Abstract
Recent studies on G-protein-coupled receptors revealed that they can dimerize. However, the role of each subunit in the activation process remains unclear. The γ-amino-n-butyric acid type B (GABAB) receptor is comprised of two subunits: GB1 and GB2. Both consist of an extracellular domain (ECD) and a heptahelical domain composed of seven transmembrane α-helices, loops and the C-terminus (HD). Whereas GB1 ECD plays a critical role in ligand binding, GB2 is required not only to target GB1 subunit to the cell surface but also for receptor activation. Here, by analysing chimeric GB subunits, we show that only GB2 HD contains the determinants required for G-protein signalling. However, the HD of GB1 improves coupling efficacy. Conversely, although GB1 ECD is sufficient to bind GABAB ligands, the ECD of GB2 increases the agonist affinity on GB1, and is necessary for agonist activation of the receptor. These data indicate that multiple allosteric interactions between the two subunits are required for wild-type functioning of the GABAB receptor and highlight further the importance of the dimerization process in GPCR activation.
Keywords: baclofen/dimerization/GPCRs/G-protein/signal transduction
Introduction
G-protein-coupled receptors (GPCRs) recognize various stimuli, including light, odours, pheromones, tastes, ions and a large variety of hormones and neurotransmitters (Bockaert and Pin, 1999). These proteins, which are encoded by >1% of the mammalian genes, are therefore involved in a large variety of physiological regulations and functions. Several subfamilies of GPCRs have been defined based on their proposed general structure and sequence similarity (Kolakowski, 1994; Bockaert and Pin, 1999). All possess seven α-helices, which contain extra- and intracellular loops connecting them, as well as the C-terminal tail. The intracellular loops and C-terminus are involved in the recognition and activation of the heterotrimeric G-proteins (Bourne, 1997). Helices, loops and C-terminus are referred to herein as the ‘heptahelical domain’ (HD). For a long time, it has been assumed that one molecule of GPCR interacts with one molecule of G-protein. However, recent studies have revealed that these receptors can form dimers, either homodimers or heterodimers (Salahpour et al., 2000). Therefore, in light of this emergent concept, the role of each subunit in the recognition and activation of the G-protein needs to be identified.
γ-amino-n-butyric acid (GABA) is a major neurotransmitter in the mammalian brain that controls neuronal excitability by activating ionotropic GABAA and GABAC receptors and G-protein-coupled GABAB receptors. The latter inhibits adenylyl cyclase and modulates the activity of a variety of ion channels. Two GABAB receptor types have been identified: GABAB1 (GB1) (Kaupmann et al., 1997b) and GABAB2 (GB2) (Jones et al., 1998; Kaupmann et al., 1998; White et al., 1998; Kuner et al., 1999). These proteins share 35% sequence identity and are related to the family 3 GPCRs, which also includes the metabotropic glutamate (mGlu) and calcium-sensing (CaS) receptors. These GPCRs have a large extracellular domain (ECD), which contains the agonist binding site and shares the same protein folding as bacterial periplasmic binding proteins (O’Hara et al., 1993; Galvez et al., 1999; Kunishima et al., 2000). Another specific feature of these family 3 receptors is that they are likely to function as dimers (Romano et al., 1996; Bai et al., 1999; Kunishima et al., 2000). In contrast to the mGlu and CaS receptors, which form functional homodimers, the GABAB receptor is a heteromer constituted of both GB1 and GB2 proteins (Kaupmann et al., 1998). Indeed, the coexpression of both subunits is required for a fully functional GABAB receptor (Jones et al., 1998; Kaupmann et al., 1998; White et al., 1998; Kuner et al., 1999).
Why are two subunits required for the formation of a functional GABAB receptor? The GB1 subunit binds all known GABAB ligands (Kaupmann et al., 1997b) and is responsible for the ligand recognition by the heteromeric GABAB receptor (Galvez et al., 2000a,b), whereas the GB2 subunit is necessary for the correct trafficking of GB1 to the cell surface (Couve et al., 1998; White et al., 1998). Indeed, the C-terminal tail of the GB2 subunit, by interacting with that of GB1, masks a signal responsible for the retention of GB1 in the endoplasmic reticulum (ER) (Margeta-Mitrovic et al., 2000; Calver et al., 2001; Pagano et al., 2001). However, even when this ER retention signal is mutated such that the GB1 subunit reaches the cell surface alone, no functional GABA response can be measured despite the correct binding of GABAB ligands (Margeta-Mitrovic et al., 2000; Calver et al., 2001; Pagano et al., 2001). This indicates that the GB2 subunit is necessary not only for the correct targeting of the GB1 subunit to the cell surface, but also for the correct functioning of the receptor.
The present study revealed new roles for the heterodimerization of the GABAB receptor and explained why the two subunits are required for the formation of a functional GABAB receptor. Here we show that although the ECD of GB1 is sufficient to bind GABAB ligands (Galvez et al., 1999; Malitschek et al., 1999), the ECD of GB2 is required for agonist-dependent activation of the receptor. Conversely, although the HD of GB2 contains the molecular determinants required for the recognition and activation of the same heterologous G-proteins as the wild-type heteromer, the HD of GB1 increases the coupling efficacy. These data reveal a modular organization of the GABAB receptor in which four functional and specialized domains cooperate.
