al., 1990; Barnier et al., 1995).
Of the three Raf isotypes, most biochemical studies have focused on Raf-1. Inactive Raf-1 is normally cytosolic, but Raf-1 binds to Ras.GTP in vitro and in vivo and so translocates to the plasma membrane in the presence of active Ras (Marais and Marshall, 1996 and references therein). However, binding to Ras is not sufficient for full Raf-1 activation (Traverse et al., 1993; Marais et al., 1995, 1997; Mason et al., 1999) and additional signals at the plasma membrane including phosphorylation are required (Marais and Marshall, 1996). Our previous studies in COS cells have shown that activation of Raf-1 requires phosphorylation of Y340 and/or Y341. Substitution of these residues to phenylalanine, creating RafFF, blocks activation of Raf-1 by oncogenic Ras and Src, and by ligand stimulation (Marais et al., 1995, 1997; Diaz et al., 1997; Stokoe and McCormick, 1997; Barnard et al., 1998). Recent data have also suggested that phosphorylation of Raf-1 at serine 338 is required for activation, demonstrating that complex phosphorylation events take place within this region of Raf-1 (Diaz et al., 1997; Barnard et al., 1998; Mason et al., 1999). However, the physiological importance of these phosphorylation events is unclear.
The principal functions of the Raf protein kinases appear to be participation in the highly conserved Ras/Raf/MEK/ERK intracellular signalling pathway (Marshall 1994). This pathway is activated by different classes of cell surface receptors including receptor tyrosine kinases (RTKs) and G protein coupled seven transmembrane receptors, all of which confer their biological effects through Ras (Dickson and Hafen, 1994; Marshall, 1994). ERK activation has been associated with many of the downstream consequences of Ras activation and the Raf proteins provide a vital link between activated Ras proteins and the ERKs. A variety of biochemical and genetic data point to the importance of Raf-1 as a MEK activator. Activation of an inducible version of oncogenic Raf-1 induces the rapid activation of MEK and ERK as well as immediate early gene expression in NIH 3T3 cells (Samuels et al., 1993; Kerkhoff and Rapp, 1997). Immunoprecipitated endogenous Raf-1 can phosphorylate MEK1 and -2 in vitro (Howe et al., 1992; Kyriakis et al., 1992; Marais et al., 1998) and the Raf/MEK/ERK cascade can be reconstituted in vitro using proteins expressed in Sf9 cells (Macdonald et al., 1993). Kinase inactive Raf-1 cannot activate MEK in this system. Finally, dominant-negative Raf-1 mutants block growth factor and oncogenic ras-stimulated activation of ERKs in fibroblasts (Schaap et al., 1993; Chao et al., 1994; Troppmair et al., 1994).
Intriguingly, a number of observations do not entirely fit with the view that the endogenous Raf-1 protein is a physiologically important MEK activator (Marais and Marshall, 1996). First, only a small proportion (<10%) of the entire cellular Raf-1 is activated upon treatment of cells with growth factors (Dent et al., 1995; Reuter et al., 1995; Jelinek et al., 1996). Secondly, B-Raf has been identified as the major MEK activator in neuronal cell types and NIH 3T3 cells, despite Raf-1 also being expressed in these cells (Moodie et al., 1993, 1994; Catling et al., 1994; Jaiswal et al., 1994; Traverse and Cohen, 1994; Reuter et al., 1995). The kinase domain of B-Raf is a considerably stronger activator of MEK and has a higher affinity for MEK than the kinase domain of Raf-1 (Pritchard et al., 1995; Marais et al., 1997; Papin et al., 1998). Finally, Raf-1 and ERK activation are not coincident in some circumstances (Wood et al., 1992, 1993; Kuo et al., 1996). A pool of Raf-1 is also thought to be located in mitochondria (Wang et al., 1996) and an ERK-independent role for mitochondrial Raf-1 in apoptosis has been postulated (Neshat et al., 2000).
Many of the previous studies designed to address the involvement of Raf-1 in mediating signals between Ras and ERKs have used antisense or dominant-negative constructs overexpressed in tissue culture cell lines to inhibit its expression or activity (Kolch et al., 1991; Chao et al., 1994; Troppmair et al., 1994). These approaches have the disadvantage that they may sequester the function/expression of other Raf isotypes (A-Raf and B-Raf) or other Ras effectors. To achieve the required specificity, gene targeting has been used to ablate individual raf genes (Pritchard et al., 1996; Wojnowski et al., 1997, 1998). In this study, we describe the generation of Raf-1 deficient mice (raf-1–/–) as well as mice with a ‘knockin’ mutation of the endogenous 340/341 tyrosines to phenylalanine (raf-1FF/FF). Although there is no detectable Raf-1 activity in cells derived from either strain of mouse, ERK activation was not compromised in either. However, the raf-1–/– animals die in embryogenesis due to vascularization defects and increased apoptosis, while the raf-1FF/FF animals survive to adulthood and have an apparently normal phenotype. Our results show that the full-length Raf-1 protein is essential for normal mouse development and for protection against apoptosis, but they argue that Raf-1 kinase activity towards MEK is not necessary for these processes.
Results
Generation and phenotype analysis of raf-1–/– and raf-1FF/FF mutant mice
The generation of the mutant mice is described in the Supplementary data (available at The EMBO Journal Online.) Both mutations were established on the mixed 129Ola/C57BL6 and 129Ola/MF-1 backgrounds. For the raf-1 knockout, raf-1+/– animals were intercrossed and PCR genotype analysis was performed on tail DNAs from surviving offspring. No surviving animals with the raf-1–/– genotype were obtained (n = 14 matings) but raf-1+/– animals were born at the expected Mendelian frequency and were indistinguishable from raf-1+/+ littermates. Therefore, raf-1+/– intercrosses were set up and embryos were genotyped. On the 129Ola/C57BL6 background, at E9.5, a number of abnormal embryos were observed and these all PCR genotyped as raf-1–/– (Table I). On the 129Ola/MF-1 background, in early backcross generations, a phenotype similar to that on the 129Ola/C57BL6 background was observed (Table II). However, a number of normal embryos at E9.5–E10.5 also typed as raf-1–/– (Table II). When the raf-1 mutation was further backcrossed to the MF-1 strain, raf-1–/– embryos were observed at E12.5 up to birth but these were small and morphologically abnormal.
Table I. Genotyping data from raf-1+/– intercrosses: C57BL6 background.
| Age | raf-1+/+ | raf-1+/– | raf-1–/– normal | raf-1–/– abnormal | Resorbeda | Untypedb |
|---|---|---|---|---|---|---|
| E3.5 | 2 | 5 | 3 | 0 | – | 3 |
| E8.5 | 2 | 4 | 3 | 0 | 0 | 0 |
| E9.5 | 17 | 26 | 0 | 12 | 1 | 0 |
| E10.5 | 7 | 16 | 0 | 10 | 9 | 0 |
| E11.5 | 14 | 20 | 0 | 6 | 14 | 0 |
| E12.5 | 4 | 2 | 0 | 0 | 0 | 0 |
| E15.5 | 0 | 2 | 0 | 0 | 2 | 0 |
| Total | 46 | 75 | 6 | 28 | 26 | 3 |
aResorbed tissue had degenerated too much to dissect cleanly.
bDNA samples did not PCR amplify.
Table II. Genotyping data from raf-1+/– intercrosses: MF-1 background.
| Age | raf-1+/+ | raf-1+/– | raf-1–/– normal | raf-1–/– abnormal | Resorbeda | Untypedb |
|---|---|---|---|---|---|---|
| E8.5 | 2 | 6 | 1 | 0 | 0 | 0 |
| E9.5 | 3 | 14 | 1 | 1 | 0 | 0 |
| E10.5 | 3 | 1 | 3 | 2 | 0 | 1 |
| E12.5 | 3 | 4 | 0 | 1 | 3 | 3 |
| E13.5 | 7 | 9 | 0 | 1 | 1 | 1 |
| E14.5 | 6 | 6 | 0 | 3 | 2 | 7 |
| E18.5 | 16 | 20 | 0 | 3 | 0 | 2 |
| Birth (P1) | 3 | 10 | 0 | 5c | – | 2 |
| Total | 43 | 70 | 5 | 16 | 6 | 16 |
aResorbed tissue had degenerated too much to dissect cleanly.
bDNA samples did not PCR amplify.
cAnimals died a few hours after birth.
