Abstract
PACS-1 is a cytosolic protein involved in controlling the correct subcellular localization of integral membrane proteins that contain acidic cluster sorting motifs, such as furin and human immunodeficiency virus type 1 (HIV-1) Nef. We have now investigated the interaction of PACS-1 with heterotetrameric adaptor complexes. PACS-1 associates with both AP-1 and AP-3, but not AP-2, and forms a ternary complex between furin and AP-1. A short sequence within PACS-1 that is essential for binding to AP-1 has been identified. Mutation of this motif yielded a dominant-negative PACS-1 molecule that can still bind to acidic cluster motifs on cargo proteins but not to adaptor complexes. Expression of dominant-negative PACS-1 causes a mislocalization of both furin and mannose 6-phosphate receptor from the trans-Golgi network, but has no effect on the localization of proteins that do not contain acidic cluster sorting motifs. Furthermore, expression of dominant-negative PACS-1 inhibits the ability of HIV-1 Nef to downregulate MHC-I. These studies demonstrate the requirement for PACS-1 interactions with adaptor proteins in multiple processes, including secretory granule biogenesis and HIV-1 pathogenesis.
Keywords: adaptors/furin/Nef/PACS-1/secretory granule
Introduction
The trans-Golgi network (TGN) and endosomal system is a series of highly dynamic and interconnected membrane-bound organelles involved in the trafficking and modification of numerous proteins of the secretory and endocytic pathways (Mellman, 1996; Traub and Kornfeld, 1997). The localization and sorting of membrane proteins in the TGN/endosomal system often require the presence of specific sorting motifs within their cytosolic domains, the most well characterized of which are the tyrosine- and dileucine-based signals (Kirchhausen et al., 1997; Marks et al., 1997). Many, and potentially all, of the functions ascribed to tyrosine- and dileucine-based signals arise from the interaction of these motifs with heterotetrameric adaptor complexes (Kirchhausen, 1999).
A more recently identified family of sorting motifs is based upon clusters of acidic residues often containing consensus sites for phosphorylation by casein kinase 2 (CK2). Acidic cluster sorting motifs are found in many proteins with varied membrane trafficking itineraries (Molloy et al., 1999). These sorting motifs are known to be important for the trafficking of the proprotein convertases furin and PC6B, the mannose 6-phosphate receptors (MPRs), herpes virus envelope glycoproteins [e.g. varicella zoster virus (VZV) gE] and human immunodeficiency virus type 1 (HIV-1) Nef, a myristylated protein involved in CD4 and major histocompatibility complex I (MHC-I) downregulation (Jones et al., 1995; Chen et al., 1997; Dittie et al., 1997; Alconada et al., 1999; Piguet et al., 2000; Xiang et al., 2000). In the case of furin and VZV gE, their phosphorylatable acidic clusters are crucial for the correct subcellular localization of these proteins to the TGN (Jones et al., 1995; Alconada et al., 1999). The acidic clusters within the cytosolic domains of the cation-independent (CI) and cation-dependent (CD) MPRs have been shown to be important for transport of lysosomal hydrolases by these receptors (Mauxion et al., 1996; Chen et al., 1997). A sequence of four consecutive acidic residues within Nef has been shown to be important for its ability to mediate MHC-I downregulation in HIV-infected cells (Greenberg et al., 1998).
Recently, we identified a novel protein, termed PACS-1, through its ability to interact specifically with the cytosolic domain of furin in a CK2 phosphorylation-dependent manner (Wan et al., 1998). PACS-1 also interacts with the cytosolic domains of other proteins containing acidic clusters, such as VZV gE, CI-MPR, PC6B and Nef (Wan et al., 1998; Piguet et al., 2000; Xiang et al., 2000). Antisense studies showed that PACS-1 is important for the correct subcellular localization of both furin and CI-MPR by a retrieval-based mechanism from endosomal compartments, and also for the ability of Nef to redistribute MHC-I from the cell surface to the TGN (Wan et al., 1998; Piguet et al., 2000). PACS-1 was also shown to associate with the AP-1 adaptor complex in the cytosol, thus prompting the proposal that PACS-1 functions as a connector molecule by linking the cytosolic domains of proteins containing acidic clusters to adaptor complexes (Wan et al., 1998; Molloy et al., 1999).
Here we show that PACS-1 interacts with both AP-1 and AP-3 but not AP-2 from the cytosol, and that AP-1 binds to PACS-1 through a direct protein–protein interaction. Furthermore, we provide in vitro evidence for the formation of a ternary complex between cargo molecules containing acidic cluster motifs, PACS-1 and AP-1. We have identified a determinant on PACS-1 necessary for binding AP-1, and mutated this binding region in PACS-1 to establish its role in vivo. The mutant PACS-1 functions in a dominant-negative manner with respect to adaptor binding, and provides an effective means of disrupting the PACS-1-mediated sorting of membrane proteins. We have shown that this dominant-negative PACS-1 specifically disrupts the TGN localization of furin and CI-MPR. Further more, dominant-negative PACS-1 expression inhibits the downregulation of MHC-I by HIV-1 Nef, demonstrating a crucial role for PACS-1–adaptor complex interactions in HIV pathogenesis.
Results
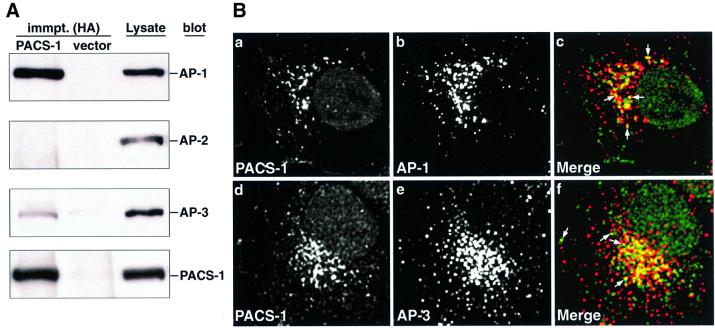
Previous data have shown that full-length rat PACS-1 can associate with the AP-1 adaptor complex in the cytosol (Wan et al., 1998). To determine whether human PACS-1 could also interact with adaptor complexes, recom binant hemagglutinin (HA)-tagged human PACS-1 was expressed in cells and immunoprecipitated with anti-HA antibodies. The resulting samples were then analyzed by western blotting using antisera directed against specific subunits of the AP-1, AP-2 and AP-3 adaptor complexes (γ-, α- and δ-adaptins, respectively). In agreement with previous data, human PACS-1 co-immunoprecipitated AP-1 (γ-adaptin; Figure 1A, lane 1). Furthermore, human PACS-1 co-immunoprecipitated AP-3 (δ-adaptin) but not AP-2 (α-adaptin; Figure 1A, lane 1). Quantitation of chemiluminescence signals demonstrated that PACS-1 co-immunoprecipitated a greater amount of AP-1 than AP-3 (2.8 and 0.7% of total AP-1 and AP-3 pools, respectively; Figure 1A, lanes 1 and 3). Control immunoprecipitates from cells infected with vector alone contained no detectable adaptor complex (Figure 1A, lane 2).
Fig. 1. PACS-1 associates with AP-1 and AP-3 adaptor complexes. (A) BSC-40 cells were infected with wild-type or recombinant vaccinia viruses expressing PACS-1HA, and proteins were immunoprecipitated from cytosol with anti-HA antibodies. Immunoprecipitated samples and cell lysates were analyzed by western blotting using antisera specific to AP-1 (anti-γ-adaptin; row 1), AP-2 (anti-α-adaptin; row 2), AP-3 (anti-δ-adaptin; row 3) and the HA tag (HA-11; row 4). (B) A7 cells were fixed and co-stained with antibodies against PACS-1 and AP-1 (anti-γ-adaptin; a–c) or AP-3 (anti-AP-3; d–f). The co-localization of PACS-1 (green) with AP-1 or AP-3 (red) is shown in the merged images (c and f, respectively; examples of co-localization are indicated by arrows).