Results
Individual chimeric GB1/2 or GB2/1 constructs are not functional
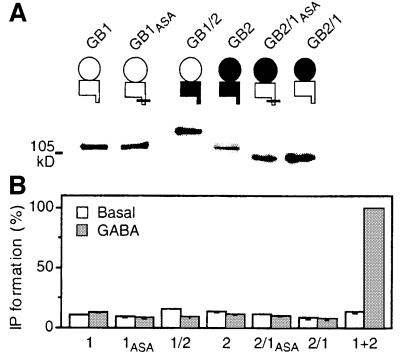
When the ER retention signal RSRR in the GB1 C-terminal tail is mutated into ASAR, the mutant receptor GB1ASA can reach the cell surface alone, bind GABAB ligands, but is still not functional (Margeta-Mitrovic et al., 2000; Calver et al., 2001; Pagano et al., 2001). We therefore speculated that the HD of GB2 was responsible for G-protein coupling, and so generated a chimeric GABAB receptor (called GB1/2) in which the HD of GB1 was replaced by that of GB2 (Figure 1A). The converse chimeric receptor (GB2/1 as well as its ER retention mutant GB2/1ASA) was also constructed. For all the constructs described, the GB1a variant (Kaupmann et al., 1997b) has been used. As shown in Figure 1A, all constructs were expressed and displayed the expected molecular weight. In addition, due to the presence of either a c-myc or an HA epitope added at their extracellular N-terminus, GB1ASA, GB2, GB1/2 and GB2/1ASA were shown to reach the cell surface (Pagano et al., 2001; data not shown). Among these, only GB1ASA and GB1/2 were able to bind the GB1 selective antagonist [125I]CGP64213, allowing us to show that both subunits bind GABA (Table I). The functionality of these constructs was then tested after their coexpression with the chimeric G-protein Gqi9 (Gαq in which the last nine C-terminal residues are replaced by those of Gαi2). This latter G-protein α-subunit has been shown to couple a wide variety of Gi-coupled receptors to PLC (Conklin et al., 1993), including the wild-type GABAB heteromeric receptor (Franek et al., 1999). When expressed alone, none of these constructs, not even the GB1/2 chimera, activated PLC upon application of GABA (Figure 1B) or baclofen (data not shown). Only a slight increase in the basal level of IP formation could be detected in cells expressing GB1/2, an activity that was inhibited by GABA. These data show that GB1/2, comprising the GABA-binding domain of GB1 and the GB2 HD, does not form a functional receptor that can be activated by GABA. Therefore, we examined whether the coexpression of any of these wild-type or chimeric subunits could form a functional receptor.
Fig. 1. Expression of the wild-type and chimeric GABAB receptor subunits. (A) Schematic representation and western blot analysis of the constructs used in this study. The GB1 and GB2 subunits are represented in white and black, respectively; the ECD is represented as a circle, whereas the HD is a rectangle with a tail showing the C-terminal tail. The horizontal bar means that the ER retention signal RSRR has been mutated into ASAR. (B) Basal (open bars) and 1 mM GABA-induced (grey bars) IP formation were measured in cells expressing the indicated constructs and Gqi9. Values correspond to the percentage of the GABA response measured in cells expressing the wild-type receptor and are the means ± SEM of at least three experiments performed in triplicate.
Table I. Affinity values of CGP64213 and GABAa.
| Subunit combination | [125I]CGP64213 binding |
|||
|---|---|---|---|---|
| CGP64213 Ki (nM) | Hill coefficient | GABA Ki (µM) | Hill coefficient | |
| GB1 | 1.4 ± 0.2b | 1.0 ± 1.1b | 22.3 ± 1.9b | 0.9 ± 0.1b |
| GB1ASA | 3.5 ± 0.8 | 1.4 ± 0.1 | 25.7 ± 3.9 | 0.7 ± 0.1 |
| GB1 + GB2 | 2.9 ± 0.2 | 1.4 ± 0.1 | 3.3 ± 0.6 | 1.0 ± 0.1 |
| GB1ASA + GB2/1ASA | 3.6 ± 0.6 | 1.4 ± 0.1 | 1.2 ± 0.4 | 0.8 ± 0.1 |
| GB1/2 | 4.0 ± 1.2 | 0.9 ± 0.2 | 8.2 ± 0.9 | 1.5 ± 0.1 |
| GB1/2 + GB2/1ASA | 2.8 ± 0.1 | 1.0 ± 0.1 | 2.5 ± 0.6 | 1.0 ± 0.2 |
| GB1/2 + GB1 | 3.9 ± 1.2 | 1.0 ± 0.2 | 6.0 ± 1.5 | 0.9 ± 0.2 |
| GB1/2 + GB2 | 3.3 ± 0.9 | 1.2 ± 0.1 | 3.3 ± 0.9 | 1.1 ± 0.3 |
aAffinity values (Ki, determined as described in Materials and methods) of CGP64213 and GABA as determined from displacement of [125I]CGP64213 binding on intact cells expressing the indicated subunit combinations.
bBinding experiments were performed on crude membranes [data from Galvez et al. (2000a)].
Values are means ± SEM of at least three independent determinations.
Coexpression of GB1/2 and GB2/1 leads to a functional GABAB receptor with wild-type properties
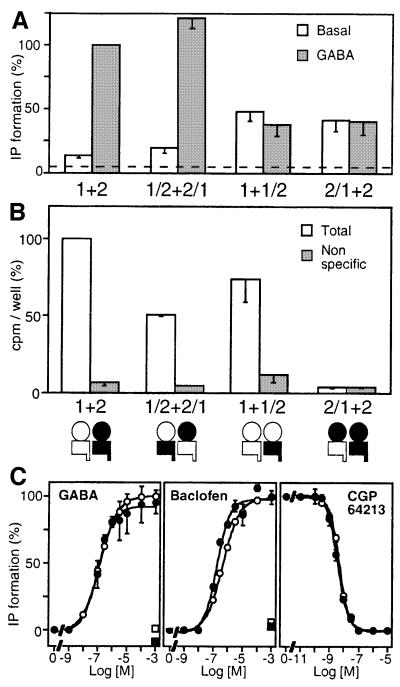
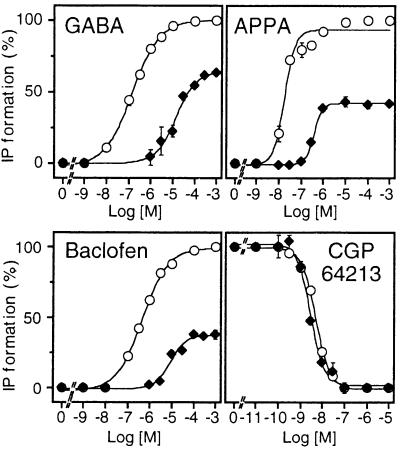
The possible formation of a functional GABAB receptor after coexpression of GB1/2 and GB2/1 was tested first, since all four modules (GB1 and GB2 ECDs and HDs) of the GABAB heteromer were conserved in that combination. As observed in cells expressing GB1 + GB2, a large GABA-induced IP formation was measured in cells expressing both GB1/2 and GB2/1 (Figure 2A). Indeed GABA, baclofen (Figure 2C) and APPA (data not shown), three GABAB agonists, stimulated IP formation to similar extents and with similar EC50 values to cells coexpressing the wild-type subunits. Moreover, the agonist-induced response could be inhibited by the GABAB antagonist CGP64213 with a similar potency to wild-type receptor (Figure 2C, Table I). Similar data were obtained when GB2/1ASA was used instead of GB2/1 (data not shown). These data revealed that both GB1/2 and GB2/1 are correctly folded. Moreover, they show that the modules can be swapped between the two subunits without affecting the function of the heteromer.