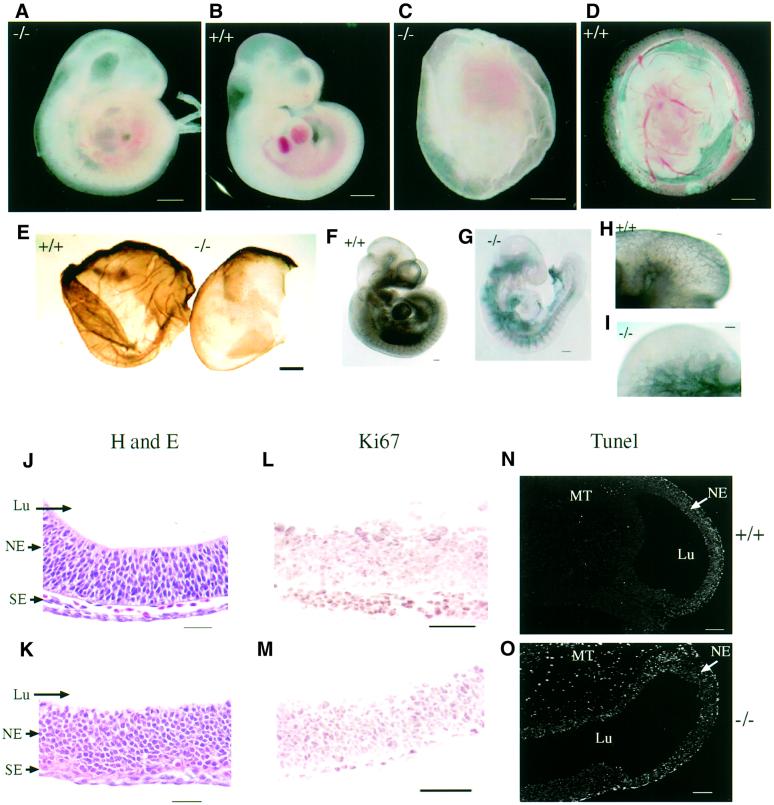
On the 129Ola/C57BL6 background, at E9.5, raf-1–/– embryos were morphologically smaller and were reduced in size by approximately one-third (Figure 1A and B). They were developmentally arrested, had fewer somites and were anaemic, but were still alive as judged by the presence of a regular heartbeat. Strikingly, all of the mutants lacked small and large blood vessels in the yolk sac (Figure 1C and D), as visualized by staining with an antibody to platelet endothelial cell adhesion molecule-1 (PECAM-1; Figure 1E). PECAM-1 staining of the raf-1–/– embryos also revealed abnormal vascular network formation (Figure 1F–I). In the head region, there was a reduction in the number of large and small blood vessels, the vessels were disorganized and there were no capillary sprouts into the neuroectoderm (Figure 1H and I). Haemorrhaging was observed in approximately one- quarter of the mutant embryos (data not shown). There was a significant reduction in the number and density of cells throughout the mutant embryos but cell size appeared larger (Figure 1J and K). Staining of the embryonic brain with the Ki67 antibody, a marker for cells in S phase, showed that there was no significant reduction in the numbers of proliferating cells in the raf-1–/– embryos (Figure 1L and M). However, the terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay indicated that there was an increase in the numbers of apoptotic cells throughout the mutant embryos (Figure 1N and O).
Fig. 1. Phenotype of the 129Ola/C57BL6 raf-1–/– embryos. (A) raf-1–/– embryo at E9.5; (B) raf-1+/+ littermate embryo at E9.5; (C) raf-1–/– embryo at E9.5 in yolk sac; (D) raf-1+/+ littermate embryo at E9.5 in yolk sac; (E) PECAM-1 immunostaining of yolk sacs from wild-type embryo at E9.5 (left) and from mutant embryo (right). (F–I) PECAM-1 staining of raf-1+/+ embryo (F and H) and raf-1–/– embryo (G and I) at E9.5. (H) and (I) are magnifications of the embryonic brain regions. (J–O) Longitudinal sections through forebrain of raf-1+/+ (J, L and N) and raf-1–/– (K, M and O) embryos at E9.5. (J) and (K) were stained with haematoxylin and eosin, (L) and (M) were immunostained with a Ki67 antibody, and (N) and (O) were processed for TUNEL staining. Scale bars: A–E, 250 µm; F and G, 100 µm; H and I, 25 µm, J–M, 50 µm; N and O, 100 µm. NE, neural ectoderm; SE, surface ectoderm; Lu, lumen of prospective forebrain; MT, cephalic mesenchyme tissue.
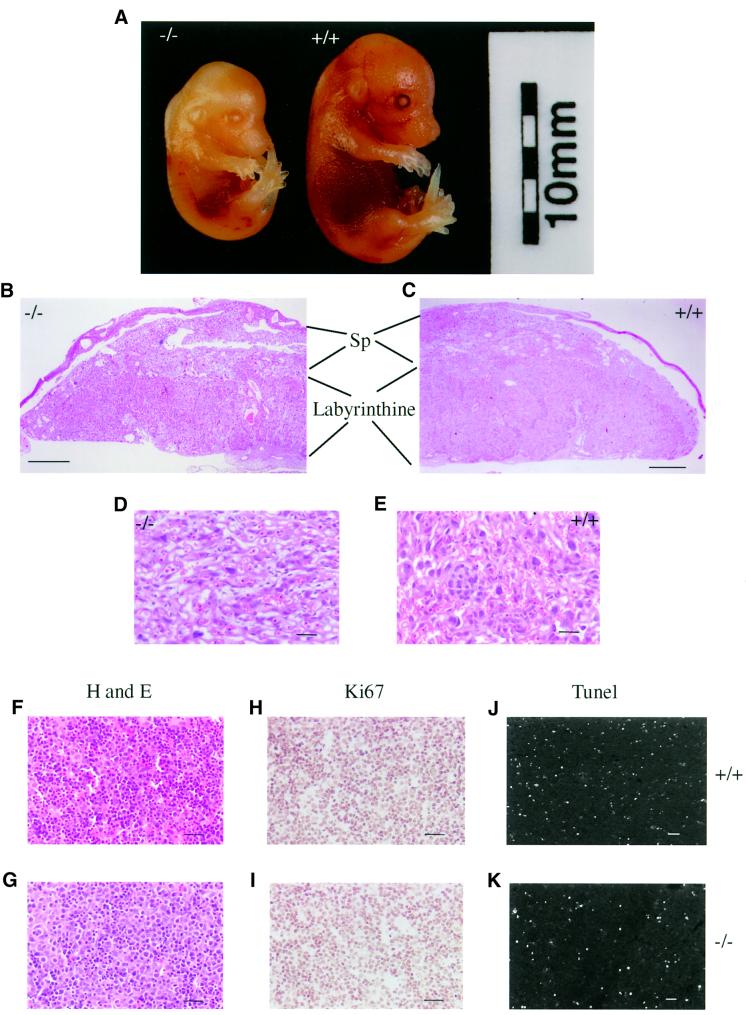
On the 129Ola/MF-1 background, the raf-1–/– embryos died within a few hours of birth. They were 50–60% lighter in weight than the raf-1+/+ embryos and they were anaemic (Figure 2A). The placenta was considerably smaller than the raf-1+/+ placenta and was disorganized (Figure 2B and C). The spongiotrophoblast layer was considerably reduced in size, a number of the mesenchymal cells of the labyrinthine layer were undifferentiated and there were fewer blood vessels (Figure 2D and E). As with the 129Ola/C57BL6 raf-1–/– embryos, there were fewer cells in the mutant liver than the wild-type liver, but the mutant cells appeared to be greater in size (Figure 2F–K). In addition, there were notably fewer areas of haemopoiesis (Figure 2F and G). There did not appear to be any difference in the number of proliferating cells in the raf-1–/– liver compared with the raf-1+/+ liver (Figure 2H and I). There was also no significant difference in the number of TUNEL positive cells in the raf-1–/– liver sections compared with the raf-1+/+ liver sections (Figure 2J and K), indicating no significant difference in levels of spontaneous apoptosis. The fact that the raf-1–/– livers are hypocellular is likely to have been caused by increased susceptibility to apoptosis at earlier developmental stages (see below).