Immunofluorescence analysis of untransfected cells with specific anti-PACS-1 antisera demonstrated a punctate distribution of endogenous PACS-1 localized mainly to a paranuclear region (Figure 1B, a and d). Double labeling of cells with antisera to PACS-1 and AP-1 or AP-3 demonstrated a partial co-localization of endogenous PACS-1 with both AP-1 and AP-3 (Figure 1B, a–f; examples of co-localization are indicated by arrows), consistent with PACS-1’s ability to interact with both of these adaptor complexes.
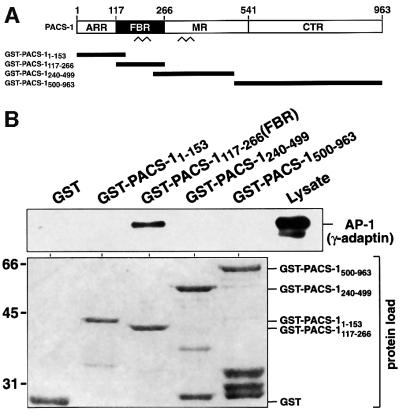
To identify the region of PACS-1 responsible for binding to adaptor complexes, the entire 963-amino-acid sequence was subdivided into four regions that cover different potential structural domains. These were the N-terminal atrophin-1-related region (ARR); the furin-binding region containing a predicted coiled-coil domain (FBR); the middle region containing a second predicted coiled-coil region (MR); and the C-terminal region, which is mostly absent from the minor isoform of the rat protein PACS-1b (CTR; Figure 2A). Glutathione S-transferase (GST) fusion proteins covering each of the four regions of PACS-1 were purified, incubated with cytosol and recovered with glutathione–agarose. Western blot analysis of recovered proteins demonstrated that only GST– PACS-1117–266 (corresponding to the FBR domain) was able to associate with AP-1 (Figure 2B). Therefore, the 150-amino-acid domain found between residues 117 and 266 inclusive is capable of binding AP-1 from cytosol (from here on, this domain is termed PACS-1FBR). Quantitation of chemiluminescent signals demonstrated that GST–PACS-1FBR interacted with ∼7.4% of the total AP-1 from lysate applied in these binding assays.
Fig. 2. AP-1 interacts with the FBR domain of PACS-1. (A) Schematic representation of PACS-1 and GST–PACS-1 fusion proteins (ARR, atrophin-1-related region; FBR, furin-binding region; MR, middle region; CTR, C-terminal region). Predicted coiled-coil domains are shown (zigzags). (B) GST–PACS-1 fusion proteins and GST alone were incubated with BSC-40 cytosol, isolated with glutathione resin and analyzed by western blotting using anti-γ-adaptin (upper panel). The load of the different GST proteins used is shown (lower panel).
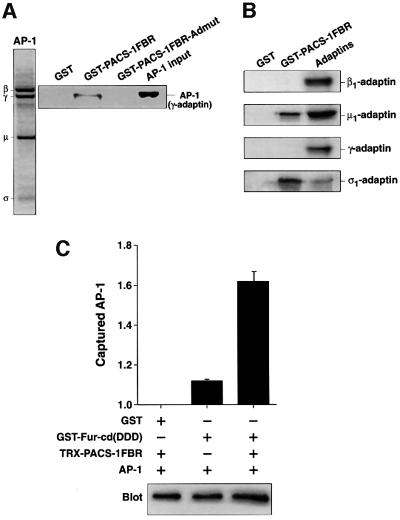
To address whether the interaction between PACS-1 and AP-1 is direct or requires other cytosolic factors, PACS-1FBR was tested for its ability to interact with purified AP-1. The purity of the AP-1 preparation is shown by SDS–PAGE followed by Coomassie Blue staining (Figure 3A, panel 1). GST–PACS-1FBR but not GST alone could interact specifically with purified AP-1 (Figure 3A, panel 2, lanes 1 and 2). Quantitation demonstrated that GST–PACS-1FBR associated with ∼7.5% of the total purified AP-1 applied in these assays. Of the four subunits of AP-1, three have been shown so far to interact with cytosolic factors: β1 with clathrin heavy chain and dileucine motifs (Shih et al., 1995; Rapoport et al., 1998); γ with γ-synergin (Page et al., 1999); and µ1 with tyrosine-based motifs (Ohno et al., 1995). To determine which of the subunits of AP-1 interacts with PACS-1, the GST– PACS-1FBR protein was tested for interaction with in vitro translated β1-, γ-, µ1- and σ1-adaptins. PACS-1FBR demonstrated a specific interaction with both µ1- and σ1-, but not with β1- or γ-adaptins (Figure 3B). Quantitation of these data demonstrated that GST–PACS-1FBR interacted with ∼27% of the applied µ1 and ∼43% of the applied σ1 in these assays.
Fig. 3. PACS-1 interacts directly with purified AP-1, in vitro translated µ1 and σ1, and forms a ternary complex between the furin cytosolic domain, PACS-1 and AP-1. (A) SDS–PAGE and Coomassie Blue staining of purified AP-1 is shown (panel 1). GST–PACS-1FBR, GST–PACS-1FBR-Admut and GST alone were incubated with purified AP-1, isolated with glutathione resin and analyzed by western blotting using anti-γ-adaptin (panel 2). (B) GST–PACS-1FBR and GST alone were incubated with 35S-labeled, in vitro translated µ1-, σ1-, β1- and γ-adaptins, isolated with glutathione resin, separated by SDS–PAGE and analyzed by autoradiography. (C) GST–Fur-cd(DDD) (phosphoryl ation mimic mutant) was incubated with purified AP-1 in the presence or absence of Trx-PACS-1FBR, isolated with glutathione resin and analyzed by western blotting using anti-γ-adaptin. Chemiluminescent signals were quantified using the NIH gel analysis software and are expressed as arbitrary units normalized to non-specific GST signal. A representative blot is shown (lower panel; the background level of AP-1’s interaction with GST alone is due to the low stringency conditions required to maintain ternary complex formation in these assays).
The FBR domain of PACS-1 interacts with the cytosolic domains of integral membrane proteins containing acidic cluster motifs. This domain also interacts with AP-1, suggesting that PACS-1 may bind cargo and adaptors simultaneously. To address this possibility, we tested the ability of the cytosolic domain of furin in which the two acidic cluster serines have been mutated to aspartates (to mimic phosphorylation and stimulate PACS-1 interaction; Wan et al., 1998) to interact with purified AP-1 in the presence or absence of the PACS-1FBR domain. A significantly greater amount of AP-1 was isolated in association with furin in the presence of PACS-1FBR than in its absence (Figure 3C). These data show that a ternary complex can form in vitro, suggesting that PACS-1 acts as a connector protein that can directly link acidic cluster motifs with AP-1.
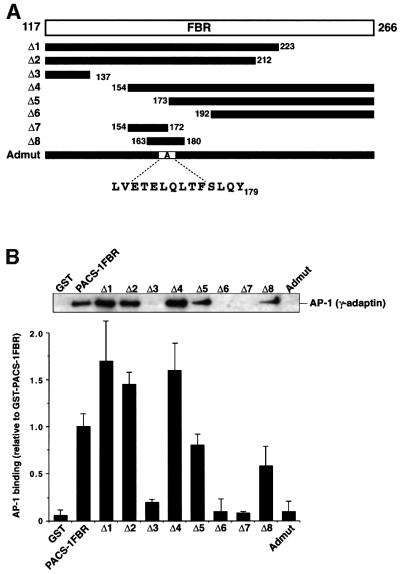
To identify residues within PACS-1 required for interactions with AP-1, a series of truncation constructs of the PACS-1FBR domain was generated (Figure 4A). Each construct was incubated with cytosol and bound AP-1 was detected by western blot analysis (Figure 4B, top). The relative interaction levels of each truncation construct with AP-1 were quantitated (Figure 4B, bottom; a value of 1 represents ∼5.0% of the AP-1 applied in these assays). Analysis of GST–PACS-1FBR Δ1 and Δ2 showed that the C-terminal 55 amino acids (residues 212–266) are not required for binding to AP-1. Truncation of the next 75 residues (Δ3) completely blocked the interaction of PACS-1FBR with AP-1. Analysis of GST–PACS-1FBR Δ4 and Δ5 showed that binding to AP-1 does not require the N-terminal 56 amino acids of PACS-1FBR (residues 117–172). Deletion of the next 20 amino acids (residues 173–192; Δ6) abolished binding. Construct Δ7, containing the 19-amino-acid PACS-1 sequence between residues 154 and 172, failed to bind AP-1. However, the overlapping construct Δ8 (PACS-1 residues 163–180) could bind AP-1 successfully. These data suggest that determinants sufficient to bind AP-1 are present within the 18-amino-acid sequence of PACS-1 encoded by the Δ8 construct.
