Abstract
Platelet adhesion on and activation by components of the extracellular matrix are crucial to arrest post-traumatic bleeding, but can also harm tissue by occluding diseased vessels. Integrin α2β1 is thought to be essential for platelet adhesion to subendothelial collagens, facilitating subsequent interactions with the activating platelet collagen receptor, glycoprotein VI (GPVI). Here we show that Cre/loxP-mediated loss of β1 integrin on platelets has no significant effect on the bleeding time in mice. Aggregation of β1-null platelets to native fibrillar collagen is delayed, but not reduced, whereas aggregation to enzymatically digested soluble collagen is abolished. Furthermore, β1-null platelets adhere to fibrillar, but not soluble collagen under static as well as low (150 s–1) and high (1000 s–1) shear flow conditions, probably through binding of αIIbβ3 to von Willebrand factor. On the other hand, we show that platelets lacking GPVI can not activate integrins and consequently fail to adhere to and aggregate on fibrillar as well as soluble collagen. These data show that GPVI plays the central role in platelet–collagen interactions by activating different adhesive receptors, including α2β1 integrin, which strengthens adhesion without being essential.
Keywords: collagen/Cre/loxP/GPVI/α2β1 integrin/platelets
Introduction
Damage to the integrity of the vessel wall results in exposure of the subendothelial extracellular matrix (ECM), which triggers adhesion and aggregation of platelets (Weiss, 1975). The consequence of this process is the formation of a thrombus, which prevents blood loss at sites of injury or leads to occlusion and irreversible tissue damage or infarction in diseased vessels. Integrins play a central role in adhesion and aggregation of platelets (Phillips et al., 1991; Shattil et al., 1998). Integrins are heterodimeric transmembrane receptors composed of an α and a β subunit. Resting platelets express their integrins in a low affinity state. After activation, mediated by other platelet receptors, integrins shift to a high affinity state and bind their ligands efficiently (Phillips et al., 1991; Shattil et al., 1998).
The ECM of the vessel walls is rich in collagens, which play essential roles in thrombus formation by providing a substrate for platelet adhesion and by activating platelets (Baumgartner, 1977). Besides GPIb-V-IX and αIIbβ3 integrin, which interact indirectly with collagen via von Willebrand factor (vWF) (Savage et al., 1998), a large number of collagen receptors have been identified on platelets, including most importantly α2β1 integrin (Santoro, 1986) and glycoprotein VI (GPVI) (Moroi et al., 1989). A current model of platelet adhesion to collagen suggests that the GPIb–vWF interaction mediates initial tethering of platelets at high shear, followed by α2β1 integrin-mediated firm adhesion, which halts platelet translocation and allows collagen interactions with GPVI, finally resulting in platelet activation and thrombus growth (Sixma et al., 1997; Barnes et al., 1998; Santoro, 1999).
The first indication that α2β1 integrin may be an essential platelet collagen receptor came from patients with excessive post-traumatic bleeding and menorrhagia. The platelets of these patients expressed markedly reduced levels of α2 integrin and displayed profound defects in collagen-induced adhesion/aggregation (Nieuwenhuis et al., 1985; Kehrel et al., 1988). Subsequent in vitro studies, however, gave rise to conflicting data on the role of α2β1 in platelet–collagen interactions. Some studies showed that inhibition of α2β1 markedly reduced or abolished adhesion and aggregate formation in stasis and flow (Saelman et al., 1994; Kamiguti et al., 1997; Verkleij et al., 1998), while others reported only minor effects of such treatment on adhesion to collagen (Morton et al., 1994; Savage et al., 1999; Siljander and Lassila, 1999) and collagen-induced aggregation (Coller et al., 1989; Hers et al., 2000), suggesting the involvement of other receptors. These conflicting results may at least partly be explained by the use of different collagen preparations. In vivo, collagen molecules are secreted by cells, processed and finally assembled into long, insoluble fibrils. However, collagens can be solubilized, e.g. by pepsin treatment, and such soluble collagen preparations are frequently used for in vitro analysis of platelets. Experimental evidence suggests that the interaction of platelets with soluble collagen is exclusively mediated by α2β1 integrin (Nakamura et al., 1998).
Another collagen receptor on platelets is GPVI. GPVI belongs to the immunoglobulin superfamily (Clemetson et al., 1999; Jandrot-Perrus et al., 2000) and is non-covalently associated with the FcRγ chain, which serves as the signal-transducing part of the receptor in human as well as mouse platelets (Gibbins et al., 1997; Tsuji et al., 1997; Nieswandt et al., 2000a). GPVI-deficient patients suffer from a mild bleeding diathesis and their platelets respond poorly to collagen (Moroi et al., 1989; Arai et al., 1995) or the collagen-related peptide (CRP) (Kehrel et al., 1998), which contains a glycine–proline–hydroxyproline repeat motif and mimics many of the signaling actions of collagen (Asselin et al., 1997). Platelets from FcRγ chain-deficient mice (Takai et al., 1994) lack GPVI (Nieswandt et al., 2000a) and fail to respond to collagen or CRP (Poole et al., 1997). We recently generated the monoclonal antibody (mAb) JAQ1 (Nieswandt et al., 2000a) and showed that it blocks the CRP binding site on mouse GPVI, and inhibits platelet aggregation induced by low, but not high, concentrations of collagen, suggesting that collagen can activate platelets by a second mechanism (Schulte et al., 2001). In vivo application of JAQ1 induces virtually complete internalization and degradation of GPVI on mouse platelets. Such GPVI-depleted mice have significantly prolonged bleeding times and their platelets fail to aggregate in response to low and high concentrations of collagen, suggesting that GPVI is essential for both activation pathways induced by collagen (Nieswandt et al., 2001).
To study the role of α2β1 and GPVI in platelet adhesion to collagen and to understand how α2β1 becomes activated in vivo, we generated mice with β1-null platelets using the cre/loxP system. We show that mice with β1-null platelets display no increased bleeding tendency. Furthermore, we demonstrate that α2β1 is dispensable for platelet adhesion, activation, and thrombus growth on fibrillar collagen under static and flow conditions, whereas these processes are abolished in the absence of GPVI.
Results
Mice with β1-null platelets have normal platelet counts and bleeding time
To generate mice lacking β1 integrin expression on platelets, mice carrying a β1 integrin gene flanked by loxP sites (Potocnik et al., 2000) were crossed with mice expressing the cre recombinase under the control of the inducible Mx promoter (Kühn et al., 1995). Deletion of the β1 gene from the mice carrying Mx-cre was induced in 4- or 5-week-old animals by three intraperitoneal (i.p.) injections of polyinosinic–polycytidylic acid (pI–pC) at 2-day intervals. Mx-cre-mediated deletion of the floxed β1 gene in various tissues 1 week after the first pI–pC injection was quantified by Southern blot analysis of genomic DNA by comparing the band intensities between the floxed and null alleles. Mx-cre-mediated deletion was almost complete in liver and bone marrow, and ∼70% complete in spleen, 40% in kidney, 40% in heart, and very little in tail and brain (data not shown).