Fig. 2. Phenotype of the 129Ola/MF-1 raf-1–/– embryos. (A) Photograph of raf-1–/– embryo (left) and raf-1+/+ littermate embryo (right) at E15.5. (B and C) Cross-sections of placentas from raf-1–/– (B) and raf-1+/+ (C) embryos showing the labyrinthine and spongiotrophoblast (Sp) layers. (D and E) Cross-sections of labyrinthine layer of placentas from raf-1–/– (D) and raf-1+/+ (E) embryos. (F–K) Cross-sections of liver from littermate raf-1–/– (G, I and K) and raf-1+/+ embryos at E15.5. These sections were either stained with haematoxylin and eosin (F and G), immunostained with the Ki67 antibody (H and I) or processed for TUNEL staining (J and K). Scale bars: B and C, 400 µm; D–K, 50 µm.
To assess the raf-1FF/FF phenotype, raf-1+/FF animals on the 129Ola/C57BL6 and 129Ola/MF-1 backgrounds were intercrossed and PCR genotype analysis was performed on tail DNAs from surviving offspring. Of 148 animals assessed, 45 typed as raf-1+/+, 67 as raf-1+/FF and 36 as raf-1FF/FF (n = 19 matings). Therefore, there was no significant difference in the ratio of raf-1+/+ to raf-1FF/FF animals surviving to adulthood (P = 0.15). The raf-1FF/FF animals on both genetic backgrounds survived for >1 year, were normal in weight and had no behavioural abnormalities. T-cell development was normal in these animals as judged by CD4 and CD8 staining of T cells (data not shown).
Analysis of proliferation and apoptosis in raf-1–/– and raf-1FF/FF MEFs
Mouse embryonic fibroblasts (MEFs) were derived from the raf-1–/– and raf-1FF/FF embryos and characterized for their ability to proliferate and undergo apoptosis in vitro. We consistently failed to culture raf-1–/– MEFs from E10.5 on the 129Ola/C57BL6 background as most of the cells were dead following embryo homogenization. This may be because of the apparent spontaneous apoptosis of the raf-1–/– embryos observed on this genetic background (Figure 1N and O). However, we successfully obtained raf-1–/– MEFs from E14.5 embryos on the 129Ola/MF-1 background. Raf-1FF/FF MEFs were isolated from E14.5 embryos resulting from raf-1+/FF intercrosses on both genetic backgrounds. Sibling raf-1+/+ MEFs were also isolated in each case.
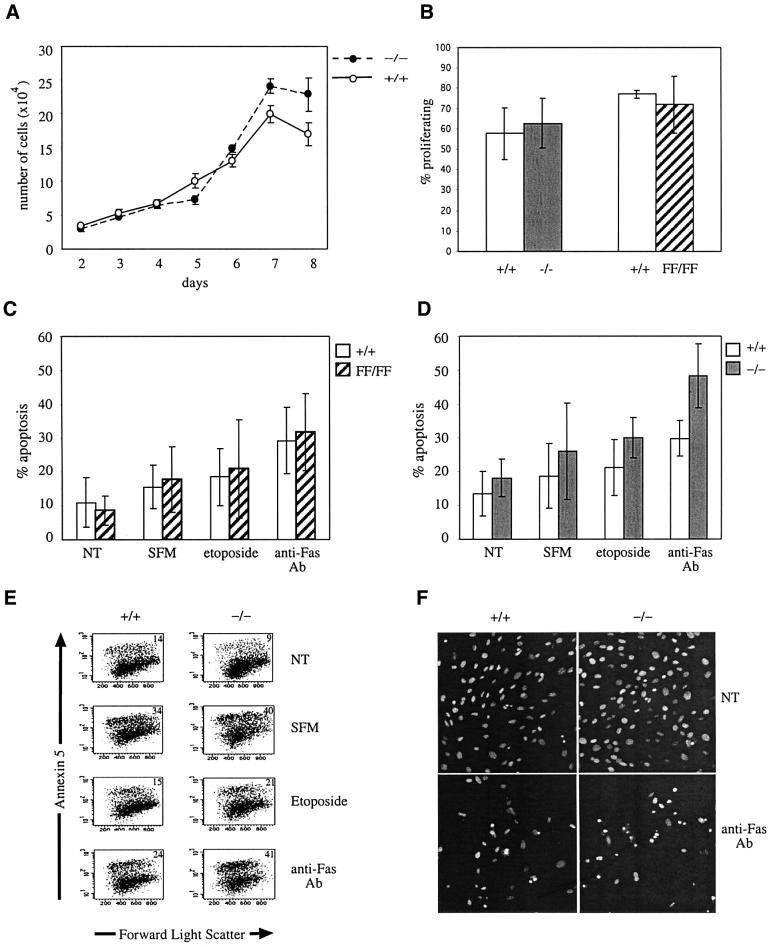
There was no detectable difference between the growth rates of the raf-1–/– cells compared with raf-1+/+ cells over 8 days in culture (Figure 3A), or the raf-1FF/FF cells compared with the raf-1+/+ cells (data not shown). Cell proliferation was measured by assessing bromodeoxyuridine (BrdU) incorporation in MEFs that had been made quiescent and then stimulated with 10% fetal calf serum (FCS). Again, the raf-1–/– and raf-1FF/FF MEFs showed no difference in their ability to undergo DNA synthesis as measured by this assay compared with raf-1+/+ MEFs (Figure 3B).
Fig. 3. Proliferation and apoptosis analysis of MEFs. (A) Growth curves of raf-1+/+ MEFs (open circles) compared with raf-1–/– MEFs (closed circles) over 8 days in culture are shown. (B) DNA synthesis of raf-1–/– and raf-1FF/FF primary MEFs compared with raf-1+/+ cells induced by 10% serum. The data represent pooled data from four experiments of cells with each genotype. (C and D) Levels of apoptosis in raf-1FF/FF cells compared with raf-1+/+ cells (C) and in raf-1–/– cells compared with raf-1+/+ cells (D). Cells were either not treated (NT) or treated with serum-free media (SFM), etoposide or anti-Fas antibody for 20 h. The percentage of cells undergoing apoptosis was quantified by flow cytometric analysis of annexin V staining. Each experiment was performed seven times and the data show mean values ± standard deviation. (E) An example of flow cytometric analysis of annexin V staining of cells for data presented in (C) and (D). The percentage of annexin V positive cells is indicated. (F) Hoechst 33258 staining of apoptotic cells. raf-1+/+ cells (left panels) and raf-1–/– cells (right panels) were either untreated (top panels) or treated with anti-Fas antibody (bottom panels).
Except for the C57BL6 raf-1–/– MEFs, the primary MF-1 raf-1–/– and raf-1FF/FF MEFs did not show evidence of spontaneous apoptosis under normal growth conditions (Figure 3C–E). Apoptosis was induced by treatment of raf-1+/+, raf-1–/– and raf-1FF/FF MEFs with etoposide, anti-Fas antibody or by serum withdrawal, and cell death was assessed by annexin V or Hoechst 33258 staining. The raf-1FF/FF cells showed no increase or decrease in programmed cell death (PCD) upon treatment with these apoptotic agents compared with raf-1+/+ cells (Figure 3C). In contrast, raf-1–/– cells showed a significant increase in PCD (Figure 3D–F). Upon treatment with etoposide, the raf-1+/+ cells showed 21.1% PCD whereas the raf-1–/– cells showed 30.0% PCD (n = 7; 95% CI for difference 0.5–17.2%, P = 0.04; Figure 3D and E). Upon treatment with anti-Fas antibody, the raf-1+/+ cells showed 29.9% PCD whereas the raf-1–/– cells showed 48.3% PCD (n = 7; 95% CI for difference 9.5–27.4%, P = 0.0007; Figure 3D and E). The raf-1–/– cells did not show a significant increase in PCD upon serum withdrawal as this treatment induced 26.0% PCD for the raf-1–/– cells and 18.7% for the raf-1+/+ cells (n = 7; 95% CI for difference –6.8 to 21.4%, P = 0.284; Figure 3D and E). Hoechst 33258 staining confirmed that the raf-1–/– cells were more susceptible to PCD induced by the anti-Fas antibody than the raf-1+/+ cells, as assessed by nuclear morphology (Figure 3F).