Fig. 2. Functional analysis of GABAB receptor combinations in which the heteromeric nature of the HDs is conserved. (A) Basal (open bars) and 1 mM GABA-induced (grey bars) IP formation were measured in cells expressing GB1 + GB2, GB1/2 + GB2/1, GB1 + GB1/2 or GB2/1 + GB2 and the chimeric G-protein Gqi9. The dotted line indicates the basal IP formation measured in mock-transfected cells. Values correspond to the percentage of the GABA response measured in cells expressing the wild-type receptor. (B) Total (open bars) and non-specific (grey bars; 1 mM GABA) [125I]CGP64213 binding measured in intact cells expressing GB1 + GB2, GB1/2 + GB2/1, GB1 + GB1/2 or GB2/1 + GB2. Values correspond to the amount of bound radioactivity per well expressed as a percentage of the total binding measured in cells expressing the wild-type receptor. (C) Dose–effect curves of GABA, baclofen and CGP64213 on cells expressing GB1 + GB2 (open circles) or GB1/2 + GB2/1 (filled circles). The effect of CGP64213 was analysed in the presence of 0.1 µM GABA. Values correspond to the percentage of the maximal agonist-induced IP formation measured in cells expressing the wild-type receptor. Values are means ± SEM of at least three experiments performed in triplicate.
Heteromeric nature of ECD is required for agonist activation, but not for G-protein coupling
We then examined the possible formation of a functional receptor after coexpression of two subunits such that the wild-type heteromeric nature of the HDs was conserved, but not that of the ECDs (Figure 2A). In cells expressing GB2/1 + GB2, GABA did not stimulate IP formation, but a large increase in the basal IP formation was detectable (Figure 2A). Surprisingly, the same high basal activity and no agonist-induced response was also observed in cells expressing GB1 and GB1/2, even though both subunits expressed alone or in combination clearly bind GABAB ligands (Figure 2B and Table I) and form heterodimers. Indeed, the GB1 subunit could be immunoprecipitated from cells expressing both GB1 and GB1/2 using a GB2 C-terminal antibody (data not shown). The basal activity observed with the combination GB1 + GB1/2 could not be inhibited by the competitive antagonists CGP64213 (data not shown). However, a significant inhibition of basal IP formation was induced by 1 mM GABA (Figure 2A), an effect antagonized by CGP64213 (data not shown). Taken together, these data revealed that either of these two combinations of GABAB subunits can activate Gqi9, but that the heteromeric nature of the ECDs is required for the activation by GABA.
Coexpression of GB2 and GB1/2 leads to a functional GABA receptor that couples to the same heterologous G-proteins as the wild-type receptor
In order to determine whether the heteromeric nature of HDs is important or not for coupling, combinations in which the heteromeric nature of the ECDs, but not that of the HDs, is maintained were tested (Figure 3).
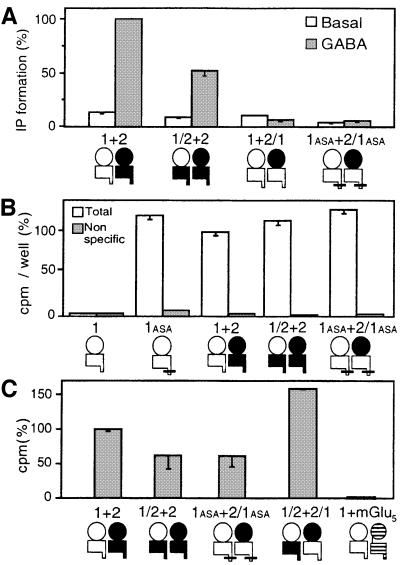
Fig. 3. Functional and biochemical analysis of GABAB receptor combinations in which the heteromeric nature of the ECDs is conserved. (A) Basal (open bars) and 1 mM GABA-induced (grey bars) IP formation were measured in cells expressing GB1 + GB2, GB1/2 + GB2, GB1 + GB2/1 or GB1ASA + GB2/1ASA and the chimeric G-protein Gqi9. Values correspond to the percentage of the GABA response measured in cells expressing the wild-type receptor. (B) Total (open bars) and non-specific (grey bars; 1 mM GABA) [125I]CGP64213 binding measured in intact cells expressing GB1, GB1ASA, GB1 + GB2, GB1ASA + GB2/1ASA or GB1/2 + GB2. Values correspond to the amount of bound radioactivity per well expressed as a percentage of the total binding measured in cells expressing the wild-type receptor. (C) Specific [125I]CGP71872 labelling immunoprecipitated with the HA antibody in cells expressing GB1 + HA-GB2, GB1ASA + HA-GB2/1ASA, GB1/2 + HA-GB2, GB1/2 + HA-GB2/1 or GB1 + HA-mGlu5. Non-specific labelling was determined in the presence of 1 mM CGP54624A. Specific 125I-labelled material immunoprecipitated is plotted as a percentage of that obtained in cells expressing GB1 + HA-GB2. Values are means ± SEM of at least three experiments performed in triplicate.