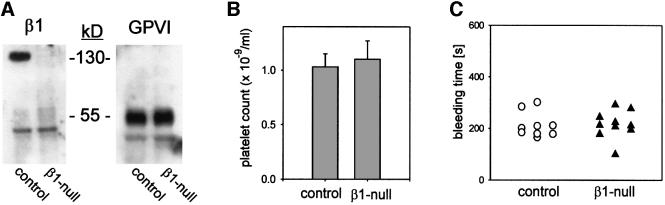
By flow cytometry, the level of β1 integrin expression was undetectable on circulating platelets 2 weeks after the last pI–pC injection in all mice used in this study. Expression of the α2, α5 and α6 subunits was also lost, while β3 as well as other major platelet receptors, including GPVI, CD9 and all subunits of the GPIb-V-IX complex, were unchanged (Table I). Western analyses of platelet lysates confirmed the loss of the β1 integrin and normal expression of GPVI (Figure 1A). Integrins α1, α4, α7, β4 and β7 were undetectable on normal or β1-null platelets (not shown).
Table I. Surface expression of different glycoproteins on control and β1-null platelets.
| Control | β1-null | |
|---|---|---|
| GPIIa (β1] | 128.3 ± 13.2 | 7.3 ± 2.1 |
| GPIa (α2) | 39.1 ± 9.5 | 8.4 ± 3.3 |
| GPIc (α5) | 27.2 ± 5.1 | 6.6 ± 1.4 |
| GPIc′ (α6) | 87.3 ± 10.2 | 5.9 ± 1.7 |
| GPVI | 43.5 ± 10.7 | 45.2 ± 12.9 |
| GPIIb/IIIa | 347.8 ± 17.5 | 359.5 ± 21.4 |
| GPIIIa | 169.7 ± 10.2 | 176.1 ± 16.2 |
| GPIb-IX | 295.8 ± 21.2 | 287.7 ± 26.1 |
| GPV | 143.4 ± 15.9 | 149.5 ± 14.3 |
| CD9 | 545.9 ± 41.1 | 537.7 ± 51.8 |
The surface expression of the indicated glycoproteins was detected by flow cytometry on control and β1-null platelets. Platelets were gated by forward scatter/side scatter (FSC/SSC) characteristics. Results are expressed as mean log fluorescence ± SD for six mice per group.
Fig. 1. Characterization of mice with β1-null platelets. (A) Western blot analysis of β1 integrin and GPVI in control and β1-null platelets. (B) Platelet counts are expressed as the mean count ± SD for groups of six mice. (C) Tail bleeding times were determined in groups of nine control and 10 β1-null mice. Each point represents one individual.
Integrins play important roles in blood cell differentiation and proliferation (Fässler et al., 1996). To test platelet production in mutant mice, blood was isolated and platelets were counted. No significant differences were found between control and mutant mice (Figure 1B). Bleeding time was determined next, since several reports showed increased bleeding in patients with reduced expression of α2β1 integrin on platelets (Nieuwenhuis et al., 1985; Kehrel et al., 1988). To restrict the Mx-cre-induced deletion of the β1 integrin gene to the hematopoietic system, bone marrow derived from mice carrying floxed β1 gene and the Mx-cre transgene was transferred into irradiated normal recipient mice. Four weeks after transfer, the β1 integrin gene was deleted by pI–pC injections as described above and the absence of the protein on circulating platelets was confirmed by flow cytometry (data not shown). Surprisingly, no significant differences were found in the bleeding time between control and β1-null bone marrow chimeric mice (Figure 1C). These results show that β1-null platelets develop normally and display no major defects in vivo.
Collagen-induced aggregation of β1-null platelets is delayed, but not reduced
α2β1 integrin was reported to play a major role during collagen-induced platelet aggregation in vitro (Nieuwenhuis et al., 1985; Kehrel et al., 1988). To test this directly, we induced aggregation of normal and β1-null platelets using fibrillar type I collagen. While no difference was observed in the dose–response characteristics and in the maximum aggregation of control and mutant platelets, β1-null platelets displayed a significant delay in the onset of aggregation that became more evident when lower concentrations of collagen were used (Figure 2A and B). This finding was due to the lack of α2β1 but not other β1 integrins, since a blocking mAb against the α2 integrin subunit revealed a similar delayed onset of platelet aggregation (not shown).
Fig. 2. Aggregation of β1-null platelets in response to fibrillar collagen. (A) Heparinized platelet-rich plasma (prp) from control and β1-null mice was stimulated with the indicated concentrations of fibrillar collagen, and light transmission was recorded in a standard aggregometer. (B) The delay in platelet aggregation is expressed as time (s) between addition of collagen and maximum shape change. Results are given as mean ± SD (n = 6). (C) Washed platelets were stimulated with fibrillar collagen (5 µg/ml) and samples were lysed at the indicated time points. Protein tyrosine phosphorylation was detected by immunoblotting.
Collagen-induced platelet aggregation leads to tyrosine phosphorylation of several intracellular signaling molecules (Watson and Gibbins, 1998). To determine the phosphorylation pattern, lysates from normal and β1-null platelets were analyzed by western blotting. Although the phosphorylation pattern was comparable between normal and β1-null platelets, the onset of phosphorylation was significantly delayed in the absence of β1 integrin (Figure 2C).
In addition to collagen, agonists such as CRP, snake venom toxins, thrombin and ADP can induce platelet aggregation. Therefore, these agonists were used to test whether the loss of β1 integrin was associated with a general delay or other defects in platelet aggregation. The GPVI-specific agonists CRP and convulxin, as well as thrombin and ADP, induced aggregation of normal and β1-null platelets to the same extent (Figure 3) and with the same kinetics (not shown).
Fig. 3. Normal aggregation of β1-null platelets in response to various agonists. Platelets from control (filled circles) and β1-null (open circles) mice were stimulated with different concentrations of ADP (A), thrombin (B), CRP (C) and convulxin (D). Results are given as percent aggregation and are expressed as mean ± SD for groups of six mice.
Altogether, these data indicate that the signaling machinery leading to platelet activation is not altered in β1-null platelets, that GPVI-mediated platelet activation is not affected by the absence of β1 integrins and that α2β1 integrin facilitates fibrillar collagen-induced platelet activation, but is not essential.