Raf kinase activities in the mutant cells
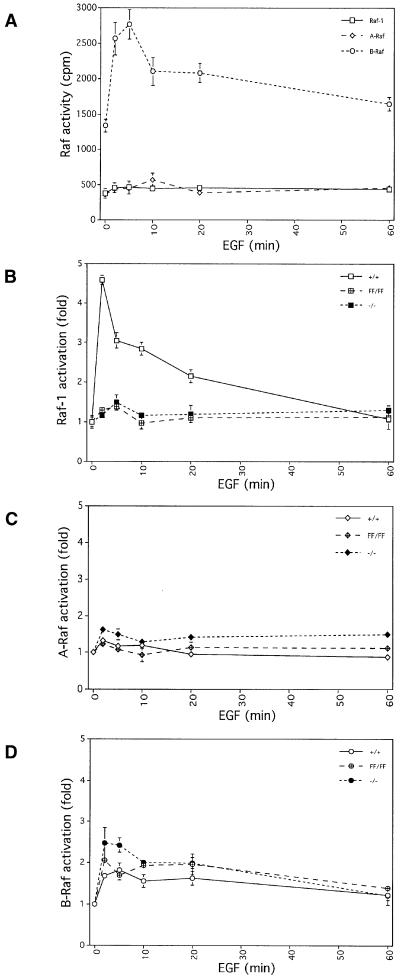
For measuring Raf kinase activities, primary MEFs were immortalized with the SV40 large T antigen and permanent cell lines were derived. Only cells expressing similar levels of the T antigen and with similar growth rates were analysed and compared (data not shown). We first compared the activities of the endogenous Raf proteins in raf-1+/+ MEFs using the immunoprecipitation kinase cascade assay (Marais et al., 1997). For these initial studies, the conditions were optimized for B-Raf activity, since B-Raf is the most active isotype under our assay conditions (Mason et al., 1999). In agreement with our previous studies, B-Raf had elevated basal activity in unstimulated cells, whereas both Raf-1 and A-Raf were inactive (Figure 4A). When stimulated with epidermal growth factor (EGF), B-Raf activity in raf-1+/+ cells was increased by ∼2-fold and returned to basal levels by 60 min (Figure 4A). However, we did not detect any activation of Raf-1 or A-Raf in this assay (Figure 4A), despite numerous reports previously describing the activation of Raf-1 in growth factor stimulated cells (Marshall, 1994 and references therein). We obtained similar results using two other B-Raf-specific antibodies, generated to peptides from different regions of B-Raf, but did not detect B-Raf activity with these antibodies in MEFs derived from B-raf–/– embryos (Wojnowski et al., 1997; our unpublished data). However, despite this extremely strong kinase activity of B-Raf, the protein could not be detected in these cells with these antibodies even by 35S-labelling or by immunoprecipitation combined with western analysis (data not shown). By contrast, A-Raf and Raf-1 are present at high levels in MEFs as detected by western analysis.
Fig. 4. Raf kinase activities. (A) Time course of activation of immunoprecipitated A-Raf, B-Raf and Raf-1 in the kinase cascade assay in raf-1+/+ cells following stimulation with EGF. The conditions for the assay were the same as those used for routinely measuring B-Raf activity. (B) Time course showing the fold Raf-1 activation in raf-1+/+, raf-1–/– and raf-1FF/FF MEFs following EGF stimulation. Standard conditions for measuring Raf-1 activity were utilized. (C) Time course showing the fold A-Raf activation in MEFs following EGF stimulation. The conditions were the same as for the Raf-1 assay. (D) Time course showing the fold B-Raf activation in MEFs following EGF stimulation. The B-Raf assay conditions were utilized. Each experiment was performed in triplicate and error bars show standard deviations.
The above results suggest that the specific activity of B-Raf towards MEK as measured by this kinase cascade assay is far greater than either Raf-1 or A-Raf. To examine Raf-1 and A-Raf activation, we increased the sensitivity of the assay by using 10-fold more lysate (1 mg) and by increasing the first incubation period from 15 to 30 min (Materials and methods). These have been developed as standard conditions for measuring Raf-1 activity (Marais et al., 1997). Immortalized raf-1+/+, raf-1–/– and raf-1FF/FF MEFs were made quiescent and stimulated with EGF, platelet-derived growth factor (PDGF), phorbol 12-myristate 13-acetate (PMA) or lysophosphatidic acid (LPA) over a time course of up to 60 min. Raf-1 kinase activity was stimulated 4- to 6-fold in raf-1+/+ cells following 2–5 min treatment with EGF and returned to basal levels within 60 min (Figure 4B). As expected, no Raf-1 kinase activity was detected in the raf-1–/– cells (Figure 4B). In the raf-1FF/FF MEFs, no activation of Raf-1 was detected in response to EGF; the level of activity observed was comparable to that in the raf-1–/– cells and to background levels in assays performed without substrate. The same Raf-1 activation results were obtained upon stimulation with PDGF, PMA and LPA (data not shown). In raf-1+/+ cells, A-Raf kinase activity was much lower than Raf-1 activity and was stimulated only by 1.3-fold upon 2 min treatment with EGF. Similar levels of A-Raf activity were detected in the raf-1–/– and raf-1FF/FF cells compared with raf-1+/+ cells (Figure 4C). We compared B-Raf activation in the raf-1+/+, raf-1–/– and raf-1FF/FF cells using the assay conditions described above for B-Raf. The levels of basal B-Raf kinase activity in the raf-1–/– and raf-1FF/FF cells were similar to that observed in raf-1+/+ cells (Figure 4D). The time course of activation by EGF was similar in the three cell lines, although the level of induction of B-Raf in raf-1–/– and raf-1FF/FF was elevated slightly compared with raf-1+/+ cells.
ERK activation in the mutant cells
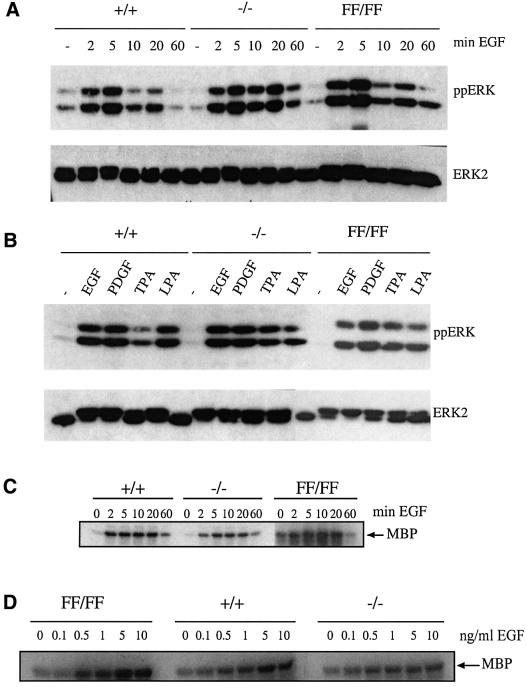
To measure ERK activation, cells were made quiescent and stimulated with EGF and levels of phospho-ERK were assessed. In the raf-1+/+ cells, phospho-ERK increased following 2 min of EGF treatment and continued to increase up to 5 min of treatment but reached basal levels after 60 min (Figure 5A). For the raf-1–/– and raf-1FF/FF cells, the level of phospho-ERK was similar to that in the raf-1+/+ cells (Figure 5A). ERK phosphorylation was assessed in cells that were made quiescent and then stimulated with EGF, PDGF, serum, LPA and PMA for 10 min (Figure 5B). In the raf-1+/+ cells, ERK phosphorylation was induced following treatment with all stimuli. A similar level of ERK phosphorylation was observed in the raf-1–/– and raf-1FF/FF cells with all stimuli (Figure 5B). ERK activation was also measured by using myelin basic protein (MBP) as a substrate for immunoprecipitated p42ERK. As with the ERK phosphorylation data, ERK activation increased in all cells following 2 min EGF treatment and reached maximum at 10 min (Figure 5C). There was no difference observed in either the time course or level of ERK activity between the raf-1+/+, raf-1–/– or raf-1FF/FF cells following EGF treatment (Figure 5C). ERK activation was also assessed upon stimulation of quiescent cells with sub-saturating amounts of EGF. MEFs of each genotype were made quiescent and then stimulated with varying concentrations of EGF from 0 to 10 ng/ml for 10 min. With increasing doses of EGF, a greater level of ERK activation was observed in all cell lines (Figure 5D). However, no difference was observed in the level of MBP phosphorylation upon treatment with any given dose of EGF between the raf-1+/+, raf-1–/– and raf-1FF/FF cells (Figure 5D).