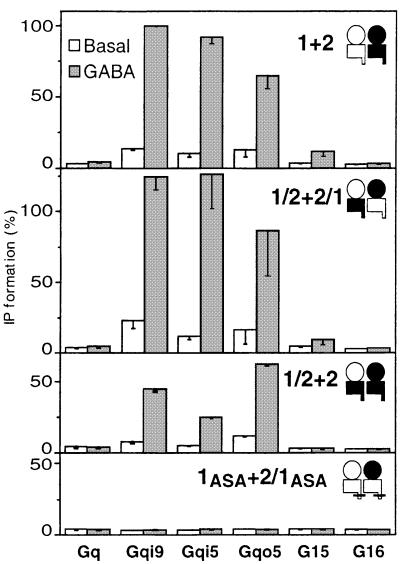
Coexpression of GB2 and GB1/2 leads to a functional GABAB receptor that possesses GB2 HD only (Figure 3A). The wild-type GABAB receptor has been reported to couple not only to Gqi9, but also to Gqi5 and Gqo5, two chimeric Gαq proteins in which the five C-terminal residues have been replaced by those of Gαi and Gαo, respectively (Franek et al., 1999). However, it does not efficiently couple to the promiscuous G-proteins G15 and G16 (Figure 4), although these two G-proteins were activated by mGlu8 receptors (data not shown), as previously described (Blahos et al., 2001). Like the wild-type and the heteromeric GB1/2 + GB2/1 receptors, the coexpression of GB2 and GB1/2 leads to a receptor that can efficiently activate Gqi9, Gqi5 and Gqo5, but not G15 and G16 (Figure 4).
Fig. 4. Coupling of various combinations of GABAB subunits to different G-proteins. Basal (empty bars) or 1 mM GABA-induced (grey bars) IP formation were measured in cells expressing the indicated combinations of subunits and the indicated G-proteins. Values are expressed as a percentage of the GABA-induced response measured in cells expressing GB1, GB2 and Gqi9. Values are the means ± SEM of three experiments performed in triplicate.
In contrast, the coexpression of the subunits GB1 and GB2/1 did not lead to a functional receptor (Figure 3A) even when the ER retention signal of both subunits was mutated (GB1ASA + GB2/1ASA). Such an absence of coupling was observed whether GABA, APPA or baclofen was used as agonist, and with all the G-proteins tested (Figure 4). This absence of coupling is not due to a low level of expression of the receptor at the cell surface. Indeed, a similar amount of specific binding of the membrane GB1-specific impermeant radioligand [125I]CGP64213 was detected on intact cells expressing GB1 + GB2 or GB1ASA + GB2/1ASA (Figure 3B). Moreover, the affinities of CGP64213 determined on cells expressing GB1 + GB2 or GB1ASA + GB2/1ASA were similar (Table I). Taken together, these data indicate that within the heterodimers, the GB2 HD is necessary to allow coupling to G-proteins, and that it possesses the molecular determinants for G-protein recognition and signalling.
GB1ASA and GB2/1ASA form a heteromeric complex unable to activate G-proteins
The absence of a GABA-induced response in cells coexpressing GB1ASA and GB2/1ASA is not due to the inability of these two subunits to form a heteromeric complex. Indeed, we found that material specifically labelled with the GB1 photo-affinity ligand [125I]CGP71872 (Kaupmann et al., 1997b, 1998; Malitschek et al., 1999) was co-immunoprecipitated with the HA-tagged GB2/1ASA in cells coexpressing this subunit and GB1ASA (Figure 3C). After separation on acrylamide gels, this immunoprecipitated 125I-labelled material appears as a single band with the expected molecular weight of the GB1ASA protein (data not shown). As a control experiment, the coexpression of GB1 and HA-tagged mGlu5 receptor did not lead to the co-immunoprecipitation of specific [125I]CGP71872 labelling with the HA antibody. Moreover, we found that the amount of [125I]CGP71872 specific labelling that could be co-immunoprecipitated in cells coexpressing GB1ASA and HA-GB2/1ASA represented ∼70% of that obtained in cells expressing GB1 + HA-GB2 or GB1/2 + HA-GB2/1 (Figure 3C). Therefore, GB1ASA and GB2/1ASA form heteromers like GB1 and GB2, and like GB1/2 and GB2/1.
Although they can interact with each other, can GB1 and GB2/1 cross-talk with each other? It has been reported that the affinity of GABAB agonists on GB1 was increased in the presence of GB2 (Kaupmann et al., 1998). Here we show that the affinity of GABA measured in intact cells expressing GB1ASA was increased 5- to 8-fold in the presence of either GB2 or GB2/1ASA (Table I). This indicates that the cross-talk between GB1ASA and GB2 can also be observed between GB1ASA and GB2/1ASA. Then, GB1ASA and GB2/1ASA cannot activate the heterologous G-proteins even though they are targeted to the cell surface, interact with and ‘talk’ to each other.
Heteromeric receptors with the HD of GB2 only are not as efficient as the wild-type receptor in coupling to Gqi9
In order to identify any possible role of the HD of GB1 in the G-protein coupling of the wild-type receptor, the functional coupling of the GB1/2 + GB2 combination was compared further with that of the wild-type combination. The maximal effects obtained with saturating concentration of agonists on cells expressing GB1/2 + GB2 were found to be about half of those obtained with cells expressing the wild-type or GB1/2 + GB2/1 combinations (Figures 2, 4 and 5). Moreover, the EC50 values of agonists were always higher than those measured in cells expressing the wild-type receptor (Figure 5). These differences are not due to a different level of GB1/2 + GB2 at the cell surface. Indeed, a similar amount of [125I]CGP64213 binding was measured in intact cells expressing either GB1 + GB2 or GB1/2 + GB2 (Figure 3B). Moreover the amount of [125I]CGP71872-labelled GB1/2 co-immunoprecipitated with GB2 represented 70% of that immunoprecipitated in cells expressing GB1 + GB2 (Figure 3C), indicating that the amount of heteromers in GB1/2 + GB2-expressing cells is not drastically different from that in GB1 + GB2-expressing cells. Finally, the GB1/2 subunit is in a high agonist affinity state in the presence of GB2 (Table I). Taken together, these data reveal that the heteromeric GB1/2 + GB2 receptor is less efficient in activating Gqi9 than the wild-type GABAB receptor, suggesting that the presence of the HD of GB1 is necessary for full coupling to G-proteins of the heterodimer.