GPVI mediates platelet activation by two different pathways, only one of which involves α2β1
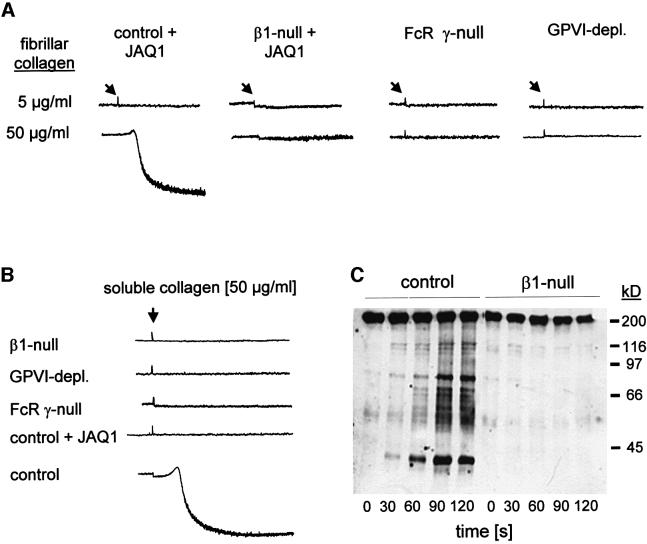
We recently reported the generation of the mAb JAQ1 against mouse GPVI (Nieswandt et al., 2000a). JAQ1 can completely block platelet aggregation in response to low concentrations of CRP and collagen. The inhibitory effect of JAQ1 can be overcome with high doses (greater than ∼7 µg/ml) of collagen, but not CRP. These findings suggested that collagen can activate platelets by a mechanism(s) that is independent of the CRP binding site on GPVI (Schulte et al., 2001). To test whether α2β1 integrin is involved in this activation process, normal and β1-null platelets were incubated with 20 µg/ml JAQ1 Fab fragments and subsequently stimulated with low (5 µg/ml) or high (50 µg/ml) concentrations of fibrillar collagen. While low concentrations of collagen failed to induce aggregation of normal or β1-null platelets in the presence of JAQ1, high collagen concentrations induced aggregation of normal but not β1-null platelets (Figure 4A), suggesting that an α2β1-dependent pathway mediates this activation. The presence of such a pathway was confirmed by the finding that aggregation was also abolished when normal platelets were pre-incubated with JAQ1 and a blocking antibody against α2 integrin (not shown). On the other hand, GPVI-depleted and FcRγ chain-deficient platelets failed to aggregate in response to high concentrations of fibrillar collagen, confirming earlier reports (Poole et al., 1997; Nieswandt et al., 2001) and demonstrating that GPVI is strictly required for the α2β1-dependent pathway.
Fig. 4. GPVI mediates two activation pathways, one of which involves α2β1. (A) Heparinized prp from control and β1-null mice was pre-incubated with 20 µg/ml JAQ1 Fab fragments and then stimulated with fibrillar collagen (5 or 50 µg/ml). As a control, prp from GPVI-depleted and FcRγ chain-deficient mice was stimulated with collagen in the absence of JAQ1. (B) Prp from the indicated mice was stimulated with soluble collagen (50 µg/ml). Where indicated, the prp was pre-treated with JAQ1 Fab fragments (20 µg/ml) for 5 min. (C) Washed platelets from control and β1-null mice were stimulated with soluble collagen (50 µg/ml) and samples were lysed at different time points. Protein tyrosine phosphorylation was detected by immunoblotting.
Cells secrete monomeric procollagen, which is converted into collagen by the proteolytic removal of the N- and C-propeptides and assembled into cross-striated fibrils that occur in the extracellular matrix of connective tissue (Kadler et al., 1996). Soluble collagen is normally not found in tissues, but is frequently used for in vitro analysis of platelets (Morton et al., 1994; Savage et al., 1999; Siljander and Lassila, 1999). It has been reported that platelet activation by soluble collagen is greatly dependent on α2β1 integrin (Siljander and Lassila, 1999). Indeed, soluble collagen induced irreversible aggregation of normal, but not of β1-null platelets (Figure 4B). The aggregation response, however, was also abolished with normal platelets pre-treated with JAQ1 as well as with GPVI-depleted and the FcRγ chain-deficient platelets, demonstrating a critical role of GPVI in this activation process. These results were further confirmed by tyrosine phosphorylation studies showing that phosphorylation induced by soluble collagen I was abolished in β1-null (Figure 4C) as well as GPVI-depleted and FcRγ chain-deficient platelets (not shown).
In summary, these data show that GPVI is the central collagen receptor for platelet activation, which only requires cooperation with α2β1 when signaling through the CRP binding site is insufficient or blocked.
Adhesion to fibrillar collagen is GPVI-, but not α2β1-dependent
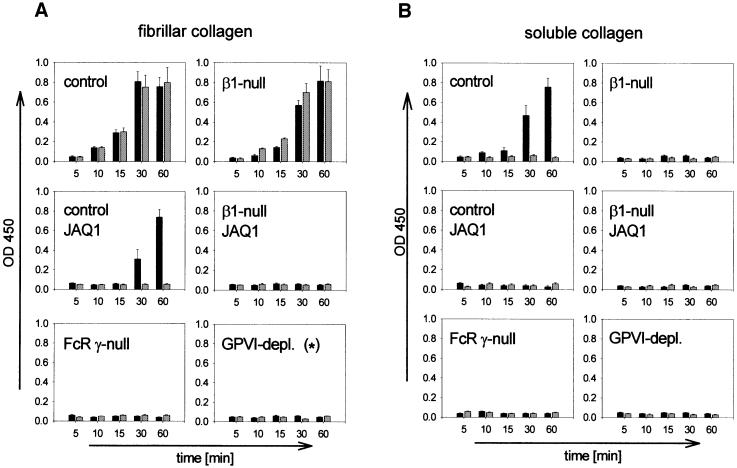
A current model suggests that, in vivo, platelets adhere firmly to collagen through α2β1 integrin, which allows subsequent collagen–GPVI interactions leading to platelet activation (Sixma et al., 1997; Barnes et al., 1998; Santoro, 1999). To test this model, normal and β1-null platelets were isolated and allowed to adhere to fibrillar collagen under static conditions. Integrin α2β1 binding depends on the presence of Mg2+/Ca2+ (Onley et al., 2000) and therefore the adhesion assays were performed in the presence or absence of these divalent cations. Both normal and β1-null platelets adhered with and without Mg2+/Ca2+ to fibrillar collagen in a time-dependent manner (Figure 5A). When the CRP binding site on GPVI was blocked with JAQ1 Fab fragments (20 µg/ml), normal platelets adhered to fibrillar collagen in the presence, but not in the absence of Mg2+/Ca2+, suggesting that the attachment is mediated by α2β1 integrin. This was confirmed by the abrogated adhesion of β1-null platelets in the presence of JAQ1. We have recently demonstrated that GPVI-depleted platelets display markedly delayed and reduced, but not abolished, adhesion to fibrillar collagen under static conditions, which is mediated by α2β1 integrin (Nieswandt et al., 2001). This low level of adhesion, however, is based on residual GPVI activity as it is abrogated in the presence of JAQ1 (Figure 5A). The strict requirement for GPVI was confirmed by the finding that FcRγ chain-deficient platelets were unable to adhere to fibrillar collagen in the absence and presence of Mg2+/Ca2+.
Fig. 5. Platelet adhesion to fibrillar and soluble collagen under static conditions. Washed platelets from the indicated mice were allowed to adhere under static conditions to fibrillar (A) or soluble (B) collagen immobilized in microtiter plates. The experiments were performed in the presence (black bars) or absence (gray bars) of Mg2+/Ca2+ (1 mM each). Where indicated, platelets were pre-incubated with JAQ1 Fab fragments (20 µg/ml). Adherent platelets were quantitated fluorimetrically. The data shown are from a single experiment, representative of four identical experiments and expressed as the mean of triplicate readings ± SD for the indicated times. (*) To exclude any residual GPVI activity, the platelets were used in the presence of (10 µg/ml) JAQ1.