Fig. 5. ERK activation. (A) Stimulation of ERK phosphorylation in raf-1+/+, raf-1–/– and raf-1FF/FF MEFs over a time course of EGF treatment. (B) Stimulation of ERK phosphorylation in raf-1+/+, raf-1–/– and raf-1FF/FF MEFs following treatment with different stimuli for 10 min. The blots in (A) and (B) were incubated with an anti-phosphoERK antibody (top panels) and an anti-ERK2 antibody (bottom panels) to control for protein loading. (C) ERK activation in raf-1+/+, raf-1–/– and raf-1FF/FF MEFs over a time course of EGF treatment as measured by the immunocomplex MBP kinase assay. (D) ERK activation in raf-1+/+, raf-1–/– and raf-1FF/FF MEFs following stimulation with different concentrations of EGF for 10 min as measured by the MBP kinase assay.
Discussion
The Ras/Raf/MEK/ERK cascade links the activation of cell surface receptors at the membrane with downstream events in the nucleus (Marshall, 1994). A wealth of biochemical data have indicated a role for the Raf-1 protein kinase in this pathway (Dent et al., 1992; Howe et al., 1992; Kyriakis et al., 1992; Samuels et al., 1993). Genetic data in Drosophila and Caenorhabditis elegans have provided evidence that the Raf homologues in these species stimulate MEK and ERK activation and mediate many of the proliferative and differentiation signals induced by RTKs and Ras (Dickson and Hafen, 1994; Eisenmann and Kim, 1994). Inhibition of Raf-1 in fibroblasts using antisense or dominant-negative expressing constructs disrupts proliferation induced by serum, transformation induced by oncogenic Ras and growth factor activation of the ERKs (Kolch et al., 1991; Schaap et al., 1993; Chao et al., 1994; Troppmair et al., 1994). In this study we show that, although Raf-1 is not the only physiological MEK activator, it is essential for mouse development.
The raf-1–/– embryo phenotype reported here shows defects in vascularization and placenta development as well as increased apoptosis of many tissues. On the mixed inbred 129Ola/C57BL6 background there was an earlier lethal phenotype than on the mixed 129Ola/MF-1 background. This strain-specific phenotype is almost identical to that described by Wojnowski et al. (1998), who generated homozygous mice with a hypomorphic allele for raf-1 that had 10% residual kinase activity on inbred (C57BL6) and outbred (CD-1) genetic backgrounds. In the manuscript by Mikula et al. (2001), raf-1–/– embryos were established primarily on the inbred 129Sv background and these also showed increased apoptosis of fetal liver cells and MEFs as well as similar placental deficiencies. However, those embryos survived to a later stage of development (E12.5–16.5) than the embryos on the 129Ola/C57BL6 background and they did not possess yolk sac vascularization defects. These differences in phenotype are likely to be attributable to different genetic modifiers in each strain, the nature of which is not known.
On the 129Ola/C57BL6 background, the most striking defect in the raf-1–/– embryos is the absence of endothelial cells in the yolk sac and disorganization of blood vessels in the embryo (Figure 1E–I). The establishment and modelling of blood vessels is controlled by ligands such as vascular endothelial growth factors (VEGFs), angiopoietins and ephrins that modulate the activity of RTKs (Risau, 1997). Vasculogenesis appears to be mediated by VEGF through the VEGF–R2 RTK while angiogenesis is mediated by VEGF through the VEGF–R1 RTK as well as angiopoietins at later stages of development (Fong et al., 1995; Sato et al., 1995; Shalaby et al., 1995; Hanahan et al., 1997). The lack of vascularization in the yolk sac of the raf-1-deficient mice suggests a link between Raf-1 and VEGF–R2 signalling and/or VEGF/VEGFR expression in vivo that cannot be compensated by other Raf proteins. VEGF–R2 has also been implicated in the determination of the haematoangioblast progenitor (Shalaby et al., 1995; Risau, 1997) and the data presented by Mikula et al. (2001) confirm that 129Sv/raf-1–/– embryos have defects in early stages of haemopoiesis. In cultured endothelial cell lines, VEGF induces Raf-1/ERK activities and stimulates mitogenesis (Takahashi et al., 1999). How ever, the fact that the raf-1FF/FF mutant mice do not show defects in vasculogenesis and the fact that ERK activation is not disrupted in the raf-1–/– MEFs argue that the role of Raf-1 in this process is largely ERK independent.
The placental and yolk sac deficiencies could clearly contribute to the generalized growth retardation and developmental delay observed in the raf-1–/– embryos on both genetic backgrounds (Figures 1 and 2). However, in addition to this, the raf-1–/– embryos and MEFs clearly demonstrate increased levels of apoptosis, whereas there is no defect in the ability of these cells to undergo proliferation (Figures 1–3). Therefore, either endogenous Raf-1 has no role in the regulation of the cell cycle or its function in proliferation is compensated by other MEK activators. A role for Raf-1 in apoptosis has been indicated from a number of biochemical studies (Wang et al., 1996; Pritchard and McMahon, 1997; Lau et al., 1998) and Ras has been reported to induce apoptosis through activation of Raf-1/ERKs (Dudek et al., 1997; Kauffmann-Zeh et al., 1997; Downward, 1998). Activated Ras is also thought to suppress apoptosis through PKB/Akt and recently it has been shown that Akt can suppress Raf-1 activity by direct phosphorylation of serine 259 (Rommel et al., 1999; Zimmermann and Moelling, 1999). The anti-apoptotic protein Bcl-2 has been shown to interact with Raf-1 and this interaction appears to be independent of Raf-1 kinase activity (Wang et al., 1994). One function of this interaction may be to target Raf-1 to the outer mitochondrial membrane where it could promote resistance to apoptosis through phosphorylation of BAD, a pro-apoptotic protein (Wang et al., 1996). This inhibition of BAD appears to require PI3-kinase but is ERK-independent (Neshat et al., 2000). The fact that the raf-1FF/FF animals have no increased apoptosis also suggests that the role of Raf-1 in suppression of apoptosis is ERK independent in vivo.
The raf-1FF/FF animals have no detectable mutant phenotype although we cannot yet exclude the possibility that they are not more or less susceptible to stressful situations such as bacterial infections or tumorigenesis. In addition, the raf-1FF/FF phenotype has so far only been examined on mixed genetic backgrounds and further backcrossing to the MF-1 and C57BL6 strains may reveal phenotypic heterogeneity between strains, as has been revealed for the raf-1–/– animals. The raf-1FF/FF animals express a mutant version of Raf-1 that shows no detectable activity towards MEK in the immunoprecipitation kinase cascade assay (Figure 4B), a result that supports our previous data in COS cells, demonstrating that overexpressed RafFF has virtually no MEK kinase activity (Marais et al., 1995). It is possible that RafFF possesses extremely low levels of MEK kinase activity that are beyond experimental detection but are sufficient to allow survival of the RafFF animals. This would then suggest that in some cell types, Raf-1 is the limiting MEK activator, and would explain the difference in survival between the raf-1–/– and raf-1FF/FF animals. However, in the studies of Wojnowski et al. (1998), 10% residual Raf-1 activity was found to be insufficient for mouse survival and we were unable to detect this level of Raf-1 activity in the raf-1FF/FF MEFs. We also observed no difference in the level or profile of ERK activation in the raf-1–/– and raf-1FF/FF MEFs compared with the raf-1+/+ cells (as also shown by Mikula et al., 2001) and, furthermore, MEK activity was detected in both the raf-1–/– and raf-1FF/FF cells (data not shown; see also Mikula et al., 2001). Thus, Raf-1 activity is not required for MEK and ERK activation, at least in some cell types, presumably because other Raf isoforms or MEK activators (such as the MEKK proteins) compensate for the loss of Raf-1. We did find a slight increase in B-Raf activity in the raf-1–/– MEFs, which may in part compensate for the loss of Raf-1. Taken together these data show that Raf-1 is not required for ERK activation at least in some cell types and yet animals expressing RafFF, a version of Raf-1 that is unable to activate MEK, survive.