Fig. 5. Effects of various concentrations of agonists and antagonists on IP formation in cells expressing GB1 + GB2 (open circles) or GB1/2 + GB2 (filled diamonds) together with Gqi9. Values are the percentage of the maximal GABA-induced formation of IP measured in GB1 + GB2-expressing cells. The effect of CGP64213 was tested in the presence of 0.1 µM GABA (GB1 + GB2) or 15 µM GABA (GB1/2 + GB2). Values are the means ± SEM of at least three experiments performed in triplicate.
Discussion
Among all GPCRs, the GABAB receptor is the only one known so far that requires the presence of two subunits, GB1 and GB2, for efficient coupling to G-proteins. We and others have recently shown that GB1 binds GABAB ligands, and that GB2 is necessary for GB1 insertion in the plasma membrane. The present study revealed new roles of both subunits for the formation of a fully functional receptor. We show that the HD of GB2 contains the molecular determinants for the recognition and activation of G-proteins. However, for an efficient coupling to G-proteins, the GB1 HD is necessary. In addition, we show that both GB1 and GB2 ECDs are required for GABA-induced activation of the receptor.
Functional specialization of GABAB subunits
We recently reported that the GABA binding site located within the ECD of GB1 plays a critical role in the recognition of GABAB ligands not only by the GB1 subunit expressed alone, but also by the heteromeric GABAB receptor (Galvez et al., 2000a,b). Here we showed that the HD of GB2 can activate the same G-proteins as those activated by the wild-type receptor. In contrast, such coupling could not be measured in combinations of subunits that have the HD of GB1 only, even though the subunits could reach the cell surface (since the ER retention signal was mutated). It seems likely, therefore, that the GB1 HD does not couple to G-proteins, or at least does not activate the G-proteins tested here.
In order to ascertain further the critical role played by the HD of GB2 in the heteromeric wild-type receptor in G-protein activation, it would be of interest to prevent GB2 coupling to G-proteins and determine whether the heteromer is functional or not. We therefore introduced point mutations in the i3 loop of GB2 (and also in GB1 as a control), similar to those that prevent G-protein coupling in mGlu (Francesconi and Duvoisin, 1998) and CaS (Pollak et al., 1993) receptors. However, all mutant subunits (R679W or R679D in GB2 and K791W or I798S in GB1) still form functional receptors when expressed either with the complementary wild-type subunit or in combination (B.Duthey, J.-P.Pin and L.Prézeau, unpublished data), showing that these mutations do not prevent the G-protein coupling of the GABAB receptor subunits.
Within the family 3 GPCRs, the second and third intracellular loops play a critical role in G-protein recognition (Pin et al., 1994; Gomeza et al., 1996) and activation (Pollak et al., 1993; Francesconi and Duvoisin, 1998; Chang et al., 2000), respectively. Indeed, the second intracellular loops of GB1 and GB2 share no sequence similarity, in agreement with the difference noticed here for the G-protein coupling properties of GB1 and GB2. This does not mean that the HD of GB1 does not play a role in the signalling of the heteromeric receptor. Indeed, the GB1 subunit can associate with the transcription factors ATF4 and ATFx (Nehring et al., 2000; White et al., 2000), suggesting that this subunit may be involved in other transduction cascades not necessarily involving G-proteins.
Cooperative interactions between different GABAB functional domains
Although the ECD of GB1 clearly binds GABAB ligands, and although the HD of GB2 can couple and activate G-proteins, a chimeric receptor comprising these two domains (GB1/2) did not lead to a functional receptor that can be activated by GABA when expressed alone. However, it did so when coexpressed with either GB2 or the converse chimera GB2/1. This observation highlights further the importance of cooperative interactions between different GABAB functional domains for a correct activation of the HD of GB2 by the agonist-occupied ECD of GB1. In agreement with this proposal, the heterodimer in which the two ECDs have been swapped between the two subunits (GB1/2 + GB2/1) behaves exactly like the wild-type receptor in all aspects studied here, i.e. coupling efficacy, G-protein selectivity, agonist and antagonist potencies and cross-talk between the two subunits. Indeed, our data indicate that cooperativity occurred at the level of both the ECDs and the HDs between the two subunits.
Concerning the ECD, we found that among all wild-type and chimeric GABAB subunits expressed either alone or in combination, only the combinations with both types of ECDs—i.e. one of GB1 and one of GB2—could form a functional receptor activated by GABA. This reveals that even though GB1 ECD binds GABA, GB2 ECD is required for GABA-induced activation of the receptor. This is in agreement with recent data showing that a small deletion in the GB2 ECD is sufficient to prevent activation of the heteromer (Jones et al., 2000). Moreover, it was reported that the GB2 subunit increased agonist affinity on GB1 (Kaupmann et al., 1998). Our data show here that this is observed with both GB2 and GB2/1, suggesting that the GB2 ECD is not only required for GABAB receptor activation, but also responsible for the increased agonist affinity on GB1.
Concerning the HDs, we showed that although heteromeric receptors comprising the GB2 HD only can couple to G-proteins, its coupling efficacy is lower than that measured for the wild-type receptor. In addition, the two other combinations of subunits comprising both types of HDs (GB1ASA + GB1/2 and GB2/1ASA + GB2), although they cannot be activated by GABA, display a large constitutive activity not seen with GB1ASA or GB2 expressed alone. Taken together these data show that the HD of GB1 largely increases G-protein coupling efficacy when associated with the HD of GB2.