Fig. 4. Sequence determinants required for PACS-1 interaction with AP-1. (A) Schematic representation of the FBR region of PACS-1 (residues 117–266) and the various mutants generated (Δ1–Δ8 and Admut). (B) GST fusion proteins of each of these constructs were incubated with lysate, isolated with glutathione resin and analyzed by western blotting using anti-γ-adaptin. Interaction between each GST fusion protein and AP-1 was quantified using the NIH gel analysis software and is expressed relative to AP-1’s interaction with GST–PACS-1FBR.
A further series of GST fusion proteins of PACS-1FBR was constructed that contained alanine substitutions of single or 3–4 sequential amino acids covering the potential AP-1 interaction domain (residues 163–180). None of these mutations was able to abolish the interaction with AP-1 (including the mutation of a potential tyrosine-based motif Y179PHF; data not shown). This suggests that the interaction site(s) within PACS-1 for adaptors is dispersed and no single amino acid is absolutely required. A mutant PACS-1FBR was constructed containing eight consecutive residues substituted with alanine (E168TELQLTF175→ AAAAAAAA). This construct (termed Admut for adaptor mutant) failed to bind AP-1 from cell lysates (Figure 4B). Furthermore, this construct also failed to bind purified AP-1 (Figure 3A). These data show that this eight-amino-acid stretch is essential for AP-1 binding to the FBR domain of PACS-1.
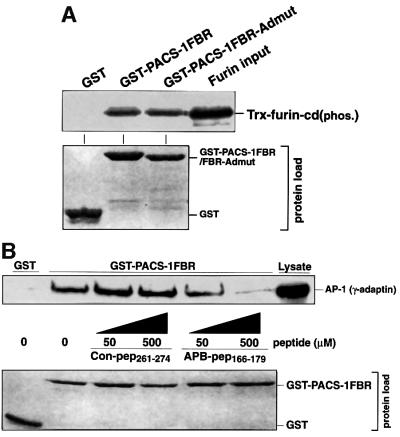
The mutation of eight consecutive residues to alanine is a significant change to the protein sequence of this domain and potentially could inhibit the interaction of PACS-1FBR with AP-1 due to some structural deformation. Therefore, GST–PACS-1FBR and GST–PACS- 1FBR-Admut were tested for their ability to interact with the phosphorylated furin cytosolic domain. Both of these constructs, but not GST alone, were able to interact with the phosphorylated furin cytosolic domain to a similar level (Figure 5A, upper panel; both GST–PACS-1FBR and GST–PACS-1FBR-Admut interacted with ∼20% of applied Trx-furin-cd). These data imply that the structure of PACS-1FBR is not so significantly altered as to preclude cargo binding; therefore, this eight alanine mutation within PACS-1 allows cargo and adaptor binding to be uncoupled.
Fig. 5. Cargo binding and peptide competition. (A) GST–PACS-1FBR, GST–PACS-1FBR-Admut and GST alone were incubated with CK2-phosphorylated Trx-furin-cd, isolated with glutathione resin and analyzed by western blotting using anti-tetra-His. The load of the GST proteins used in these binding assays is shown (lower panel). (B) GST–PACS-1FBR and GST were incubated with lysate in the presence of 50 or 500 µM control peptide (Con-pep261–274) or competing peptide (ABP-pep166–179), isolated with glutathione resin and analyzed by western blotting using anti-γ-adaptin. The load of the GST proteins used in these binding assays is shown (lower panel).
Peptide competition assays were performed to verify the adaptor interaction site within PACS-1. The ability of PACS-1FBR to interact with AP-1 from the cytosol in the presence or absence of increasing concentrations of two peptides, one covering the potential AP-1 interaction domain (L166VETELQLTFSLQY179: APB-pep166–179) and another covering the C-terminal end of the FBR domain (E261GIKSKLSDRSPDI274: Con-pep261–274), was analyzed. The presence of 50 µM ABP-pep166–179 reduced the interaction level of PACS-1FBR with AP-1, and 500 µM ABP-pep166–179 almost completely abolished the interaction. In contrast, the control peptide (Con-pep261–274) failed to interfere with the interaction between PACS-1FBR and AP-1 at any concentration tested (Figure 5B, upper panel).
To analyze the importance of this eight-amino-acid sequence for the interaction of full-length PACS-1 with adaptors in vivo, the eight alanine substitution of residues 168–175 was introduced into full-length PACS-1 and the ability of this construct (PACS-1Admut) to co-immunoprecipitate adaptors from cells was determined. As previously demonstrated in Figure 1, wild-type PACS-1 successfully co-immunoprecipitated with both AP-1 and AP-3 from cell lysates (Figure 6, lane 1). However, PACS-1Admut was unable to co-immunoprecipitate either AP-1 or AP-3 (Figure 6, lane 2). These data demonstrate that the eight-amino-acid sequence identified in the in vitro binding assays is necessary for the in vivo interaction of PACS-1 with both AP-1 and AP-3.

Fig. 6. PACS-1Admut can not co-immunoprecipitate AP-1 or AP-3. BSC-40 cells were infected with wild-type or recombinant vaccinia viruses expressing PACS-1 or PACS-1Admut, and proteins were immunoprecipitated with anti-HA. Samples were analyzed by western blotting using antisera specific to AP-1 (anti-γ-adaptin; row 1), AP-3 (anti-δ-adaptin; row 2) and the HA-tag (HA-11; row 3).
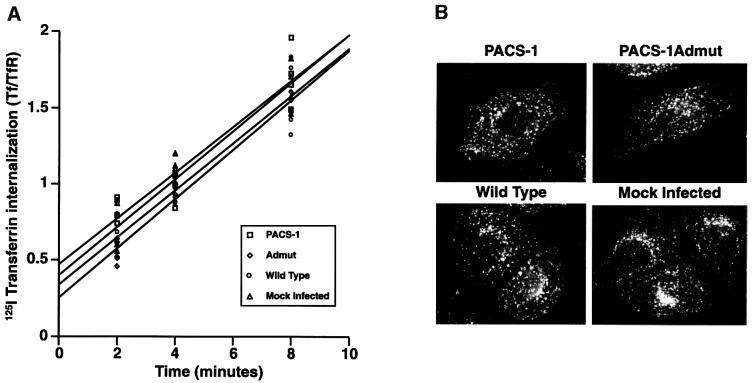
As noted above, PACS-1 does not associate with AP-2 and is not thought to play a role in endocytosis directly. To confirm this hypothesis, the internalization of transferrin was assessed in the presence or absence of PACS-1 or PACS-1Admut expression. The rate of [125I]transferrin internalization was not significantly affected by expression of either PACS-1 or PACS-1Admut as compared with internalization in the control conditions (Figure 7A). Consistently, the subcellular localization of internalized transferrin was unchanged in the presence of wild-type PACS-1 or PACS-1Admut expression (Figure 7B).