Even in the raf-1+/+ MEFs, the kinase activity of endogenous Raf-1 towards MEK in the immunocomplex kinase cascade assay is difficult to measure unless sensitive assay conditions are utilized (Figure 4A and B). In contrast, immunoprecipitated B-Raf from MEFs has extremely strong kinase activity towards MEK under much less sensitive assay conditions (Figure 4A), even though the B-Raf protein is difficult to detect (data not shown). In B-raf–/– MEFs, ERK activation was reduced compared with wild-type MEFs (M.Hüser, unpublished; Wojnowski et al., 2000). Taken together, these data provide strong evidence that B-Raf, rather than Raf-1, is the primary Raf isotype that activates MEK/ERKs in MEFs, if not more cell types. B-Raf regulation differs from that of Raf-1. Unlike Raf-1 and A-Raf, B-Raf does not require tyrosine phosphorylation for activation, and B-Raf is reported to be activated by Rap1a, notably in PC12 cells (York et al., 1998) and by TC21 (Rosario et al., 1999). B-Raf also has strong basal kinase activity towards MEK that is independent of Ras, although oncogenic Ras does stimulate B-Raf activity further through a mechanism involving translocation of B-Raf to the membrane (Marais et al., 1997; Mason et al., 1999). Thus, although the induction of B-Raf activity by growth factors in MEFs appears to be weak (2- to 3-fold), it is possible that it is the relocation of active B-Raf to the plasma membrane in the presence of active Ras that gives the large induction of MEK/ERK activity.
What then is the role of Raf-1? Our data imply that the MEK kinase activity of Raf-1 is not essential for normal mouse development, but that the protein is required. A number of other substrates for Raf-1 have been reported including the dual specificity phosphatase Cdc25A (Galaktionov et al., 1995) and the ankyrin repeat protein Tvl-1 (Lin et al., 1999). Possibly, the raf-1–/– phenotype may be manifested through disruption of the activities of these other substrates, whereas RafFF may be able to phosphorylate and activate these substrates. Alternatively, the main function of Raf-1 may not be as a kinase. Raf-1 forms part of a multiprotein complex in the cell including HSP90, p50 and 14-3-3 proteins, and binding of these scaffold proteins is thought to be important for maintaining the stability of Raf-1 (Stancato et al., 1993; Wartmann and Davis, 1994; Reuter et al., 1995). Many of the proteins that interact with Raf-1 also interact with KSR (kinase suppressor of Ras), a putative Ras-effector protein kinase that was identified by genetic studies in Drosophila and C.elegans (Kornfeld et al., 1995; Sundaram and Han, 1995; Therrien et al., 1995). Studies in C.elegans have shown that the kinase activity of KSR is not important for its function (Stewart et al., 1999). Thus, at least in some circumstances, Raf-1, like KSR, may not require its kinase activity for function, and like KSR, one of its main functions may be as a scaffold protein (Denouel-Galy et al., 1998; Yu et al., 1998).
During evolution, three highly conserved raf genes have arisen from a single prototypic gene as a result of genome duplication followed by functional adaptation. Biochemical data indicate that the three mammalian Raf enzymes are differentially utilized and the results presented here suggest that the Raf-1 protein has evolved such that its kinase activity towards MEK is not essential for mouse development, or that sufficient redundancy exists to overcome the need for Raf-1 kinase activity, but not for Raf-1 protein. A-Raf has extremely weak, if any, activity towards MEK (Figure 4 and Marais et al., 1997) and is primarily located in mitochondria (Yuryev et al., 2000), suggesting that it too has evolved alternative functions. Interestingly, Drosophila raf is more homologous to B-raf in many domains than A-raf or raf-1. It is now important to identify the non-MEK kinase functions of Raf-1 and A-Raf.
Materials and methods
Histology
Tissues and embryos were prepared, sectioned and then either stained with haematoxylin and eosin or processed for Ki67 immunostaining or TUNEL assay (Monkley et al., 2000). The Ki67 antibody was a rabbit polyclonal antibody supplied by Dianova (Germany). Embryos and yolk sacs for whole mount staining with PECAM-1 were processed by the method described by Schlaeger et al. (1995).
Derivation of MEFs and immortalization
Individual embryos were dissected and cut, washed several times with cold phosphate-buffered saline (PBS) and then incubated at 4°C for 2 (raf-1/C57BL6) or 6 h (raf-1/MF-1 and rafFF) in 0.25% (w/v) trypsin. An aliquot of Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FCS and 100 U/ml penicillin/streptomycin was added and the suspension was transferred to tissue culture plates and cultured. Primary cells were immortalized with the ZIPTEX virus expressing the SV40 large T antigen (Sladek and Jacobberger, 1992). Virus and cells were co-incubated in 8 µg/ml polybrene for 4–24 h and immortalized cells were grown out by continuous culture.
Proliferation and apoptosis assays
For growth curves, 2 × 104 primary cells were plated and counted at 24 h intervals in triplicate using a haemocytometer. For DNA synthesis assays the method described by Treinies et al. (1999) was followed. To induce apoptosis, primary cells at 80% confluency on 6 cm dishes were treated with 50 µm etoposide, 50 ng/ml anti-Fas antibody with 0.5 µm cycloheximide or serum-free medium for 20 h in a 37°C humidifying incubator. Suspension and attached cells were collected, incubated for 20 min at 37°C in fresh media and stained with annexin V (Bender MedSystems). Stained cells were assessed by FACS analysis (Becton Dickinson). Alternatively, treated cells were stained with 5 µg/ml Hoechst 33258 in PBS for 1 h at 37°C. Data were analysed by using the two-tailed unpaired t-test.
Kinase assays and immunoblotting
MEFs were made quiescent by culturing in DMEM containing 0.5% (v/v) FCS for at least 24 h. They were stimulated by addition of 10 ng/ml EGF, 50 ng/ml PDGF, 40 µM PMA, 200 ng/ml LPA or 20% (v/v) FCS and incubated over a time course of 0–60 min. Protein lysates were prepared as described in Luckett et al. (2000) for the ERK analysis or Marais et al. (1997) for the Raf kinase assays. Western blots were prepared and processed as described previously (Luckett et al., 2000). Primary antibodies were a mouse monoclonal antibody against Thr202/Tyr204 phospho-p44/42 ERK (New England Biolabs) and a rabbit polyclonal antibody for ERK2 (Leevers and Marshall, 1992). Raf proteins were immunoprecipitated for 2 h at 4°C from 0.1–1 mg cell extract with 2 µg of anti-Raf-1 antibody (Transduction Laboratories), 4 µg of anti-B-Raf rabbit polyclonal antibody (Mason et al., 1999), 2 µg of anti-B-Raf goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and 2 µg of anti-A-Raf mouse monoclonal antibody (Transduction Laboratories). p42 ERK was immunoprecipitated by using 5 µl rabbit anti-p42ERK polyclonal antibody (no. 122; Treinies et al., 1999). The activity of each Raf protein was assessed by using the Raf kinase cascade assay using glutathione S-transferase (GST)–MEK, GST–ERK and MBP together with [γ-32P]ATP as sequential substrates (Marais et al., 1997). For B-Raf, 0.1 mg of lysate was used and the first kinase incubation period with GST–MEK and GST–ERK was for 15 min whereas for Raf-1 and A-Raf, 1 mg of lysate was used and the first incubation period was extended to 30 min. ERK activity was measured using the MBP kinase assay (Samuels et al., 1993).
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Jim Norman, David Critchley, Gerry Cohen and Chris Marshall for critical reading of the manuscript, members of the Marais and Pritchard laboratories for their support, Lee Topping for help with statistics and the Division of Biomedical Services for their help. We also thank Phil Soriano for providing the ZIPTEX virus, Hugh Paterson for providing the DNA synthesis protocol and reagents, Martin McMahon for providing the C-terminal Raf-1 antibody and Rich Murray for providing targeting vectors. This work was supported by a Royal Society fellowship to C.P., BBSRC studentships to J.L. and K.M., and project grants from the Cancer Research Campaign and The Wellcome Trust. We thank these organizations for their invaluable support.