To test whether this defect was based on impaired integrin activation, adhesion was analyzed in the presence of Mn2+ cations, which are known to activate β1 and β3 integrins directly (Humphries, 2000). As shown in Figure 6A, FcRγ chain-deficient platelets strongly adhered to fibrillar collagen in the presence of Mn2+ and comparable results were obtained with GPVI-depleted platelets (not shown). This adhesion was nearly abolished when α2 integrin was blocked with an antibody, whereas blockage of αIIbβ3 had no significant effect. A different picture was found when the collagen substrate had previously been exposed to vWF. Under these conditions, blockage of either α2β1 or αIIbβ3 only had minor effects, whereas concurrent blockage of both receptors abolished adhesion, suggesting that αIIbβ3 mediated adhesion via vWF. When the collagen substrate had been pre-exposed to plasma, even the blockage of both α2β1 and αIIbβ3 only had partial inhibitory effects, indicating that additional platelet receptors may interact with adhesive plasma proteins immobilized on collagen under these experimental conditions.
Fig. 6. GPVI-mediated integrin activation is a prerequisite for platelet adhesion to collagen. (A) Washed FcRγ chain-deficient platelets were allowed to adhere to fibrillar collagen immobilized in microtiter plates in the presence of Mn2+ with or without addition of blocking antibodies against α2β1 or αIIbβ3. Where indicated, the collagen substrate had been pre-exposed to vWF (20 µg/ml) or plasma for 30 min. Adherent platelets were quantitated fluorimetrically. The data shown are from a single experiment, representative of three identical experiments, and are expressed as the mean of triplicate readings ± SD for the indicated times. (B) Flow cytometric analysis of collagen-induced integrin activation on control, FcRγ chain-deficient or GPVI-depleted platelets. Diluted prp was stimulated with 30 µg/ml fibrillar collagen for 5 min at room temperature. Activated β1 integrin was detected directly with 9EG7–FITC, and activated αIIbβ3 indirectly by quantitation of surface-bound fibrinogen. Platelets were gated by FSC/SSC characteristics and Fl2 positivity (anti-CD9PE). The results shown are representative of six individual experiments. Note that only a subpopulation of the control platelets is activated. This is based on the fact that fibrillar collagen is insoluble and, therefore, not all platelets come in contact with this agonist. The percentage of activated control platelets varied between 10.3 and 19.6 in these experiments.
These data suggested that the GPVI–collagen interaction is essential for the activation of integrins, which then mediate firm adhesion directly or indirectly via adhesive ligands. To test this hypothesis further, we incubated normal, GPVI-depleted and FcRγ chain- deficient platelets with fibrillar collagen and determined activation of β1 integrin with the fluorescein isothiocyanate (FITC)-conjugated mAb 9EG7, which specifically recognizes the activated form of the β1 subunit (Lenter et al., 1993). Indeed, activated β1 integrin was detectable on (a subpopulation of) normal, but not GPVI-depleted or FcRγ chain-deficient platelets. In addition to β1 integrin, fibrillar collagen also activated αIIbβ3, as determined by measuring the amount of surface-bound fibrinogen (Figure 6B).
Adhesion assays were also performed on immobilized soluble collagen. This process has been reported to be entirely α2β1 dependent (Morton et al., 1994). Normal platelets adhered to soluble collagen in the presence, but not in the absence of Mg2+/Ca2+, suggesting that the attachment is mediated by α2β1 integrin (Figure 5B). This was confirmed by the abolished adhesion of β1-null platelets. However, normal platelets pre-treated with JAQ1, as well as GPVI-depleted and FcRγ chain-deficient platelets, also failed to adhere to soluble collagen, demonstrating that this interaction depends on both α2β1 integrin and GPVI (Figure 5B).
Adhesion to fibrillar collagen at low and high shear is GPVI-, but not α2β1-dependent
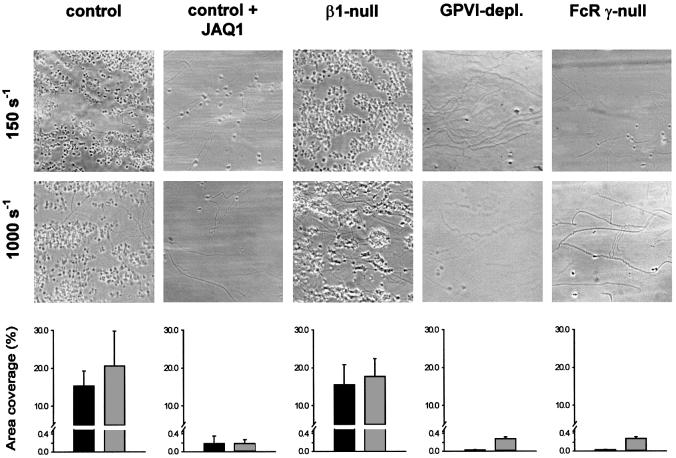
To define further the role of the two collagen receptors under more physiological conditions, we used whole blood to study platelet adhesion to collagen under conditions of low and high shear stress (150 and 1000 s–1, respectively). At low shear, normal and β1-null platelets formed large aggregates on fibrillar collagen within 5 min, indicating that integrin α2β1 is not essential for adhesion under these conditions. Blocking the CRP binding site on GPVI by addition of 20 µg/ml JAQ1 Fab fragments drastically reduced the adhesion of normal platelets and completely blocked the formation of aggregates (Figure 7). β1-null platelets did not adhere in the presence of JAQ1 (not shown). These data demonstrate that adhesion and subsequent thrombus formation are predominantly dependent on the CRP binding epitope on GPVI, and to a very minor part facilitated by α2β1. Also, this minor α2β1-dependent adhesion required GPVI, since adhesion of GPVI-depleted and FcRγ chain-deficient platelets was abolished (Figure 7).
Fig. 7. GPVI, but not α2β1, is essential for platelet adhesion to collagen under flow. Whole blood from the indicated mice was loaded with calcein and perfused at wall shear rates of 150 s–1 (10 min) or 1000 s–1 (4 min) over a collagen-coated surface. Blood from control mice was assessed in the absence or presence of 20 µg/ml JAQ1 Fab fragments. Upper panels: representative phase-contrast microscope images after perfusion. Lower panels: surface area coverage of calcein fluorescence at the end of the perfusion period (mean ± SEM, n = 3–6). Similar results were obtained when analyzing surface area coverage of platelets from phase-contrast images.
At high shear stress (1000 s–1), platelet adhesion greatly depends on interactions between GPIbα and vWF (Savage et al., 1998). Under these conditions, adhesion and thrombus formation occurred significantly faster than under low shear conditions (<2 min), and no significant difference was found between normal and β1-null platelets. In contrast, pre-incubation of platelets with JAQ1 Fab fragments almost completely abrogated adhesion and formation of aggregates. A comparable picture was found with GPVI-depleted and FcRγ chain-deficient platelets, confirming the critical role of GPVI in the adhesion process at high shear.
Adhesion under low and high shear flow conditions was also tested on soluble collagen. In agreement with the results obtained in the static assay, normal platelets adhered under both conditions in the absence but not in the presence of JAQ1 Fab fragments, whereas no adhesion occurred with β1-null, FcRγ-null or GPVI-depleted platelets, confirming the critical role of both α2β1 and GPVI for adhesion to soluble collagen under flow conditions (not shown).