Fig. 7. PACS-1 or PACS-1Admut expression does not affect endocytosis. (A) Cells were infected with wild-type or recombinant vaccinia viruses expressing PACS-1 or PACS-1Admut, or were mock infected. The rate of 125I-labeled transferrin internalization was monitored. Each time point was performed in quadruplicate and corrected for non-specific uptake. The following linear equations for each data set were determined, with the gradient functions representing the rate of transferrin uptake: PACS-1, y = 0.150x + 0.473; Admut, y = 0.162x + 0.254; wild-type, y = 0.155x + 0.341; mock infected, y = 0.158x + 0.400. (B) Cells were infected with wild-type or recombinant vaccinia viruses expressing PACS-1 or PACS-1Admut, or were mock infected. Cells were incubated with rhodamine-labeled transferrin, fixed and analyzed by fluorescence microscopy.
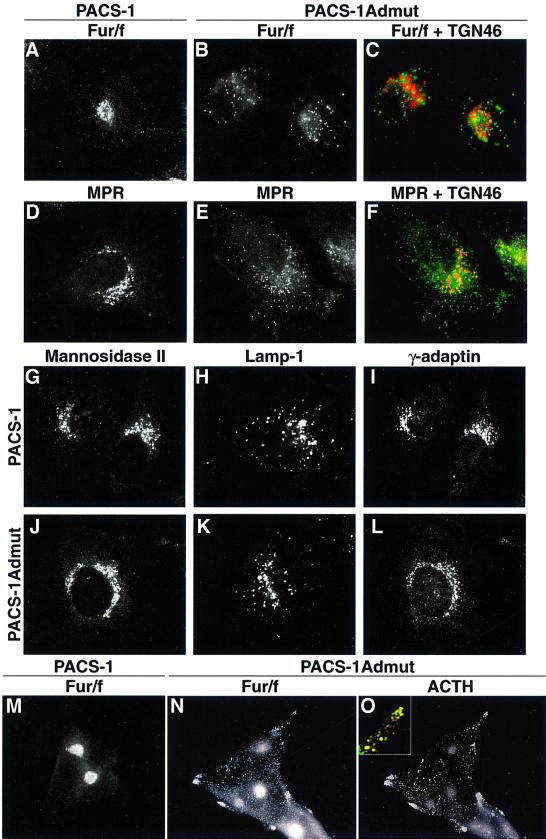
The data presented above suggest that PACS-1Admut could act as a dominant-negative protein for processes requiring PACS-1 connector function. PACS-1 and PACS- 1Admut were co-expressed with an epitope (FLAG)-tagged furin (fur/f) in cells, and the distribution of both furin and TGN46 was analyzed by immunofluorescence. In the presence of PACS-1, furin demonstrated a paranuclear, TGN-like staining pattern indistinguishable from the normal localization of furin (Figure 8A). In the presence of PACS-1Admut, furin demonstrated a dispersed punctate distribution, whereas TGN46 remained in a normal TGN-like pattern (Figure 8B and C). Similarly, the distribution of both endogenous CI-MPR and TGN46 was analyzed in cells expressing either PACS-1 or PACS-1Admut. In the presence of PACS-1, CI-MPR demonstrated a normal TGN-like staining pattern, whereas in the presence of PACS-1Admut, CI-MPR showed a dispersed punctate distribution while TGN46 remained in a normal TGN-like localization (Figure 8D–F). Further analysis of cells expressing PACS-1 or PACS-1Admut demonstrated that the localization of mannosidase II (medial Golgi marker), lamp-1 (lysosome marker) and γ-adaptin was unaffected by expression of either of these two proteins (Figure 8G–L). Analysis of the neuroendocrine cell line AtT-20 showed that furin was mislocalized away from the TGN to a dispersed punctate distribution which showed significant co-localization with adrenocorticotrophic hormone (ACTH) by the presence of PACS-1Admut (Figure 8M–O). These data suggest that furin is mislocalized to secretory granules by the presence of PACS-1Admut in neuroendocrine cells. In all cases above, no difference was detected in the distribution of any protein analyzed between cells expressing wild-type PACS-1 or control cells (infected with empty vectors or mock infected; data not shown).
Fig. 8. Expression of dominant-negative PACS-1 disrupts the subcellular localization of furin and CI-MPR. A7 or AtT-20 cells were infected with recombinant vaccinia viruses expressing PACS-1 or PACS-1Admut alone (D–L) or together with vaccinia viruses expressing FLAG-tagged furin (A–C and M–O). Cells were fixed, permeabilized and double labeled with anti-FLAG tag (M1; A–C and M–O) or anti-CI-MPR (D–F) and anti-TGN46 (C and F) or anti-ACTH (O). Further cells were labeled with anti-mannosidase II (G and J), anti-lamp-1 (H and K) or anti-γ-adaptin (I and L). Cells were analyzed by fluorescence microscopy, and merged images of green and red signals are shown (C, F and O inset).
Taken together, these data demonstrate that PACS- 1Admut does indeed act as a bona fide dominant-negative with respect to proteins containing acidic cluster motifs (furin and CI-MPR), causing their mislocalization away from the TGN, while not affecting other secretory pathway proteins (TGN46, mannosidase II and lamp-1). These data are consistent with a functional role for PACS-1 in the active retrieval of proteins such as furin and CI-MPR to the TGN from post-TGN/endosomal compartments. The mislocalization of furin to secretory granules caused by PACS-1Admut in neuroendocrine cells is in agreement with previous observations demonstrating that the binding site for PACS-1 within furin (the phosphorylated acidic cluster) is required for efficient retrieval of furin from immature secretory granules (ISGs) (Dittie et al., 1997).
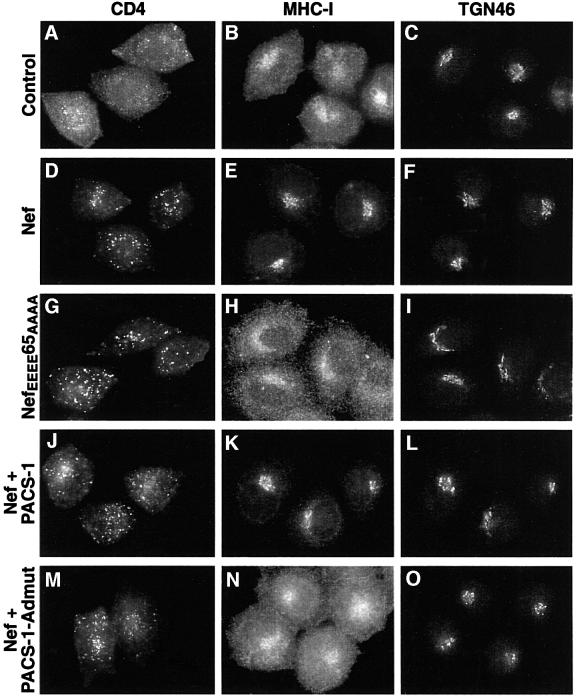
Recent data have shown that PACS-1 is also involved in viral pathogenesis, specifically in the HIV-1 Nef-mediated downregulation of MHC-I from the cell surface to the TGN (Piguet et al., 2000). These data showed that PACS-1 interacts directly with an acidic cluster motif within Nef (EEEE65), and that this interaction is crucial for MHC-I downregulation. In contrast, neither this tetraglutamic acid Nef motif nor PACS-1 is required for downregulation of CD4 by Nef (Piguet et al., 2000). To determine whether the connector function of PACS-1 is required for Nef-mediated MHC-I downregulation, the distribution of CD4, MHC-I and TGN46 in HeLa-CD4 cells expressing different combinations of Nef and PACS-1 or PACS- 1Admut was analyzed. Control cells showed a predominantly cell-surface localization of both CD4 and MHC-I, and a paranuclear distribution of TGN46, as expected (Figure 9A–C). In the presence of Nef, CD4 showed a punctate distribution reminiscent of late endosomes/lysosomes and MHC-I showed a TGN-like paranuclear localization (Figure 9D–F), demonstrating the different downregulation pathways of these two proteins induced by Nef. An indistinguishable distribution of both MHC-I and CD4 was observed with co-expression of both Nef and PACS-1 (Figure 9J–L). In contrast, cells co-expressing Nef and PACS-1Admut, MHC-I showed a cell-surface distribution while CD4 showed a ‘downregulated’ punctate distribution (Figure 9M–O). This effect was identical to the staining patterns observed for CD4 and MHC-I in the presence of NefEEEE65AAAA [a mutant Nef protein unable to interact with PACS-1 (Piguet et al., 2000); Figure 9G–I]. These data show that PACS-1Admut can block the ability of Nef to downregulate MHC-I from the cell surface to the TGN, but not its ability to downregulate CD4. As this observation is identical to that for NefEEEE65AAAA, these data point to the formation of a complex between HIV-1 Nef, PACS-1 and adaptor complexes that is essential for MHC-I downregulation in HIV-1-infected cells.