Insights in GABAB receptor activation
As already mentioned, the ECD of the two GABAB subunits share structural similarity with those of mGlu and CaS receptors (Kaupmann et al., 1997b; Galvez et al., 1999, 2000a), and with some bacterial amino acid binding proteins (PBPs). The resolution of the structure of these PBPs (Quiocho, 1990) and that of the mGlu1 ECD (Kunishima et al., 2000) revealed that they comprise two lobes separated by a large cleft where the ligand binds. These two lobes close upon agonist binding, a change in conformation that is likely to be critical for the activation of the receptor (Bessis et al., 2000; Galvez et al., 2000a; Kunishima et al., 2000). However, the binding domain of mGlu receptor dimerizes and a more drastic change in the general conformation of the ECD dimer is observed upon binding of glutamate (Kunishima et al., 2000). Indeed, this change in conformation is such that the C-terminal tails of the two protomers, which are distant in the absence of ligand, become closer in the presence of agonist. This is observed even though a single binding domain closes. The HD region is linked to the C-terminus of each ECD; it has been proposed, therefore, that the agonist binding favours the interaction of the two HDs leading to the stabilization of their active state. This is in contrast to the previously proposed mechanism of activation of family 3 GPCRs in which a closed form of one ECD would stabilize the activated state of its associated HD (Pin et al., 1999). Accordingly, it is possible that a change in conformation of a possible heterodimeric form of the GB1 and GB2 ECDs is indeed responsible for GABAB receptor activation.
The above hypothesis easily explains our finding that both GB1 and GB2 ECDs are required for agonist activation of the receptor. Our recent proposal that GABA binds on the GB1 ECD only (Galvez et al., 2000a) is also in agreement with Kunishima’s hypothesis, since the closure of a single mGlu1 ECD is sufficient to reach the proposed active state of the dimeric ECD (Kunishima et al., 2000). Coexpression of GB1 with GB1/2, which form a dimeric receptor that cannot be activated by agonists even though both subunits bind GABA, shows that the stabilization of a closed form of the GB1 ECD is not sufficient to activate the receptor. Moreover, this suggests that the two GB1 ECDs cannot interact with each other in such a way that they activate the receptor. Interestingly, the coexpression of GB1 and GB1/2 as well as the coexpression of GB2/1 and GB2 lead to the formation of constitutively active receptors that contain either GB1 ECDs only, or GB2 ECDs only, but neither could be activated by agonists. According to the above described hypothesis for receptor activation, the constitutive activity observed may result from an incorrect association of the homodimeric ECDs, which therefore does not prevent the two HDs from activating G-proteins. Interestingly, this observation indicates that the heteromeric nature of the ECDs is required not only for the agonist activation but also to maintain the receptor in its inactive state.
In conclusion, the GABAB receptor appears to be a multidomain protein, with complex allosteric cross-talk required for the normal functioning of the receptor. Although the ECD of GB1 appears to be the main determinant for agonist recognition, the GB2 subunit increases agonist affinities. Moreover, although the HD of GB2 appears to be the main determinant for G-protein recognition and activation, the HD of GB1 increases its coupling efficacy. Finally, our data are consistent with the proposal that a general change in conformation within the ECD dimer of family 3 GPCRs is responsible for the stabilization of an active dimeric form of the HDs. Such a proposal highlights further the importance of the dimerization process in GPCR activation.
Materials and methods
Construction of chimeric receptors
The N-terminus c-myc- and HA-tagged GB1a and GB2 coding sequences described previously (Pagano et al., 2001) have been subcloned into the pRK5 expression vector. To construct the GB1/2 and GB2/1 chimeric receptors, a unique Bsu36I restriction site was introduced in the region coding for the tripeptide Pro434–Pro435–Ala436 and Pro405– Pro406–Lys407 located at the C-terminal end of the extracellular sequence of the c-myc- or HA-GB1a and GB2 subunits, respectively. The fragment between the EcoRI site preceding the start codon and the introduced Bsu36I site was swapped between the two plasmids, therefore creating the GB1/2 and GB2/1 chimera-expressing plasmids. The construction of the HA-mGlu5 receptor expression vector has been described previously (Ango et al., 1999).
Cell culture, immunofluorescence and expression in HEK 293 cells
Human embryonic kidney (HEK) 293 cells were cultured and transfected by electroporation as described previously (Franek et al., 1999). After electroporation, the cells were plated on poly-ornithine-coated dishes. Serum, culture media and other solutions used for cell culture were from Life Technologies, SARL (Cergy Pontoise, France). Immunofluorescence on intact cells using the c-myc or HA antibodies was performed as described previously (Pagano et al., 2001).
Ligand binding assay
Ligand binding experiments were performed on intact HEK 293 cells plated in 24-well plates just after electroporation (107 cells per plate). The day after, cells were equilibrated to 4°C, washed three times with ice-cold binding buffer (20 mM Tris–HCl pH 7.4, 118 mM NaCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 4.7 mM KCl and 1.8 mM CaCl2) and incubated in the presence of 0.1 nM [125I]CGP64213 with or without unlabelled ligands at the indicated concentration (200 ml final volume, for 3 h at 4°C). The incubation was terminated by washing three times with ice-cold binding buffer. The cells were then disrupted with 400 ml 0.1 M NaOH and the bound radioactivity was counted. The amount of protein in each well was measured by the Bradford method. Non-specific binding was determined in the presence of 1 mM GABA. The concentration of [125I]CGP64213 used in displacement experiments (0.1 nM) was ∼10-fold lower than the affinity of this radioligand on GB1. Ki values were calculated according to the equation IC50 = Ki (1 + [L]/Kd); Kd was assumed to be equal to Ki in the case of CGP64213. GABA was obtained from Sigma (L’Isle d’Abeau, France). [125I]CGP64213 was synthesized as described elsewhere (Kaupmann et al., 1997a) and labelled to a specific radioactivity of >2000 Ci/mmol (ANAWA AG, Wangen, Switzerland). l-Baclofen was synthesized in the research laboratories of Novartis Pharma in Basel (Froestl et al., 1995). Displacement curves were fitted as described previously (Galvez et al., 2000a).
Western blotting
Forty-eight hours after transfection, the cells were washed twice in ice-cold Tris–Krebs buffer containing proteases inhibitors (Roche Diagnostics, Meylan, France), scrapped and centrifuged to collect membranes. Samples (10 µg) were loaded on a Tricine–SDS gel for electrophoresis and transferred on nitrocellulose membranes (Amersham Pharmacia Biotech, Orsay, France). After incubation in phosphate-buffered saline (PBS)/5% milk, the membranes were incubated with the monoclonal anti-HA antibody (1/3000; Roche Diagnostics) at room temperature for 2 h. After washing, the membranes were incubated overnight at 4°C with the anti-mouse HRP antibody (Roche Diagnostics). Signal was revealed using an ECL chemioluminescent assay (Amersham Pharmacia Biotech, Orsay, France).