Discussion
The collagen receptor α2β1 integrin on platelets was considered to play a pivotal role in hemostasis. In the present study, we established mice that lack β1 integrins on platelets and found, very unexpectedly, that they have no increased bleeding tendency. Furthermore, β1-null platelets can be efficiently activated with collagen in vitro, although the onset of activation is delayed. A similar delay has been observed using α2β1 function-blocking antibodies (Coller et al., 1989; Hers et al., 2000). β1-null platelets can adhere to fibrillar collagen under static as well as low and high shear flow conditions. These findings are in sharp contrast to observations made in patients with low or no expression of α2β1 on platelets. They suffered from severe bleedings and their platelets failed to respond to collagen in vitro (Nieuwenhuis et al., 1985; Kehrel et al., 1988). Possible explanations for the discrepancy could be species-specific differences or, more likely, that the few patients reported so far had additional defects on their platelets. Mice with β1-deficient platelets clearly demonstrate that α2β1 integrin is dispensable for hemostasis under normal conditions in vivo. It is possible, however, that α2β1-mediated adhesion of platelets to collagen is crucial if additional components such as vWF or fibronectin are absent.
We found, however, that α2β1 integrin was essential for adhesion to soluble collagen under static and flow conditions (Figure 5 and data not shown). These results confirm the role of α2β1 as a collagen receptor on platelets and may at least partly explain the conflicting data on the determinants of the platelet–collagen interaction reported in the literature. Numerous studies of platelet adhesion under both static and flow conditions were performed with type I collagen solubilized by pepsin, which cleaves collagen in the non-triple helical region where covalent cross-links are found that are essential for the assembly of collagen molecules into the typical banded structure as well as the insolubility of collagen (Kadler et al., 1996; Savage et al., 1999). Such soluble collagen has served as a powerful tool to characterize the recognition sites for individual collagen receptors within the molecule. Although collagens are degraded under certain pathological circumstances, they are still predominantly present in fibrillar form in normal vessels. These data suggest that platelet interactions with fibrillar collagen in vivo must be different and require investigation.
Since our results strongly suggested that α2β1 plays a supportive rather than an essential role in platelet interactions with native, fibrillar collagen, we analyzed GPVI, which binds fibrillar collagens as a low affinity and signal transducing receptor. Importantly, we found that GPVI function is not impaired in the absence of α2β1, which is in contrast to earlier reports showing that blockage of α2β1 function reduced platelet responses to the GPVI-specific agonist convulxin and CRP (Jandrot-Perrus et al., 1997; Hers et al., 2000; Kamiguti et al., 2000). An explanation for these different results could be that certain anti-α2β1 agents may have impaired GPVI function by yet unidentified mechanisms (Saelman et al., 1994; Kamiguti et al., 1997; Verkleij et al., 1998).
Interference with GPVI function revealed an essential role of this receptor in adhesion of normal as well as β1-null platelets to collagen. A function-blocking antibody to GPVI or the absence of the receptor on platelets efficiently inhibited platelet adhesion and aggregation to collagen under flow conditions. Under static conditions, GPVI was also essential for adhesion to fibrillar as well as soluble collagen, suggesting that GPVI–collagen interactions are a prerequisite for integrin-mediated adhesion.
We have recently shown that JAQ1 is unable to inhibit activation of platelets by high concentrations of fibrillar collagen, indicating the presence of a second, GPVI-independent collagen receptor (Schulte et al., 2001). We reasoned that α2β1 could be this receptor since it is present on resting platelets in a low affinity binding state requiring high levels of collagens for interaction. Indeed, β1-null platelets fail to respond to high concentrations of fibrillar collagen in the presence of JAQ1. Interestingly, however, while this response is independent of the CRP binding site that is blocked by JAQ1, it is still dependent on the presence of GPVI, as high collagen concentrations are unable to aggregate GPVI-depleted as well as FcRγ chain-deficient platelets despite normal expression of α2β1. A possible explanation for this finding could be that, in addition to the CRP binding site, GPVI has a second, low affinity binding site for fibrillar collagen mediating activation of α2β1 integrin, which then contributes to signaling. Alternatively, binding of the multivalent fibrillar collagen to α2β1 could co-aggregate α2β1 and GPVI within lipid rafts. It has been shown recently that receptor aggregates in lipid rafts can signal in a ligand-independent manner (Harder and Kuhn, 2000; Janes et al., 2000). In contrast, soluble monomeric collagen may not be able to mediate the clustering of its receptors in lipid rafts due to the lack of highly repetitive recognition sites within the molecule. It is important to note, however, that it is currently not clear whether α2β1 integrin and GPVI are present in lipid rafts.
Resting platelets express integrins in a low affinity binding state to avoid interactions with fibrinogen or plasma fibronectin (Phillips et al., 1991; Shattil et al., 1998). When platelets become activated, their integrins shift to a high affinity state and bind ligands. In our present study, we provide multiple lines of evidence that stimulation of GPVI can shift α2β1 and αIIbβ3 integrins from a low to high affinity state, thereby confirming and extending the observation of Moroi and co-workers (Jung and Moroi, 1998; Moroi et al., 2000). First, GPVI- and FcRγ chain-deficient platelets failed to adhere to collagen despite normal expression of α2β1 and αIIbβ3 (Nieswandt et al., 2000a, 2001), which are in a low affinity state, as demonstrated by fluorescence-activated cell sorting analysis (Figure 6B). Secondly, binding of these platelets to collagen via α2β1 and αIIbβ3–vWf could be induced by Mn2+, which is known to activate β1 and β3 integrins directly (Humphries, 2000). These findings, together with the observation that collagen induced the activation of both integrins in a GPVI-dependent manner (Figure 6C), demonstrate that GPVI triggers integrin-mediated platelet–collagen interactions.
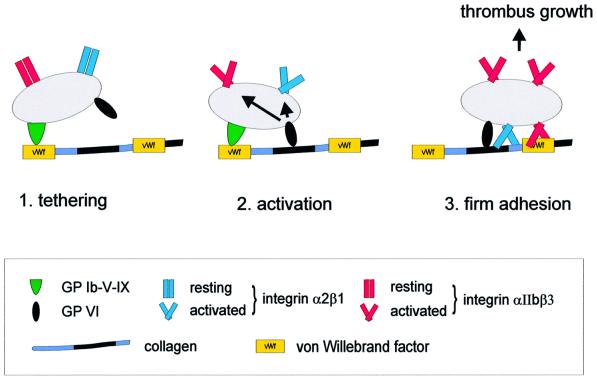
A previous model incorporated the idea that α2β1 integrin is essential for shear-resistant thrombus growth by mediating firm platelet adhesion to collagen. This first binding event would then allow a second step involving low affinity interactions of GPVI with collagen, leading to platelet activation and thrombus formation (Sixma et al., 1997; Barnes et al., 1998; Santoro, 1999). However, our analysis of β1-null platelets presented here demonstrates clearly that they can adhere to collagen under low as well as high shear stress. The number and size of platelet aggregates did not differ significantly between normal and β1-null platelets. In contrast, no adhesion occurred in the absence of GPVI. These findings establish a new sequence of events in the initial phase of hemostasis and thrombosis (Figure 8). First, platelets tether at sites of injury by GPIbα–vWF interactions, which are essential under conditions of high shear (Savage et al., 1998). In a second step, the platelets use GPVI to bind to collagen and thereby become activated and upregulate the activity of integrins, including α2β1 and α2IIbβ3. High affinity binding of α2β1 to collagen strengthens firm adhesion without, however, being essential for this process. This revised model of platelet–collagen interactions clearly establishes GPVI as the central collagen receptor on platelets and may have important implications for the development of new anti-thrombotic therapies.