Fig. 9. Expression of dominant-negative PACS-1 disrupts the ability of HIV-1 Nef to downregulate MHC-I. HeLa-CD4 cells were infected with vaccinia virus alone (A–C), viruses expressing Nef (D–F) or NefEEEE65AAAA (G–I), or co-infected with viruses expressing Nef and PACS-1 (J–L) or Nef and PACS-1Admut (M–O). Cells were fixed, permeabilized and labeled with anti-CD4 (column 1) or double labeled with anti-MHC-I (column 2) and anti-TGN46 (column 3) followed by fluorescently labeled secondary antisera. The expression of PACS-1 alone had no effect on MHC-I or CD4 localization (data not shown).
Discussion
The trafficking itineraries of numerous membrane proteins have been shown to rely on the presence of short sequences of amino acids within their cytosolic domains (Kirchhausen et al., 1997). One such class of motifs, based around a cluster of acidic residues, often containing consensus sites for CK2 phosphorylation, has been shown to interact with the protein PACS-1 (Wan et al., 1998; Molloy et al., 1999; Piguet et al., 2000; Xiang et al., 2000). In this report, we show that PACS-1 also interacts with specific adaptor complexes (AP-1 and AP-3) and that PACS-1 can mediate the formation of a ternary complex between itself, AP-1 and membrane protein cargo molecules containing acidic cluster motifs. These data suggest that PACS-1 functions in a connector capacity by linking acidic cluster motifs to adaptor complexes and their associated vesicle-generating machinery.
The proposed ability of PACS-1 to function as a connector by linking cargo to adaptor complexes, thus allowing the inclusion of such cargo into transport vesicles, is similar to activities reported for some proteins involved in endocytosis. The internalization of many activated G-protein-coupled receptors (GPCRs) requires the function of β-arrestin (Lefkowitz, 1998). The incorporation of GPCRs into clathrin-coated pits and subsequent endocytic vesicles requires the ability of β-arrestin to interact with GPCRs and with both the AP-2 adaptor complex and clathrin (Goodman et al., 1997; Laporte et al., 1999, 2000). Therefore, β-arrestin functions as a connector molecule by linking the cytosolic domains of cargo (GPCRs) to adaptors and the associated vesicle-generating machinery (AP-2 and clathrin). Another protein that has also been shown to function in a connector capacity is the HIV-1 protein Nef in the downregulation of CD4. Nef has been shown to interact with both the cytosolic domain of CD4 and the AP-2 adaptor complex, thereby causing the incorporation of CD4 into clathrin-coated pits and resulting endocytic vesicles (Greenberg et al., 1997; Piguet et al., 1998). Therefore, Nef also functions as a connector molecule by linking cargo (CD4) to adaptors and associated vesicle-generating machinery (AP-2 and clathrin). These data suggest that PACS-1 shares functional similarity with both β-arrestin and Nef through its ability to connect cargo to adaptor complexes. Importantly, however, PACS-1 connects proteins to AP-1 and AP-3, unlike β-arrestin and Nef, which have been shown to functionally connect proteins to AP-2. The association of Nef with AP-2 is required specifically to downregulate CD4 but not MHC-I. Together with our results showing a lack of assocation of PACS-1 with AP-2, these data suggest that MHC-I is downregulated by Nef and PACS-1 in a pathway that does not require AP-2.
As we have reported previously, numerous proteins that are involved in many different membrane traffic steps contain acidic cluster motifs, and thus potential PACS-1 interaction sites (Molloy et al., 1999). Therefore, PACS-1 may be involved in several different trafficking events. Such a variety of roles for PACS-1 could be reconciled with differential binding of PACS-1 to various adaptor complexes. We have demonstrated the ability of PACS-1 to associate with both AP-1 and AP-3, but not AP-2. Also while they have not been tested, AP-4 (Dell’Angelica et al., 1999; Hirst et al., 1999) and the epithelium-specific AP-1B (Folsch et al., 1999) may also interact with PACS-1. Which adaptor complex PACS-1 binds to could be determined indirectly by some sequence determinant within cargo molecules (perhaps through subtle differences in the sequence context of acidic cluster motifs) or by the presence of spatially distinct pools of PACS-1. Further specificity in the PACS-mediated trafficking events may arise through PACS-1 homologs or functional equivalents that have different adaptor and/or compartment specificity.
Through deletion analysis of PACS-1, we have identified a short sequence of 18 amino acids that is necessary and sufficient for adaptor binding. Mutation of eight consecutive residues within this sequence abolishes the ability of PACS-1 to interact with adaptors, but importantly, this mutation has no effect upon the interaction of PACS-1 with acidic cluster motifs. Thus, we have generated a mutant PACS-1 protein that can interact with cargo molecules but can not connect such cargo to adaptor proteins. Interestingly, the mutational analysis of the adaptor-binding region in PACS-1 demonstrated that replacement of single or 3–4 consecutive residues with alanines throughout this entire region failed to inhibit PACS-1–adaptor interactions (data not shown). These data imply that the interaction between PACS-1 and adaptors involves multiple contact sites, and that no single contact is absolutely required. Large contact sites between proteins have also been identified between other mediators of vesicle biogenesis. One such example is the recently published data identifying the interaction sites within amphiphysin 1 for both clathrin and AP-2: 40 and 19 amino acids, respectively (Slepnev et al., 2000). A large, multi-contact interaction site between PACS-1 and adaptors is consistent with our observations that PACS-1FBR can interact with both in vitro translated µ1 and σ1, suggesting that PACS-1 may contact both of these AP-1 subunits simultaneously. Interestingly, a similar dual interaction has been observed between synaptotagmin and AP-2, with synaptotagmin binding to both µ2- and α-adaptins (Haucke et al., 2000). However, there is homology between σ1 and the N-terminal region of µ1 (Traub, 1997), suggesting that PACS-1 may interact individually with a similar motif on both adaptins. It will be intriguing to determine whether PACS-1 does indeed contact both µ1 and σ1 during interaction with AP-1 in vivo, and whether these adaptins interact simultaneously or individually with PACS-1.
Previously, we have shown that furin is localized to the TGN through an acidic cluster/PACS-1-dependent retrieval step from an endocytic compartment (Wan et al., 1998). In this report, we show that the expression of mutant PACS-1 (Admut), defective in adaptor binding, causes furin to be redistributed away from its normal TGN location. Furthermore, we show a remarkably similar effect on CI-MPR, with PACS-1Admut expression also causing a redistribution of CI-MPR away from the TGN. AP-3 has recently been associated with the trafficking of certain proteins to lysosomes in mammalian cells, namely lamp-1, limp-II and tyrosinase (Höning et al., 1998; Le Borgne et al., 1998). Even though these lysosomal proteins do not contain acidic cluster motifs within their cytosolic domains (and so are unlikely to interact with PACS-1), given the interaction of PACS-1 with AP-3 it was deemed possible that PACS-1Admut could affect such lysosomal trafficking. However, there was no discernible effect of PACS-1Admut expression on the subcellular localization of lamp-1, suggesting that the ability of PACS-1 to interact with AP-1 and AP-3 is not required for AP-3-dependent lysosomal targeting. Other proteins that are also thought to be independent of PACS-1, namely TGN46, mannosidase II and internalized transferrin, were unaffected by expression of PACS-1Admut. Together, these data show that PACS-1Admut expression can cause a ‘dominant-negative’ phenotype with respect to PACS-1-dependent protein traffic. We have also shown that PACS-1Admut expression causes furin to be mislocalized from the TGN to ACTH-positive secretory granules in neuroendocrine cells. These data further suggest that the ability of PACS-1 to interact with adaptor complexes is crucial for retrieving furin from maturing ISGs in these cell types, consistent with previous data (Dittie et al., 1997).