Photo-affinity labelling and co-immunoprecipitations
Crude membranes from HEK 293 cells expressing the different combinations of receptors were prepared and labelled with the GB1 ECD specific photo-affinity ligand [125I]CGP71872 as previously described (Kaupmann et al., 1997b). Briefly, 24 h after transfection, the cells were washed, homogenized in cold Tris–Krebs buffer (20 mM Tris–HCl pH 7.4, 118 mM NaCl, 5.6 mM glucose, 1.2 mM KH2PO4, 1.2 mM MgSO4, 4.7 mM KCl and 1.8 mM CaCl2), and centrifuged for 20 min at 40 000 g. The pellet was resuspended in Tris–Krebs buffer. Membranes (50 µg protein) were incubated in the dark for 1 h at room temperature with 0.8 nM [125I]CGP71872. Non-specific binding was determined in the presence of 1 mM CGP54626A. Binding was stopped by two washes with cold Tris–Krebs buffer before UV cross-linking. For immunoprecipitation, labelled membranes (200 mg) were resuspended in 100 ml buffer [50 mM HEPES pH 7.5, 150 mM NaCl, 1% (w/v) deoxycholate and protease inhibitors] at 4°C and then centrifuged at 100 000 g for 1 h. After a 5-fold dilution in protein buffer [20 mM HEPES pH 7.5, 150 mM NaCl, 0.1% Triton X-100, protease inhibitors (Roche Diagnostics, Meylan, France)], the supernatants were incubated overnight at 4°C with anti-HA monoclonal antibody (clone 12CA5, 1/250; Roche Diagnostics) and protein A–Sepharose. The pellets were washed five times with cold protein buffer 0.1% Triton X-100. The radioactivity associated with the pellet was counted and the labelled immunoprecipitated receptors detected by SDS–PAGE and autoradiography.
Determination of inositol phosphate (IP) accumulation
HEK 293 cells were transfected as described above with the receptor constructs in pRK5 (2 mg), Gqi9 expression vector (2 mg) and carrier DNA (pRK6, 4 mg) (Franek et al., 1999). Determination of IP accumulation was performed 15 h after transfection as described elsewhere (Franek et al., 1999).
Acknowledgments
Acknowledgements
The authors wish to thank Drs J.Bockaert, F.Carroll, T.Durroux, B.Mouillac and M.L.Parmentier (CCIPE, Montpellier, France) for constructive discussion and critical reading of the manuscript. The authors also wish to thank Dr Wolfgang Froestl (Novartis, Basel, Switzerland) for the supply of GABAB ligands. This work was supported by grants from the CNRS, the Action Incitative Physique et Chimie du Vivant (PCV00-134) from the CNRS (J.-P.P.), the program Molécules et Cibles Thérapeutiques from INSERM and CNRS (J.-P.P.) and Novartis Pharma (Basel, Switzerland).
References
- Ango F., Albani-Torregrossa,S., Joly,C., Robbe,D., Michel,J.-M., Pin,J.-P., Bockaert,J. and Fagni,L. (1999) A simple method to transfer plasmid DNA into neuronal primary cultures: func tional expression of mGluR5 in cerebellar granule cells. Neuropharmacology, 38, 793–803. [DOI] [PubMed] [Google Scholar]
- Bai M., Trivedi,S., Kifor,O., Quinn,S.J. and Brown,E.M. (1999) Intermolecular interactions between dimeric calcium-sensing receptor monomers are important for its normal function. Proc. Natl Acad. Sci. USA, 96, 2834–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis A.-S., Bertrand,H.-O., Galvez,T., De Colle,C., Pin,J.-P. and Acher,F. (2000) 3D-model of the extracellular domain of the type 4a metabotropic glutamate receptor: new insights into the activation process. Protein Sci., 9, 2200–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blahos J., Fischer,T., Brabet,I., Stauffer,D., Rovelli,G., Bockaert,J. and Pin,J.-P. (2001) A novel site on the Gα protein that recognizes heptahelical receptors. J. Biol. Chem., 276, 3262–3269. [DOI] [PubMed] [Google Scholar]
- Bockaert J. and Pin,J.-P. (1999) Molecular tinkering of G-protein coupled receptors: an evolutionary success. EMBO J., 18, 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H.R. (1997) How receptors talk to trimeric G proteins. Curr. Opin. Cell Biol., 9, 134–142. [DOI] [PubMed] [Google Scholar]
- Calver A.R. et al. (2001) The C-terminal domains of the GABAB receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J. Neurosci., 21, 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W., Chen,T.H., Pratt,S. and Shoback,D. (2000) Amino acids in the second and third intracellular loops of the parathyroid Ca2+-sensing receptor mediate efficient coupling to phospholipase C. J. Biol. Chem., 275, 19955–19963. [DOI] [PubMed] [Google Scholar]
- Conklin B.R., Farfel,Z., Lustig,K.D., Julius,D. and Bourne,H.R. (1993) Substitution of three amino acids switches receptor specificity of Gqα to that of Giα. Nature, 363, 274–276. [DOI] [PubMed] [Google Scholar]
- Couve A., Filippov,A.K., Connolly,C.N., Bettler,B., Brown,D.A. and Moss,S.J. (1998) Intracellular retention of recombinant GABAB receptors. J. Biol. Chem., 273, 26361–26367. [DOI] [PubMed] [Google Scholar]
- Francesconi A. and Duvoisin,R.M. (1998) Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J. Biol. Chem., 273, 5615–5624. [DOI] [PubMed] [Google Scholar]
- Franek M., Pagano,A., Kaupmann,K., Bettler,B., Pin,J.-P. and Blahos,J.,II (1999) The heteromeric GABA-B receptor recognizes G-protein α subunit C-termini. Neuropharmacology, 38, 1657–1666. [DOI] [PubMed] [Google Scholar]