References
- Barnard D., Diaz,B., Clawson,D. and Marshall,M. (1998) Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene, 17, 1539–1547. [DOI] [PubMed] [Google Scholar]
- Barnier J.V., Papin,C., Eychene,A., Lecoq,O. and Calothy,G. (1995) The mouse B-raf gene encodes multiple protein isoforms with tissue-specific expression. J. Biol. Chem., 270, 23381–23389. [DOI] [PubMed] [Google Scholar]
- Catling A.D., Reuter,C.W., Cox,M.E., Parsons,S.J. and Weber,M.J. (1994) Partial purification of a mitogen-activated protein kinase kinase activator from bovine brain. Identification as B-Raf or a B-Raf-associated activity. J. Biol. Chem., 269, 30014–30021. [PubMed] [Google Scholar]
- Chao T.O., Foster,D.A., Rapp,U.R. and Rosner,M.R. (1994) Differential Raf requirement for activation of mitogen-activated protein kinase by growth factors, phorbol esters, and calcium. J. Biol. Chem., 269, 7337–7341. [PubMed] [Google Scholar]
- Denouel-Galy A., Douville,E.M., Warne,P.H., Papin,C., Laugier,D., Calothy,G., Downward,J. and Eychene,A. (1998) Murine Ksr interacts with MEK and inhibits Ras-induced transformation. Curr. Biol., 8, 46–55. [DOI] [PubMed] [Google Scholar]
- Dent P., Haser,W., Haystead,T.A.J., Vincent,L.A., Roberts,T.M. and Sturgill,T.W. (1992) Activation of the mitogen-activated protein kinase kinase by v-raf in NIH3T3 cells and in vitro. Science, 257, 1404–1407. [DOI] [PubMed] [Google Scholar]
- Dent P., Jelinek,T., Morrison,D.K., Weber,M.J. and Sturgill,T.W. (1995) Reversal of Raf-1 activation by purified and membrane-associated protein phophatases. Science, 268, 1902–1906. [DOI] [PubMed] [Google Scholar]
- Diaz B., Barnard,D., Filson,A., Macdonald,S., King,A. and Marshall,M. (1997) Phosphorylation of Raf-1 serine 338–serine 339 is an essential regulatory event for Ras-dependent activation and biological signalling. Mol. Cell. Biol., 17, 4509–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson B. and Hafen,E. (1994) Genetics of signal transduction in invertebrates. Curr. Opin. Genet. Dev., 4, 64–70. [DOI] [PubMed] [Google Scholar]
- Downward J. (1998) Ras signalling and apoptosis. Curr. Opin. Genet. Dev., 8, 49–54. [DOI] [PubMed] [Google Scholar]
- Dudek H., Datta,S.R., Franke,T.F., Birnbaum,M.J., Yao,R., Cooper,G.M., Segal,R.A., Kaplan,D.R. and Greenberg,M.E. (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science, 275, 661–665. [DOI] [PubMed] [Google Scholar]
- Eisenmann D.M. and Kim,S.K. (1994) Signal transduction and cell fate specification during Caenorhabditis elegans vulval development. Curr. Opin. Genet. Dev., 4, 508–516. [DOI] [PubMed] [Google Scholar]
- Fong G.-H., Rossant,J., Gertsenstein,M. and Breitman,M.L. (1995) Role of the flt-1 receptor tyrosine kinase in regulating the assembly of vasacular endothelium. Nature, 376, 66–70. [DOI] [PubMed] [Google Scholar]
- Galaktionov K., Jessus,C. and Beach,D. (1995) Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev., 9, 1046–1058. [DOI] [PubMed] [Google Scholar]
- Hanahan D. (1997) Signaling vascular morphogenesis and maintenance. Science, 277, 48–50. [DOI] [PubMed] [Google Scholar]
- Howe L.R., Leevers,S.J., Gomez,N., Nakielny,S., Cohen,P. and Marshall,C.J. (1992) Activation of the MAP kinase pathway by the protein kinase raf. Cell, 71, 335–342. [DOI] [PubMed] [Google Scholar]
- Jaiswal R.K., Moodie,S.A., Wolfman,A. and Landreth,G. (1994) The mitogen-activated protein kinase cascade is activated by B-Raf in response to nerve growth factor through interaction with p21ras. Mol. Cell. Biol., 14, 6944–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek T., Dent,P., Sturgill,T.W. and Weber,M.J. (1996) Ras-induced activation of Raf-1 is dependent on tyrosine phosphorylation. Mol. Cell. Biol., 16, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann-Zeh A., Rodriguez-Viciana,P., Ulrich,E., Gilbert,C., Coffer,P., Downward,J. and Evan,G. (1997) Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature, 385, 544–548. [DOI] [PubMed] [Google Scholar]
- Kerkhoff E. and Rapp,U.R. (1997). Induction of cell proliferation in quiescent NIH3T3 cells by oncogenic c-Raf-1. Mol. Cell. Biol., 17, 2576–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W., Heidecker,G., Lloyd,P. and Rapp,U.R. (1991) Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature, 349, 426–428. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Hom,D.B. and Horvitz,H.R. (1995) The ksr-1 gene encodes a novel protein kinase involved in ras-mediated signaling in C.elegans. Cell, 83, 903–913. [DOI] [PubMed] [Google Scholar]
- Kuo W.-L., Abe,M., Rhee,J., Eves,E.M., McCarthy,S.A., Yan,M., Templeton,D.J., McMahon,M. and Rich Rosner,M. (1996) Raf, but not MEK or ERK is sufficient for differentiation of hippocampal neuronal cells. Mol. Cell. Biol., 16, 1458–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J.M., App,H., Zhang,X.-F., Banerjee,P., Brautigan,D.L., Rapp,U.R. and Avruch,J. (1992) Raf-1 activates MAP kinase-kinase. Nature, 358, 417–421. [DOI] [PubMed] [Google Scholar]
- Lau Q.C., Brusselbach,S. and Muller,R. (1998) Abrogation of c-Raf expression induces apoptosis in tumor cells. Oncogene, 16, 1899–1902. [DOI] [PubMed] [Google Scholar]
- Leevers S.J. and Marshall,C.J. (1992) Activation of extracellular signal-regulated kinase ERK2 by p21ras oncoprotein. EMBO J., 11, 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.H., Makris,A., McMahon,C., Bear,S.E., Patriotis,C., Prasad,V.R., Brent,R., Golemis,E.A. and Tsichlis,P.N. (1999) The ankyrin repeat-containing adaptor protein Tvl-1 is a novel substrate and regulator of Raf-1. J. Biol. Chem., 274, 14706–14715. [DOI] [PubMed] [Google Scholar]
- Luckett J.C.A., Hüser,M.B., Giagtzoglou,N., Brown,J.E. and Pritchard,C.A. (2000) Expression of the A-raf proto-oncogene in the normal adult and embryonic mouse. Cell Growth Differ., 11, 163–171. [PubMed] [Google Scholar]
- Macdonald S.G., Crews,C.M., Wu,L., Driller,J., Clark,R., Erikson,R.L. and McCormick,F. (1993) Reconstitution of the Raf-1–MEK–ERK signal transduction pathway in vitro. Mol. Cell. Biol., 13, 6615–6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R. and Marshall,C.J. (1996) Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv., 27, 101–125. [PubMed] [Google Scholar]
- Marais R., Light,Y., Paterson,H.F. and Marshall,C.J. (1995) Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J., 14, 3136–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R., Light,Y., Paterson,H.F., Mason,C.S. and Marshall,C.J. (1997) Differential regulation of Raf-1, A-Raf and B-Raf by oncogenic ras and tyrosine kinases. J. Biol. Chem., 272, 4378–4383. [DOI] [PubMed] [Google Scholar]
- Marais R., Light,Y., Mason,C., Paterson,H., Olson,M.F. and Marshall,C.J. (1998) Requirement of Ras–GTP–Raf complexes for activation of Raf-1 by protein kinase C. Science, 280, 109–112. [DOI] [PubMed] [Google Scholar]
- Marshall C.J. (1994) MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev., 4, 82–89. [DOI] [PubMed] [Google Scholar]
- Mason C.S., Springer,C.J., Cooper,R.G., Superti-Furga,G., Marshall,C.J. and Marais,R. (1999) Serine and tyrosine phophorylations cooperate in Raf-1, but not B-Raf activation. EMBO J., 18, 2137–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikula M. et al. (2001) Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J., 20, 1952–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkley S.J. et al. (2000) Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev. Dyn., 219, 560–574. [DOI] [PubMed] [Google Scholar]
- Moodie S.A., Willumsen,B.M., Weber,M.J. and Wolfman,A. (1993) Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science, 260, 1658–1661. [DOI] [PubMed] [Google Scholar]