Fig. 8. Model for platelet adhesion to collagen. 1. The initial tethering to the reactive surface is mediated predominantly by GPIbα–vWF interactions and is important at high shear rates (>500 s–1), but may not be relevant at lower shear rates (Savage et al., 1998). 2. GPVI–collagen interactions lead to cellular activation followed by shifting of integrins to a high affinity state. This activation step is proposed to be a strict prerequisite for adhesion. 3. Firm adhesion of platelets to collagen through activated α2β1 (directly) and αIIbβ3 (indirectly via vWF or other ligands). Integrin α2β1–collagen interactions are not essential for this process. This scheme only includes interactions examined in the current study (with the exception of GPIbα– vWF) and does not exclude the involvement of other receptor–ligand interactions.
Materials and methods
Animals
Generation of mice with β1-null platelets. To produce mice carrying the β1-null allele in megakaryocytes, β1(fl/fl) mice (Potocnik et al., 2000) were crossed with transgenic mice carrying the Mx-cre transgene (mx-cre+) (Kühn et al., 1995). Deletion of the β1 gene was induced in 4- to 5-week-old [β1(fl/fl)/Mx-cre+] mice by three i.p. injections of 250 µg of pI–pC at 2-day intervals. Control β1(fl/fl) mice received the same treatment and were derived from the same litters. For experiments, mice were used at least 2 weeks after pI–pC injection.
Bone marrow chimeras were generated by tail vein injection of 106 bone marrow cells derived from [β1(fl/–)/Mx-cre+]c into lethally irradiated (6 MV X-rays, 10 Gy, 6.2 Gy/min) congenic B6.SJL mice. Control mice received bone marrow cells derived from [β1(fl/+)/Mx-cre] mice. Four weeks after the transfer, deletion of the β1 gene was induced by pI–pC injections as described above. Bleeding times were tested 3 weeks and 6 months later.
Generation of GPVI-deficient platelets. Wild-type mice were injected with 100 µg of JAQ1 i.p. and platelets were harvested on day 7. As reported previously, GPVI was not detectable on those platelets by flow cytometry and western blot analysis (Nieswandt et al., 2001). For static adhesion assays on fibrillar collagen, these platelets were used in the presence of 10 µg/ml JAQ1 to block any residual GPVI activity.
FcRγ chain-deficient mice. FcRγ chain-deficient mice (Takai et al., 1994) were obtained from Taconics, Germantown.
Chemicals and antibodies
Fibrillar type I collagen from equine tendon (Horm; Nycomed, Munich, Germany), high molecular weight heparin, soluble, non-fibrillar type I collagen from rat tail (both from Sigma, Deisenhofen, Germany), calcein acetoxymethyl ester (Molecular Probes, Leiden, The Netherlands) and d-Phe-Pro-Arg chloromethyl ketone (PPACK; Calbiochem, San Diego, CA) were purchased. Purified human vWF was a generous gift from G.Dickneite (Marburg, Germany). All other reagents were from sources described previously (Nieswandt et al., 2001).
The following antibodies were used: hamster anti-β1 integrin (Ha31/8), hamster anti-α2 integrin (Ha1/29), FITC–anti-α4 integrin (R1-2), biotinylated anti-α5 integrin (5H10-27), FITC–anti-α6 integrin (GoH3), FITC–anti-α1 integrin (Ha2/5), rat anti-β1 integrin (9EG7), rat anti-β4 integrin (346-11A), biotinylated anti-β7 integrin (M293) (all PharMingen), mouse hybridoma supernatant against α7 integrin (3C12; obtained from K.von der Mark, Erlangen, Germany), rat anti-mouse P-selectin mAb RB40.34 (from D.Vestweber, Münster, Germany) and polyclonal rabbit antibodies to human fibrinogen (Dako, Glostrup, Denmark). Rat anti-mouse αIIbβ3 (JON1, JON/A), β3 integrin (EDL4), GPIb-IX (p0p1), GPV (DOM1), CD9 (ULF1) and GPVI (JAQ1) were generated, produced and modified in our laboratories as described (Bergmeier et al., 2000; Nieswandt et al., 2000a,b). Fab fragments of JAQ1 were prepared as described (Nieswandt et al., 2001). FITC–anti-hamster IgG (PharMingen), FITC–anti-mouse IgG (Jackson Laboratories), streptavidin–phycoerythrin (Southern Biotechnologies) and rabbit anti-FITC–horseradish peroxidase (Dako) were used as secondary reagents.
Platelet preparation and platelet aggregation
Mice were bled under ether anesthesia from the retro-orbital plexus. Platelet counting, preparation of platelet-rich plasma (prp) and determination of platelet aggregation were carried out as described (Nieswandt et al., 2001).
Immunoblotting and flow cytometry
Platelet preparation, immunoblotting and flow cytometry were carried out as described (Nieswandt et al., 2001). Tyrosine phosphorylation was determined as described elsewhere (Schulte et al., 2001).
Bleeding time experiments
Mice were restrained and a 0.5 cm segment of the tail tip was cut off with a scalpel. Tail bleeding was monitored by gently absorbing the bead of blood with a filter paper without contacting the wound site. When no blood was observed on the paper after 15 s intervals, bleeding was determined to have ceased.
Static adhesion assay
The static adhesion assay was performed with washed platelets in the presence or absence of MgCl2/CaCl2 (each at 1 mM) on 96-well plates (Nunc, Wiesbaden, Germany) coated with fibrillar or soluble collagen (2 µg/well) as described (Nieswandt et al., 2001). For adhesion in the presence of 0.5 mM MnCl2, the experiments were performed in the presence of apyrase (1 U/ml), aspirin (1 mM) and prostacyclin (1 µM) in order to minimize Mn2+-induced platelet degranulation.
Adhesion under flow conditions
Mouse blood (1 vol.) was collected into 0.5 vols of HEPES buffer pH 7.45 containing 137 mM NaCl, 5.6 mM glucose, 5 mM HEPES, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 0.1% bovine serum albumin (BSA), 120 µM PPACK and 15 U/ml heparin. The blood was incubated with 2.5 µM calcein acetoxymethyl ester for 30–60 min. Rectangular coverslips (24 × 60 mm) were sprayed with 150 µl of fibrillar or non-fibrillar collagen (0.25 mg/ml), and blocked with HEPES buffer containing 1% BSA, as described previously (Heemskerk et al., 1997).