- Froestl W., Mickel,S.J., Hall,R.G. et al. (1995) Phosphinic acid analogues of GABA. I. New potent and selective GABA-B agonists. J. Med. Chem., 38, 3297–3312. [DOI] [PubMed] [Google Scholar]
- Galvez T. et al. (1999) Mutagenesis and modeling of the GABA-B receptor binding site suggest a Venus fly-trap mechanism for ligand binding. J. Biol. Chem., 274, 13362–13369. [DOI] [PubMed] [Google Scholar]
- Galvez T. et al. (2000a) Mapping the agonist binding site of GABAB type 1 subunit sheds light on the activation process of GABAB receptors. J. Biol. Chem., 275, 41166–41174. [DOI] [PubMed] [Google Scholar]
- Galvez T. et al. (2000b) Ca2+-requirement for high affinity GABA binding at GABAB receptors: involvement of serine 269 of the GABABR1 subunit. Mol. Pharmacol., 57, 419–426. [DOI] [PubMed] [Google Scholar]
- Gomeza J., Joly,C., Kuhn,R., Knöpfel,T., Bockaert,J. and Pin,J.-P. (1996) The second intracellular loop of mGluR1 cooperates with the other intracellular domains to control coupling to G-protein. J. Biol. Chem., 271, 2199–2205. [DOI] [PubMed] [Google Scholar]
- Jones K.A. et al. (1998) GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B) R1 and GABA(B) R2. Nature, 396, 674–679. [DOI] [PubMed] [Google Scholar]
- Jones K.A., Tamm,J.A., Craig,D.A., Ph,D., Yao,W. and Panico,R. (2000) Signal transduction by GABA(B) receptor heterodimers. Neuropsychopharmacology, 23, S41–S49. [DOI] [PubMed] [Google Scholar]
- Kaupmann K., Bettler,B., Bettiger,H., Froestl,W. and Mickel,S. (1997a) Metabotropic GABAB receptors, receptor-specific ligands and their uses. PCT Patent, WO 97/46675 A1, 11 December, 1997.
- Kaupmann K. et al. (1997b) Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature, 386, 239–246. [DOI] [PubMed] [Google Scholar]
- Kaupmann K. et al. (1998) GABA B-receptor subtypes assemble into functional heteromeric complexes. Nature, 396, 683–687. [DOI] [PubMed] [Google Scholar]
- Kolakowski L.F. (1994) GCRDb: a G-protein-coupled receptor database. Receptors Channels, 2, 1–7. [PubMed] [Google Scholar]
- Kuner R., Kohr,G., Grunewald,S., Eisenhardt,G., Bach,A. and Kornau,H.C. (1999) Role of heteromer formation in GABAB receptor function. Science, 283, 74–77. [DOI] [PubMed] [Google Scholar]
- Kunishima N., Shimada,Y., Tsuji,Y., Sato,T., Yamamoto,M., Kumasaka,T., Nakanishi,S., Jingami,H. and Morikawa,K. (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature, 407, 971–977. [DOI] [PubMed] [Google Scholar]
- Malitschek B. et al. (1999) The N-terminal domain of GABAB receptors is sufficient to specify agonist and antagonist binding. Mol. Pharmacol., 56, 448–454. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M., Jan,Y.N. and Jan,L.Y. (2000) A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron, 27, 97–106. [DOI] [PubMed] [Google Scholar]
- Nehring R.B., Horikawa,H.P.M., El Far,O., Kneussel,M., Brandstätter,J.H., Stamm,S., Wischmeyer,E., Betz,H. and Karschin,A. (2000) The metabotropic GABAB receptor directly interacts with the activating transcription factor 4. J. Biol. Chem., 275, 35185–35191. [DOI] [PubMed] [Google Scholar]
- O’Hara P.J. et al. (1993) The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron, 11, 41–52. [DOI] [PubMed] [Google Scholar]
- Pagano A. et al. (2001) Coiled-coil heteromerization masks a GABAB receptor ER retention signal and facilitates surface expression. J. Neurosci., 21, 1189–1202.11160389 [Google Scholar]
- Pin J.-P., Joly,C., Heinemann,S.F. and Bockaert,J. (1994) Domains involved in the specificity of G protein activation in phospholipase C coupled metabotropic glutamate receptor. EMBO J., 13, 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin J.-P., De Colle,C., Bessis,A.-S. and Acher,F. (1999) New perspectives for the development of selective metabotropic glutamate receptor ligands. Eur. J. Pharmacol., 375, 277–294. [DOI] [PubMed] [Google Scholar]
- Pollak M.R., Brown,E.M., Chou,Y.-H.W., Hebert,S.C., Marx,S.J., Steinmann,B., Levi,T., Seidman,C.E. and Seidman,J.G. (1993) Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyper parathyroidism. Cell, 75, 1297–1303. [DOI] [PubMed] [Google Scholar]
- Quiocho F.A. (1990) Atomic structures of periplasmic binding proteins and the high-affinity active transport systems in bacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci., 326, 341–351. [DOI] [PubMed] [Google Scholar]
- Romano C., Yang,W.-L. and O’Malley,K.L. (1996) Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J. Biol. Chem., 271, 28612–28616. [DOI] [PubMed] [Google Scholar]
- Salahpour A., Angers,S. and Bouvier,M. (2000) Functional significance of oligomerization of G-protein-coupled receptors. Trends Endocrinol. Metab., 11, 163–168. [DOI] [PubMed] [Google Scholar]
- White J.H. et al. (1998) Heterodimerisation is required for the formation of a functional GABAB receptor. Nature, 396, 679–682. [DOI] [PubMed] [Google Scholar]
- White J.H., McIllhinney,R.A., Wise,A., Ciruela,F., Chan,W.Y., Emson,P.C., Billinton,A. and Marshall,F.H. (2000) The GABAB receptor interacts directly with the related transcription factors CREB2 and ATFx. Proc. Natl Acad. Sci. USA, 97, 13967–13972. [DOI] [PMC free article] [PubMed] [Google Scholar]