Recently, cell lines have been derived from transgenic mice that lack the µ1A protein and thus have non-functional AP-1 complexes (Meyer et al., 2000). It is interesting to note that one of the main phenotypic effects observed in these cells was the disruption of MPR retrieval from endosomes to the TGN. These data are consistent with a role for PACS-1 in connecting MPRs to AP-1 in the recycling of these receptors to the TGN. Another protein that has also been shown to be important for MPR trafficking from late endosomes to the TGN is TIP47 (Diaz and Pfeffer, 1998). It will be interesting to assess whether PACS-1 and TIP47 function at different points in the same MPR retrieval pathway or whether they constitute two different retrieval mechanisms.
We have recently shown a role for PACS-1 in the downregulation of MHC-I by HIV-1 Nef (Piguet et al., 2000). An essential activity in the ability of HIV-1 to evade the host immune system is the Nef-dependent redistribution of MHC-I from the cell surface of infected cells to the TGN (Schwartz et al., 1996). This redistribution (and hence downregulation) is dependent on the ability of Nef to interact with PACS-1 (Piguet et al., 2000). We have demonstrated here that the ability of PACS-1 to interact with adaptor complexes is crucial for the ability of Nef to cause MHC-I downregulation but not CD4 downregulation. Given the connector functions of both PACS-1 and Nef, these data suggest that PACS-1 and Nef form a ‘co-connector’ complex that is able to link MHC-I to adaptor complexes, a process that ultimately retains MHC-I in the TGN. These data raise the intriguing possibility of combating HIV’s immune evasion through inhibition of PACS-1 function.
In summary, we have demonstrated a direct interaction between the cellular sorting protein PACS-1 and adaptor complexes involved in trafficking events within the TGN/endosomal system. We have identified the adaptor-binding site in PACS-1 and showed that mutation of this site prevents PACS-1’s ability to interact with both AP-1 and AP-3. This mutant PACS-1 disrupts independent trafficking pathways shown to require PACS-1 function: TGN localization of furin and CI-MPR and MHC-I downregulation by Nef. We believe these data clearly demonstrate the importance of PACS-1 in the many varied trafficking events controlled by acidic cluster motifs.
Materials and methods
Cell lines and vaccinia virus
HeLa-CD4, BSC-40, AtT-20 and A7 cells were cultured as previously described (Anderson et al., 1993; Molloy et al., 1994; Liu et al., 1997; Xiang et al., 2000).
Vaccinia viruses expressing HIV-1 Nef, NefEEEE65AAAA and PACS-1 have been described previously (Piguet et al., 2000). A recombinant vaccinia virus expressing PACS-1Admut was made as previously described (VanSlyke et al., 1995).
DNA constructs
A fragment of the first half of human PACS-1 was amplified with oligonucleotides XY-010 (see Table I) and YX-010, digested with KpnI and ApaI, blunt ended and ligated into pGEX4T-1 (Pharmacia). This recombinant vector was digested with EcoRI and the fragment encoding residues 1–153 was cloned into pGEX4T-1. A DNA fragment encoding residues 117–266 was amplified with oligonucleotides XY-011 and YX-111 and cloned into the BamHI site of pGEX3X. A DNA fragment encoding residues 240–499 was amplified with oligonucleotides XY-051 and YX-013 and cloned into the KpnI–EcoRI sites of pGEX3X. A DNA fragment encoding residues 500–963 was amplified with the oligonucleotides XY-012 and YX-012, digested with KpnI and ApaI, blunt ended and cloned into the SmaI site of pGEX4T-1. Truncation constructs of the FBR region of PACS-1 were subcloned into the BamHI site of pGEX3X after amplification with the following oligonucleotide pairs: Δ1, XY-011/ YX-050; Δ2, XY-011/YX-101; Δ3, XY-011/YX-114; Δ4, XY-105/YX- 111; Δ5, XY-106/YX-111; Δ6, XY-119/YX-111; Δ7, XY-105/ YX-100; and Δ8, XY-115/YX-124.
Table I. Oligonucleotides used in the amplification reactions.
| Oligo | Sequence |
|---|---|
| XY-010 | 5′-CGGTACCATGGCGGAACGCGGAGGGGCG |
| YX-010 | 5′-CAGGGCCCTCACACTTTGCTGGGGGAGCAGA |
| XY-011 | 5′-CGGGATCCCGGGTACCGTGCCTAGGCTATTCAGC |
| YX-111 | 5′-CGTGGATCCTCACTTGGATTTGATTCCTTCAT |
| XY-051 | 5′-AGGGTACCGTCTCTGTGCCTGTGGCA |
| YX-013 | 5′-CAGGGCCCTCACACTTTGCTGGGGGAGGCAGA |
| XY-012 | 5′-CAGGGTACCGAGGGGGTGCACACACCCCGG |
| YX-012 | 5′-GCAGGGCCCTCAGGTGGCCTTGCTGCCACTGAA |
| YX-050 | 5′-AGGGATCCTCAATCTTCAACGTTGCTGTG |
| YX-101 | 5′-ACGGGATCCTCACAAGGTCTTATAGCCCAA |
| YX-114 | 5′-ACGGAATTCTCAATCTTTGTCCATTTCTTT |
| XY-105 | 5′-CTGGATCCCGGGTACCCTTCGCTCCAACGAGATC |
| XY-106 | 5′-CTGGATCCCGGGTACCTTAACCTTCTCCCTTCAG |
| XY-119 | 5′-CTGGATCCCGGGTACCATCATGCTGCAAAGGAGA |
| YX-100 | 5′-ACGGGATCCTCATTGGAGCTCTGTTTCCAC |
| XY-115 | 5′-CTTGGATCCCCAGCTAGTGGACTGGTG |
| YX-124 | 5′-CATGAATTCTCAGTACTGAAGGGAGAAGGT |
| COL007 | 5′-GGATCCCGCTCTGGCTTTAGT |
| COL008 | 5′-CTCGAGTCAGAGGGCGCTCTGGTC |
| COL001 | 5′-GGATCCGTGCCTAGGCTATTCAGC |
| COL003 | 5′-CTCGAGTCAATCATCACTGCCTTCCTG |
An eight-amino-acid stretch of PACS-1 (E168TELQLTF175) was replaced with eight alanine residues by PCR mutagenesis using overlapping oligonucleotides encoding the mutated codons. The mutated fragment was then subcloned into the EcoNI and AvrII sites of pGEX-PACS-1117–266 (FBR) and pZVneo-PACS-1 for expression of GST– PACS-1FBR-Admut and production of recombinant vaccinia virus expressing PACS-1Admut, respectively.
The pET32-furin-cd vector was constructed by amplification of the furin cytosolic domain with oligonucleotides COL007 and COL008, followed by subcloning into the BamHI and XhoI sites of pET32a (Novagen). The pET32-PACS-1FBR vector was constructed by amplification of the PACS-1FBR domain (residues 117–294) with oligonucleotides COL001 and COL003, followed by subcloning into the BamHI and XhoI sites of pET32a.