Perfusion of whole blood was performed as described elsewhere (Briedé et al., 2001). Briefly, transparent flow chambers with a slit depth of 50 µm, equipped with the collagen-coated coverslips, were rinsed with HEPES buffer supplemented with 2 mM CaCl2 and 1 U/ml heparin, and connected to a syringe filled with the anti-coagulated blood. Perfusion was carried out at room temperature using a pulse-free pump under low shear stress conditions (10 min, flow rate of 1.12 ml/h, equivalent to a wall shear rate of 150 s–1) or at high shear stress (4 min, flow rate of 7.53 ml/h, equivalent to a wall shear rate of 1000 s–1). During perfusion, microscope phase-contrast images were recorded in real time. Thereafter, the chambers were rinsed by a 10 min perfusion with HEPES buffer pH 7.45 containing 2 mM CaCl2 and 1 U/ml heparin at the same shear stress. Both phase-contrast and fluorescence images were recorded from at least five different microscope fields (40× or 80× objectives), using a fluorescence digital imaging system equipped with two intensified, charge-coupled device cameras (Heemskerk et al., 1997). Image analysis was performed off-line using Quanticell 700/900 software (Visitech, Sunderland, UK). The platelet adhesion results are expressed as the mean percentage of the total area covered by platelets or calcein fluorescence.
Statistical evaluation
Statistical analysis was performed using the unpaired Student’s t-test.
Acknowledgments
Acknowledgements
We thank Michael Dictor and Kamin Johnson for discussion and critically reading the manuscript, U.Barnfred for constant support throughout the study and Per Nilsson for irradiating the mice used for bone marrow transplantations. The work was supported by grants from the Deutsche Forschungsgemeinschaft (to B.N.) and the Swedish National Research Foundation (to C.B. and R.F.).
References
- Arai M., Yamamoto,N., Moroi,M., Akamatsu,N., Fukutake,K. and Tanoue,K. (1995) Platelets with 10% of the normal amount of glycoprotein VI have an impaired response to collagen that results in a mild bleeding tendency. Br. J. Haematol., 89, 124–130. [DOI] [PubMed] [Google Scholar]
- Asselin J., Gibbins,J.M., Achison,M., Lee,Y.H., Morton,L.F., Farndale,R.W., Barnes,M.J. and Watson,S.P. (1997) A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase C γ2 in platelets independent of the integrin α2β1. Blood, 89, 1235–1242. [PubMed] [Google Scholar]
- Barnes M.J., Knight,C.G. and Farndale,R.W. (1998) The collagen– platelet interaction. Curr. Opin. Hematol., 5, 314–320. [DOI] [PubMed] [Google Scholar]
- Baumgartner H.R. (1977) Platelet interaction with collagen fibrils in flowing blood. I. Reaction of human platelets with α chymotrypsin-digested subendothelium. Thromb. Haemost., 37, 1–16. [PubMed] [Google Scholar]
- Bergmeier W., Rackebrandt,K., Schroder,W., Zirngibl,H. and Nieswandt,B. (2000) Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood, 95, 886–893. [PubMed] [Google Scholar]
- Briedé J.J., Heemskerk,J.W.M., van’t Veer,C., Hemker,H.C. and Lindhout,T. (2001) Contribution of platelet-derived factor Va to thrombin generation on immobilized collagen- and fibrinogen-adherent platelets. Thromb. Haemost., in press. [PubMed] [Google Scholar]
- Clemetson J.M., Polgar,J., Magnenat,E., Wells,T.N. and Clemetson,K.J. (1999) The platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcαR and the natural killer receptors. J. Biol. Chem., 274, 29019–29024. [DOI] [PubMed] [Google Scholar]
- Coller B.S., Beer,J.H., Scudder,L.E. and Steinberg,M.H. (1989) Collagen–platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood, 74, 182–192. [PubMed] [Google Scholar]
- Fässler R., Georges-Labouesse,E. and Hirsch,E. (1996) Genetic analyses of integrin function in mice. Curr. Opin. Cell Biol., 8, 641–646. [DOI] [PubMed] [Google Scholar]
- Gibbins J.M., Okuma,M., Farndale,R., Barnes,M. and Watson,S.P. (1997) Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor γ-chain. FEBS Lett., 413, 255–259. [DOI] [PubMed] [Google Scholar]
- Harder T. and Kuhn,M. (2000) Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies. J. Cell Biol., 151, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk J.W., Vuist,W.M., Feijge,M.A., Reutelingsperger,C.P. and Lindhout,T. (1997) Collagen but not fibrinogen surfaces induce bleb formation, exposure of phosphatidylserine and procoagulant activity of adherent platelets: evidence for regulation by protein tyrosine kinase-dependent Ca2+ responses. Blood, 90, 2615–2625. [PubMed] [Google Scholar]
- Hers I., Berlanga,O., Tiekstra,M.J., Kamiguti,A.S., Theakston,R.D. and Watson,S.P. (2000) Evidence against a direct role of the integrin α2β1 in collagen-induced tyrosine phosphorylation in human platelets. Eur. J. Biochem., 267, 2088–2097. [DOI] [PubMed] [Google Scholar]
- Humphries M.J. (2000) Integrin structure. Biochem. Soc. Trans., 28, 311–339. [PubMed] [Google Scholar]
- Jandrot-Perrus M., Lagrue,A.H., Okuma,M. and Bon,C. (1997) Adhesion and activation of human platelets induced by convulxin involve glycoprotein VI and integrin α2β1. J. Biol. Chem., 272, 27035–27041. [DOI] [PubMed] [Google Scholar]
- Jandrot-Perrus M. et al. (2000) Cloning, characterization and functional studies of human and mouse glycoprotein VI: a platelet-specific collagen receptor from the immunoglobulin superfamily. Blood, 96, 1798–1807. [PubMed] [Google Scholar]
- Janes P.W., Ley,S.C., Magee,A.I. and Kabouridis,P.S. (2000) The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol., 12, 23–34. [DOI] [PubMed] [Google Scholar]
- Jung S.M. and Moroi,M. (1998) Platelets interact with soluble and insoluble collagens through characteristically different reactions. J. Biol. Chem., 273, 14827–14837. [DOI] [PubMed] [Google Scholar]
- Kadler K.E., Holmes,D.F., Trotter,J.A. and Chapman,J.A. (1996) Collagen fibril formation. Biochem. J., 316, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguti A.S., Markland,F.S., Zhou,Q., Laing,G.D., Theakston,R.D. and Zuzel,M. (1997) Proteolytic cleavage of the β1 subunit of platelet α2β1 integrin by the metalloproteinase jararhagin compromises collagen-stimulated phosphorylation of pp72. J. Biol. Chem., 272, 32599–32605. [DOI] [PubMed] [Google Scholar]
- Kamiguti A.S., Theakston,R.D., Watson,S.P., Bon,C., Laing,G.D. and Zuzel,M. (2000) Distinct contributions of glycoprotein VI and α(2)β(1) integrin to the induction of platelet protein tyrosine phosphorylation and aggregation. Arch. Biochem. Biophys., 374, 356–362. [DOI] [PubMed] [Google Scholar]