- Moodie S.A., Paris,M., Kolch,W. and Wolfman,A. (1994) Association of MEK1 with p21ras.GMPPNP is dependent on B-Raf. Mol. Cell. Biol., 14, 7153–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neshat M.S., Raitano,A.B., Wang,H.-G., Reed,J.C. and Sawyers,C.L. (2000) The survival of the Bcr-Abl oncogene is mediated by Bad-dependent and independent pathways: roles for phosphatidylinositol 3-kinase and Raf. Mol. Cell. Biol., 20, 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papin C., Denouel-Galy,A., Laugier,D., Calothy,G. and Eychene,A. (1998) Modulation of kinase activity and oncogenic properties by alternative splicing reveals a novel regulatory mechanism for B-Raf. J. Biol. Chem., 273, 24939–24947. [DOI] [PubMed] [Google Scholar]
- Pritchard C. and McMahon,M. (1997) Raf revealed in life-or-death decisions. Nature Genet., 16, 214–215. [DOI] [PubMed] [Google Scholar]
- Pritchard C.A., Samuels,M.L., Bosch,E. and McMahon,M. (1995) Conditionally oncogenic forms of the A-Raf and B-Raf protein kinases display different biological and biochemical properties in NIH 3T3 cells. Mol. Cell. Biol., 15, 6430–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard C.A., Bolin,L., Slattery,R., Murray,R. and McMahon,M. (1996) Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-Raf protein kinase gene. Curr. Biol., 6, 614–617. [DOI] [PubMed] [Google Scholar]
- Reuter C.W.M., Catling,A.D., Jelinek,T. and Weber,M.J. (1995) Biochemical analysis of MEK activation in NIH3T3 fibroblasts. J. Biol. Chem., 270, 7644–7655. [DOI] [PubMed] [Google Scholar]
- Risau W. (1997) Mechanisms of angiogenesis. Nature, 386, 671–674. [DOI] [PubMed] [Google Scholar]
- Rommel C., Clarke,B.A., Zimmermann,S., Nunez,L., Rossman,R., Reid,K., Moelling,K., Yancopoulos,G.D. and Glass,D.J. (1999) Differentiation stage-specific inhibition of the Raf–MEK–ERK pathway by Akt. Science, 286, 1738–1741. [DOI] [PubMed] [Google Scholar]
- Rosario M., Paterson,H.F. and Marshall,C.J. (1999) Activation of the Raf/MAP kinase cascade by the Ras-related protein TC21 is required for the TC21-mediated transformation of NIH 3T3 cells. EMBO J., 18, 1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels M.L., Weber,M.J., Bishop,J.M. and McMahon,M. (1993) Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human Raf-1 protein kinase. Mol. Cell. Biol., 13, 6241–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T.N. et al. (1995) Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature, 376, 70–74. [DOI] [PubMed] [Google Scholar]
- Schaap D., van der Wal,J., Howe,L.R., Marshall,C.J. and van Blitterswijk,W.J. (1993) A dominant-negative mutant of raf blocks mitogen-activated protein kinase activation by growth factors and oncogenic p21ras. J. Biol. Chem., 268, 20232–20236. [PubMed] [Google Scholar]
- Schlaeger T.M., Qin,Y., Fujiwara,Y., Magram,J. and Sato,T.N. (1995) Vascular endothelial cell lineage-specific promoter in transgenic mice. Development, 121, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Shalaby F., Rossant,J., Yamaguchi,T.P., Gertsenstein,M., Wu,X.-F., Breitman,M.L. and Schuh,A.C. (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature, 376, 62–66. [DOI] [PubMed] [Google Scholar]
- Sladek T.L. and Jacobberger,J.W. (1992) Dependence of SV40 large T-antigen cell cycle regulation on T-antigen expression levels. Oncogene, 7, 1305–1313. [PubMed] [Google Scholar]
- Stancato L.F., Chow,Y.F., Hutchison,K.A., Perdew,G.H., Jove,R. and Pratt,W.B. (1993) Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J. Biol. Chem., 268, 21711–21716. [PubMed] [Google Scholar]
- Stewart S., Sundaram,M., Zhang,Y., Lee,J., Han,M. and Guan,K.-L. (1999) Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization. Mol. Cell. Biol., 19, 5523–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe D. and McCormick,F. (1997) Activation of c-raf-1 by Ras and Src through different mechanisms: activation in vivo and in vitro. EMBO J., 16, 2384–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm S.M., Cleveland,J.L. and Rapp,U.R. (1990) Expression of raf family of proto-oncogenes in normal mouse tissues. Oncogene, 5, 345–351. [PubMed] [Google Scholar]
- Sundaram M. and Han,M. (1995) The C.elegans ksr-1 gene encodes a novel Raf-related kinase involved in ras-mediated signal transduction. Cell, 83, 889–901. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Ueno,H. and Shibuya,M. (1999) VEGF activates protein kinase C-dependent, but Ras-independent Raf–MEK–MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene, 18, 2221–2230. [DOI] [PubMed] [Google Scholar]
- Therrien M., Chang,H.C., Solomon,N.M., Karim,F.D., Wassarman,D.A. and Rubin,G.M. (1995) KSR, a novel protein kinase required for RAS signal transduction. Cell, 83, 879–888. [DOI] [PubMed] [Google Scholar]
- Traverse S. and Cohen,P. (1994) Identification of a latent MAP kinase kinase kinase in PC-12 cells as B-Raf. FEBS Lett., 350, 13–18. [DOI] [PubMed] [Google Scholar]
- Traverse S., Cohen,P., Paterson,H., Marshall,C., Rapp,U. and Grand,R.J. (1993) Specific association of activated MAP kinase kinase kinase (Raf) with the plasma membranes of ras-transformed retinal cells. Oncogene, 8, 3175–3181. [PubMed] [Google Scholar]
- Treinies I., Paterson,H.F., Hooper,S., Wilson,R. and Marshall,C.J. (1999) Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal to stimulate DNA synthesis. Mol. Cell. Biol., 19, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troppmair J., Bruder,J.T., Munoz,H., Lloyd,P.A., Kyriakis,J., Banerjee,P., Avruch,J. and Rapp,U.R. (1994) Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation by oncogenes, serum, and 12-O-tetradecanoylphorbol-13-acetate requires Raf and is necessary for transformation. J. Biol. Chem., 269, 7030–7035. [PubMed] [Google Scholar]
- Wang H.-G. et al. (1994) Apoptosis regulation by interaction of bcl-2 protein and Raf-1 kinase. Oncogene, 9, 2751–2756. [PubMed] [Google Scholar]
- Wang H.-G., Rapp,U.R. and Reed,J.C. (1996) Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell, 87, 629–638. [DOI] [PubMed] [Google Scholar]
- Wartmann M. and Davis,R.J. (1994) The native structure of the activated Raf protein kinase is a membrane-bound multi-subunit complex. J. Biol. Chem., 269, 6695–6701. [PubMed] [Google Scholar]
- Wojnowski L., Zimmer,A.M., Beck,T.W., Hahn,H., Bernal,R., Rapp,U.R. and Zimmer,A. (1997) Endothelial apoptosis in Braf-deficient mice. Nature Genet., 16, 293–297. [DOI] [PubMed] [Google Scholar]
- Wojnowski L., Stancato,L.F., Zimmer,A.M., Hahn,H., Beck,T.W., Larner,A.C., Rapp,U.R. and Zimmer,A. (1998) Craf-1 protein kinase is essential for mouse development. Mech. Dev., 76, 141–149. [DOI] [PubMed] [Google Scholar]
- Wojnowski L., Stancato,L.F., Larner,A., Rapp,U.R. and Zimmer,A. (2000) Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech. Dev., 91, 97–104. [DOI] [PubMed] [Google Scholar]
- Wood K.W., Sarnecki,C., Roberts,T.M. and Blenis,J. (1992) Ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1 and RSK. Cell, 68, 1041–1050. [DOI] [PubMed] [Google Scholar]
- Wood K.W., Qi,H., D’Arcangelo,G., Armstrong,R.C., Roberts,T.M. and Halegoua,S. (1993) The cytoplasmic raf oncogene induces a neuronal phenotype in PC12 cells: A potential role for cellular raf kinases in neuronal factor signal transduction. Proc. Natl Acad. Sci. USA, 90, 5016–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York R.D., Yao,H., Dillon,T., Ellig,C.L., Eckert,S.P., McCleskey,E.W. and Stork,P.J.S. (1998) Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature, 392, 622–626. [DOI] [PubMed] [Google Scholar]
- Yu W., Fantl,W.J., Harrowe,G. and Williams,L.T. (1998) Regulation of the MAP kinase pathway by mammalian Ksr through direct interaction with MEK and ERK. Curr. Biol., 8, 56–64. [DOI] [PubMed] [Google Scholar]
- Yuryev A., Ono,M., Goff,S.A., Macaluso,F. and Wennogle,L.P. (2000) Isoform-specific localization of A-RAF in mitochondria. Mol. Cell. Biol., 20, 4870–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S. and Moelling,K. (1999) Phosphorylation and regulation of Raf by Akt (protein kinase B). Science, 286, 1741–1744. [DOI] [PubMed] [Google Scholar]