In vivo co-immunoprecipitation
BSC-40 cells were infected with vaccinia viruses encoding PACS-1, PACS-1Admut or wild-type vaccinia viruses as described previously (Molloy et al., 1994). At 16 h post-infection, cells were washed once with phosphate-buffered saline (PBS) and harvested in co-IP buffer [PBS pH 7.5, 1% NP-40, 1 mM E64, 1 mM pepstatin A, 1 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM aprotinin]. The cell lysates were incubated for 20 min on ice and centrifuged at 5000 g for 5 min at 4°C. The supernatant was incubated with anti-HA (HA.11; Babco) for 2 h at room temperature and precipitated with protein G–Sepharose. The beads were washed with co-IP buffer and analyzed by western blotting using antibodies against γ-adaptin (100.3; Sigma; 1:500), α-adaptin (100.2; Sigma; 1:200), δ-adaptin (from R.Kelly; 1:5000) or the HA epitope on the PACS proteins (HA.11; 1:1000).
Protein purification and lysate preparation
pGEX vectors encoding the various GST–PACS-1 constructs were co-transformed into the bacterial strain BL21 (DE3) pLysS (Invitrogen) with the pT-Trx plasmid (Yasukawa et al., 1995). GST fusion proteins were purified using glutathione–Sepharose following the manufacturer’s guidelines (Pharmacia). Thioredoxin (Trx) fusion proteins encoded by the vectors pET32-furin-cd and pET32-PACS-1FBR were expressed and purified using Ni-NTA–agarose following the manufacturer’s instructions (Qiagen). GST–fur-cd(DDD) was expressed and purified as previously described (Wan et al., 1998). Purified AP-1 was obtained as described (Austin et al., 2000).
BSC-40 cell lysates were prepared by harvesting cells in GST binding buffer (150 mM NaCl, 50 mM Tris pH 7.5, 2 mM MgCl2, 1% NP-40) supplemented with protease inhibitors. The lysates were incubated for 20 min on ice and centrifuged at 5000 g for 5 min at 4°C.
GST protein binding assays
Lysate and purified AP-1 interactions. BSC-40 lysates or 1 µg of purified AP-1 were incubated with 5 µg of each respective GST fusion protein in GST binding buffer for 45 min at 37°C, followed by incubation with glutathione–agarose for 30 min at room temperature. Glutathione beads were harvested by centrifugation, washed with GST binding buffer and analyzed by western blotting using antibodies against γ-adaptin.
Ternary complex analysis. A 10 µg aliquot of GST–Fur-cd(DDD) or GST alone was immobilized on 20 µl of glutathione–agarose and washed in PBS. Beads were incubated with 4 µg of purified AP-1 in the presence or absence of 5 µg of Trx-PACS-1FBR for 45 min at 37°C. Glutathione beads were harvested by centrifugation (2000 g, 2 min, room tempera ture) and washed with PBS + 1% NP-40 pH 7.0. Samples were analyzed by western blotting using antibodies against γ-adaptin. Chemilumin escence signals were quantified using the NIH image gel analysis program and data corrected for non-specific binding to GST alone.
Furin cytosolic domain interactions. Trx-furin-cd was phosphorylated with purified CK2 as previously described (Jones et al., 1995). A 5 µg aliquot of GST–PACS-1 fusion proteins was incubated with 2 µg of phosphorylated Trx-furin-cd for 45 min at 37°C, followed by incubation with glutathione–agarose for 30 min at room temperature. Glutathione beads were harvested by centrifugation (2000 g, 2 min, room tempera ture) and washed with GST binding buffer. Samples were then analyzed by western blotting using antibodies against the His tag of Trx-furin-cd (anti-tetra His; Qiagen; 1:1000).
Peptide competition analysis. Peptides containing the amino acid sequences L166VETELQLTFSLQY179 or E261GIKSKLSDRSPDI274 (Macromolecular Resources; numbering represents residue position in human PACS-1) were reconstituted in GST binding buffer + 5% dimethylsulfoxide. The peptides were added to the GST binding reactions at various concentrations.
In vitro translation
cDNA fragments encoding µ1-, σ1- and γ-adaptins were excised from the plasmids pACT2/µ1, pACT2/σ1 and pACT2/γ (from J.Bonifacino) with EcoRI–XhoI or BamHI–EcoRI. The cDNA fragment encoding β1-adaptin was excised from the plasmid pGAD10/β1 (from K.Nakayama) with EcoRI. All four cDNA fragments were subcloned into pBSSK(+) cut with the same enzymes. The recombinant pBSSK-adaptin vectors were used as templates for in vitro translation from the T7 promoter according to the manufacturer’s instructions (Promega, USA). A 10 µl aliquot of clarified translation reactions was incubated with 5 µg of GST– PACS-1FBR or GST alone in GST binding buffer for 45 min at 37°C. Samples were incubated with glutathione–agarose for 30 min at room temperature, harvested by centrifugation (2000 g, 2 min, room temperature) and washed with GST binding buffer. Bound proteins were resolved by SDS–PAGE and detected by autoradiography.
Quantitative transferrin internalization assays
A7 cells were infected with recombinant vaccinia viruses expressing either PACS-1 or PACS-1Admut, vaccinia virus alone [multiplicity of infection (m.o.i.) 5], or were mock infected. At 4 h post-infection, the rate of [125I]transferrin (Tf) uptake was measured as previously described (Liu et al., 1997).
Immunofluorescence
PACS-1, AP-1 and AP-3. A7 cells were grown and processed for immunofluorescence as previously described (Molloy et al., 1994). Primary antibodies to PACS-1 (polyclonal 599; 1:50) together with monoclonal antibodies to AP-1 (100.3; 1:50) or AP-3 (from J.Bonifacino; 1:50) were incubated for 4 h at room temperature.
Furin, TGN46, CI-MPR, mannosidase II, γ-adaptin, lamp-1 and transferrin uptake. A7 cells were grown to 80% confluency and infected with recombinant vaccinia viruses expressing either PACS-1 or PACS-1Admut, with vaccinia virus alone (all at m.o.i. 10) or co-infected with vaccinia viruses expressing fur/f (m.o.i. 3), together with the above viruses (all at m.o.i. 7), or were mock infected. Cells were processed for immunofluorescence. Primary antibodies to the FLAG tag (monoclonal M1; Kodak; 1:300), TGN46 (p12; from S.Ponnambalam; 1:100), CI- MPR (monoclonal anti-MPR/IGF2 receptor; from S.Pfeffer, 1:2), mannosidase II (from K.Moremen; 1:200), γ-adaptin (100.3; 1:50) and lamp-1 (H4A3; 1:20) were incubated for 1 h at room temperature. Alternatively, internalization of iron-loaded rhodamine–transferrin (Molecular Probes) was analyzed as previously described (Liu et al., 1997).
Furin and ACTH. AtT-20 cells were grown to 80% confluence and co-infected with recombinant vaccinia viruses expressing either PACS-1 or PACS-1Admut and fur/f (each at m.o.i. 5). Cells were fixed and processed for immunofluorescence. Primary antibodies to FLAG-tag (M1) together with polyclonal antibodies to ACTH (AS29; 1:200) were incubated overnight at 4°C.
MHC-I, CD4 and TGN46. HeLa-CD4 cells were infected with wild-type vaccinia virus (m.o.i. 10), vaccinia virus expressing NefEEEE65AAAA (m.o.i. 10) or co-infected with vaccinia viruses expressing Nef (m.o.i. 3) and either PACS-1, PACS-1Admut or wild-type vaccinia (m.o.i. 7). Cells were fixed and processed for immunofluorescence. Primary antibodies to MHC-I (W6/32; from D.Johnson; 1:100), CD4 (anti-CD4 #4; Lab Vision Corp; 1:100) and TGN46 (p12) were incubated at 4°C overnight.
Following incubation with fluorescently labeled secondary antisera (Southern Biotech), all images were captured using a 63× oil immersion objective on a Leica DM-RB microscope and processed with the Scion Image 1.62 program.
Acknowledgments
Acknowledgements
We thank J.S.Bonifacino (NIH), K.Nakayama (University of Tsukuba), R.Kelly (UCSF), S.Ponnambalam (Dundee University), S.Pfeffer (Stanford University), K.Moremen (University of Georgia) and D.Johnson (OHSU) for their generous gifts of reagents. We thank F.Cook and C.Gross for help with the quantitative transferrin uptake assays, and members of the Thomas laboratory for helpful discussions. This work was supported by NIH grants DK37274 and AI49793. C.M.C. is funded by a Prize Travelling Research Fellowship from the Wellcome Trust. F.G. is funded by the Human Frontiers of Science Program.