- Kehrel B., Balleisen,L., Kokott,R., Mesters,R., Stenzinger,W., Clemetson,K.J. and van de Loo,J. (1988) Deficiency of intact thrombospondin and membrane glycoprotein Ia in platelets with defective collagen-induced aggregation and spontaneous loss of disorder. Blood, 71, 1074–1078. [PubMed] [Google Scholar]
- Kehrel B., Wierwille,S., Clemetson,K.J., Anders,O., Steiner,M., Knight,C.G., Farndale,R.W., Okuma,M. and Barnes,M.J. (1998) Glycoprotein VI is a major collagen receptor for platelet activation: it recognizes the platelet-activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa and von Willebrand factor do not. Blood, 91, 491–499. [PubMed] [Google Scholar]
- Kühn R., Schwenk,F., Aguet,M. and Rajewsky,K. (1995) Inducible gene targeting in mice. Science, 269, 1427–1429. [DOI] [PubMed] [Google Scholar]
- Lenter M., Uhlig,H., Hamann,A., Jeno,P., Imhof,B. and Vestweber,D. (1993) A monoclonal antibody against an activation epitope on mouse integrin chain β1 blocks adhesion of lymphocytes to the endothelial integrin α6 β1. Proc. Natl Acad. Sci. USA, 90, 9051–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi M., Jung,S.M., Okuma,M. and Shinmyozu,K. (1989) A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J. Clin. Invest., 84, 1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi M., Onitsuka,I., Imaizumi,T. and Jung,S.M. (2000) Involvement of activated integrin α2β1 in the firm adhesion of platelets onto a surface of immobilized collagen under flow conditions. Thromb. Haemost., 83, 769–776. [PubMed] [Google Scholar]
- Morton L.F., Peachey,A.R., Zijenah,L.S., Goodall,A.H., Humphries,M.J. and Barnes,M.J. (1994) Conformation-dependent platelet adhesion to collagen involving integrin α2β1-mediated and other mechanisms: multiple α2β1-recognition sites in collagen type I. Biochem. J., 299, 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Jamieson,G.A., Okuma,M., Kambayashi,J. and Tandon,N.N. (1998) Platelet adhesion to native type I collagen fibrils. Role of GPVI in divalent cation-dependent and -independent adhesion and thromboxane A2 generation. J. Biol. Chem., 273, 4338–4344. [DOI] [PubMed] [Google Scholar]
- Nieswandt B., Bergmeier,W., Rackebrandt,K., Gessner,J.E. and Zirngibl,H. (2000a) Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood, 96, 2520–2527. [PubMed] [Google Scholar]
- Nieswandt B., Bergmeier,W., Schulte,V., Rackebrandt,K., Gessner,J.E. and Zirngibl,H. (2000b) Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRγ chain. J. Biol. Chem., 275, 23998–24002. [DOI] [PubMed] [Google Scholar]
- Nieswandt B., Schulte,V., Bergmeier,W., Mokhtari-Nejad,R., Rackebrandt,K., Cazenave,J.P., Ohlmann,P., Gachet,C. and Zirngibl,H. (2001) Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J. Exp. Med., 193, 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis H.K., Akkerman,J.W., Houdijk,W.P. and Sixma,J.J. (1985) Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature, 318, 470–472. [DOI] [PubMed] [Google Scholar]
- Onley D.J., Knight,C.G., Tuckwell,D.S., Barnes,M.J. and Farndale,R.W. (2000) Micromolar Ca2+ concentrations are essential for Mg2+-dependent binding of collagen by the integrin α2β1 in human platelets. J. Biol. Chem., 275, 24560–24564. [DOI] [PubMed] [Google Scholar]
- Phillips D.R., Charo,I.F. and Scarborough,R.M. (1991) GPIIb–IIIa: the responsive integrin. Cell, 65, 359–362. [DOI] [PubMed] [Google Scholar]
- Poole A., Gibbins,J.M., Turner,M., van Vugt,M.J., van de Winkel,J.G., Saito,T., Tybulewicz,V.L. and Watson,S.P. (1997) The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J., 16, 2333–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocnik A.J., Brakebusch,C. and Fässler,R. (2000) Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen and bone marrow. Immunity, 12, 653–663. [DOI] [PubMed] [Google Scholar]
- Saelman E.U. et al. (1994) Platelet adhesion to collagen types I through VIII under conditions of stasis and flow is mediated by GPIa/IIa (α2β1-integrin) Blood, 83, 1244–1250. [PubMed] [Google Scholar]
- Santoro S.A. (1986) Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell, 46, 913–920. [DOI] [PubMed] [Google Scholar]
- Santoro S.A. (1999) Platelet surface collagen receptor polymorphisms: variable receptor expression and thrombotic/hemorrhagic risk. Blood, 93, 3575–3577. [PubMed] [Google Scholar]
- Savage B., Almus-Jacobs,F. and Ruggeri,Z.M. (1998) Specific synergy of multiple substrate–receptor interactions in platelet thrombus formation under flow. Cell, 94, 657–666. [DOI] [PubMed] [Google Scholar]
- Savage B., Ginsberg,M.H. and Ruggeri,Z.M. (1999) Influence of fibrillar collagen structure on the mechanisms of platelet thrombus formation under flow. Blood, 94, 2704–2715. [PubMed] [Google Scholar]
- Schulte V., Snell,D., Bergmeier,W., Zirngibl,H., Watson,S.P. and Nieswandt,B. (2001) Evidence for two distinct epitopes within collagen for activation of murine platelets. J. Biol. Chem., 276, 364–368. [DOI] [PubMed] [Google Scholar]
- Shattil S.J., Kashiwagi,H. and Pampori,N. (1998) Integrin signaling: the platelet paradigm. Blood, 91, 2645–2657. [PubMed] [Google Scholar]
- Siljander P. and Lassila,R. (1999) Studies of adhesion-dependent platelet activation: distinct roles for different participating receptors can be dissociated by proteolysis of collagen. Arterioscler. Thromb. Vasc. Biol., 19, 3033–3043. [DOI] [PubMed] [Google Scholar]
- Sixma J.J., van Zanten,G.H., Huizinga,E.G., van der Plas,R.M., Verkley,M., Wu,Y.P., Gros,P. and de Groot,P.G. (1997) Platelet adhesion to collagen: an update. Thromb. Haemost., 78, 434–438. [PubMed] [Google Scholar]
- Takai T., Li,M., Sylvestre,D., Clynes,R. and Ravetch,J.V. (1994) FcR γ chain deletion results in pleiotrophic effector cell defects. Cell, 76, 519–529. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Ezumi,Y., Arai,M. and Takayama,H. (1997) A novel association of Fc receptor γ-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J. Biol. Chem., 272, 23528–23531. [DOI] [PubMed] [Google Scholar]
- Verkleij M.W., Morton,L.F., Knight,C.G., de Groot,P.G., Barnes,M.J. and Sixma,J.J. (1998) Simple collagen-like peptides support platelet adhesion under static but not under flow conditions: interaction via α2β1 and von Willebrand factor with specific sequences in native collagen is a requirement to resist shear forces. Blood, 91, 3808–3816. [PubMed] [Google Scholar]
- Watson S.P. and Gibbins,J. (1998) Collagen receptor signalling in platelets: extending the role of the ITAM. Immunol. Today, 19, 260–264. [DOI] [PubMed] [Google Scholar]
- Weiss H.J. (1975) Platelet physiology and abnormalities of platelet function (first of two parts). N. Engl. J. Med., 293, 531–541. [DOI] [PubMed] [Google Scholar]