References
- Alconada A., Bauer,U., Sodeik,B. and Hoflack,B. (1999) Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol., 73, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.D., Thomas,L., Hayflick,J.S. and Thomas,G. (1993) Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed α1-antitrypsin variant. J. Biol. Chem., 268, 24887–24891. [PubMed] [Google Scholar]
- Austin C., Hinners,I. and Tooze,S.A. (2000) Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem., 275, 21862–21869. [DOI] [PubMed] [Google Scholar]
- Chen H.J., Yuan,J. and Lobel,P. (1997) Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J. Biol. Chem., 272, 7003–7012. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica E.C., Mullins,C. and Bonifacino,J.S. (1999) AP-4, a novel protein complex related to clathrin adaptors. J. Biol. Chem., 274, 7278–7285. [DOI] [PubMed] [Google Scholar]
- Diaz E. and Pfeffer,S.R. (1998) TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell, 93, 433–443. [DOI] [PubMed] [Google Scholar]
- Dittie A.S., Thomas,L., Thomas,G. and Tooze,S.A. (1997) Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phos phorylation. EMBO J., 16, 4859–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H., Ohno,H., Bonifacino,J.S. and Mellman,I. (1999) A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell, 99, 189–198. [DOI] [PubMed] [Google Scholar]
- Goodman O.B. Jr, Krupnick,J.G., Gurevich,V.V., Benovic,J.L. and Keen,J.H. (1997) Arrestin/clathrin interaction. Localization of the arrestin binding locus to the clathrin terminal domain. J. Biol. Chem., 272, 15017–15022. [DOI] [PubMed] [Google Scholar]
- Greenberg M.E., Bronson,S., Lock,M., Neumann,M., Pavlakis,G.N. and Skowronski,J. (1997) Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J., 16, 6964–6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M.E., Iafrate,A.J. and Skowronski,J. (1998) The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J., 17, 2777–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V., Wenk,M.R., Chapman,E.R., Farsad,K. and De Camilli,P. (2000) Dual interaction of synaptotagmin with µ2- and α-adaptin facilitates clathrin-coated pit nucleation. EMBO J., 19, 6011–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Bright,N.A., Rous,B. and Robinson,M.S. (1999) Character ization of a fourth adaptor-related protein complex. Mol. Biol. Cell, 10, 2787–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höning S., Sandoval,I.V. and von Figura,K. (1998) A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J., 17, 1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.G., Thomas,L., Molloy,S.S., Thulin,C.D., Fry,M.D., Walsh,K.A. and Thomas,G. (1995) Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cyto plasmic tail. EMBO J., 14, 5869–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. (1999) Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol., 15, 705–732. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Bonifacino,J.S. and Riezman,H. (1997) Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr. Opin. Cell Biol., 9, 488–495. [DOI] [PubMed] [Google Scholar]
- Laporte S.A., Oakley,R.H., Zhang,J., Holt,J.A., Ferguson,S.S., Caron,M.G. and Barak,L.S. (1999) The β2-adrenergic receptor/β-arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl Acad. Sci. USA, 96, 3712–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte S.A., Oakley,R.H., Holt,J.A., Barak,L.S. and Caron,M.G. (2000) The interaction of β-arrestin with the AP-2 adaptor is required for the clustering of β2-adrenergic receptor into clathrin-coated pits. J. Biol. Chem., 275, 23120–23126. [DOI] [PubMed] [Google Scholar]
- Le Borgne R., Alconada,A., Bauer,U. and Hoflack,B. (1998) The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J. Biol. Chem., 273, 29451–29461. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R.J. (1998) G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J. Biol. Chem., 273, 18677–18680. [DOI] [PubMed] [Google Scholar]
- Liu G., Thomas,L., Warren,R.A., Enns,C.A., Cunningham,C.C., Hartwig, J.H. and Thomas,G. (1997) Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway. J. Cell Biol., 139, 1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M.S., Ohno,H., Kirchhausen,T. and Bonifacino,J.S. (1997) Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol., 7, 124–128. [DOI] [PubMed] [Google Scholar]
- Mauxion F., Le Borgne,R., Munier-Lehmann,H. and Hoflack,B. (1996) A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J. Biol. Chem., 271, 2171–2178. [DOI] [PubMed] [Google Scholar]
- Mellman I. (1996) Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol., 12, 575–625. [DOI] [PubMed] [Google Scholar]
- Meyer C., Zizioli,D., Lausmann,S., Eskelinen,E.L., Hamann,J., Saftig,P., von Figura,K. and Schu,P. (2000) µ1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J., 19, 2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy S.S., Thomas,L., VanSlyke,J.K., Stenberg,P.E. and Thomas,G. (1994) Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J., 13, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy S., Anderson,E., Jean,F. and Thomas,G. (1999) Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol., 9, 28–35. [DOI] [PubMed] [Google Scholar]
- Ohno H. et al. (1995) Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science, 269, 1872–1875. [DOI] [PubMed] [Google Scholar]
- Page L.J., Sowerby,P.J., Lui,W.W. and Robinson,M.S. (1999) γ-synergin: an EH domain-containing protein that interacts with γ-adaptin. J. Cell Biol., 146, 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V., Chen,Y.L., Mangasarian,A., Foti,M., Carpentier,J.L. and Trono,D. (1998) Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the µ chain of adaptor complexes. EMBO J., 17, 2472–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V., Wan,L., Borel,C., Mangasarian,A., Demaurex,N., Thomas,G. and Trono,D. (2000) HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nature Cell Biol., 2, 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I., Chen,Y.C., Cupers,P., Shoelson,S.E. and Kirchhausen,T. (1998) Dileucine-based sorting signals bind to the β chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J., 17, 2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O., Marechal,V., Le Gall,S., Lemonnier,F. and Heard,J.M. (1996) Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nature Med., 2, 338–342. [DOI] [PubMed] [Google Scholar]
- Shih W., Gallusser,A. and Kirchhausen,T. (1995) A clathrin-binding site in the hinge of the β2 chain of mammalian AP-2 complexes. J. Biol. Chem., 270, 31083–31090. [DOI] [PubMed] [Google Scholar]
- Slepnev V.I., Ochoa,G.C., Butler,M.H. and De Camilli,P. (2000) Tandem arrangement of the clathrin and AP-2 binding domains in amphiphysin 1 and disruption of clathrin coat function by amphi physin fragments comprising these sites. J. Biol. Chem., 275, 17583–17589. [DOI] [PubMed] [Google Scholar]
- Traub L.M. (1997) Clathrin-associated adaptor proteins—putting it all together. Trends Cell Biol., 7, 43–46. [DOI] [PubMed] [Google Scholar]
- Traub L.M. and Kornfeld,S. (1997) The trans-Golgi network: a late secretory sorting station. Curr. Opin. Cell Biol., 9, 527–533. [DOI] [PubMed] [Google Scholar]
- VanSlyke J.K., Thomas,L. and Thomas,G. (1995) Use of vaccinia virus vectors to study neuropeptide processing. In Smith,A.I. (ed.), Peptidases and Neuropeptide Processing. Vol. 23. Academic Press, San Diego, CA, pp. 45–64.
- Wan L., Molloy,S.S., Thomas,L., Liu,G., Xiang,Y., Rybak,S.L. and Thomas,G. (1998) PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell, 94, 205–216. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Molloy,S.S., Thomas,L. and Thomas,G. (2000) The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments. Mol. Biol. Cell, 11, 1257–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa T., Kanei-Ishii,C., Maekawa,T., Fujimoto,J., Yamamoto,T. and Ishii,S. (1995) Increase of solubility of foreign proteins in Escherichia coli by coproduction of the bacterial thioredoxin. J. Biol. Chem., 270, 25328–25331. [DOI] [PubMed] [Google Scholar]