Abstract
Phospholipase D (PLD) has been proposed to mediate cytoskeletal remodeling and vesicular trafficking along the secretory pathway. We recently described the activation of an ADP ribosylation factor-regulated PLD at the plasma membrane of chromaffin cells undergoing secretagogue-stimulated exocytosis. We show here that the isoform involved is PLD1b, and, using a real-time assay for individual cells, that PLD activation and exocytosis are closely correlated. Moreover, overexpressed PLD1, but not PLD2, increases stimulated exocytosis in a phosphatidylinositol 4,5-bisphosphate-dependent manner, whereas catalytically inactive PLD1 inhibits it. These results provide the first direct evidence that PLD1 is an important component of the exocytotic machinery in neuroendocrine cells.
Keywords: chromaffin cell/cytoskeleton/exocytosis/phospholipase D/secretory granule
Introduction
Membrane compartments in eukaryotic cells are in constant dynamic flux. Transport vesicles emerge from donor compartments and are targeted specifically to acceptor compartments where they deliver cargo through membrane fusion. Proteins that catalyze membrane bilayer mixing inside secretory cells have been identified, such as the SNAREs, which bring membranes into close proximity (Hanson et al., 1997; Weber et al., 1998; Chen et al., 1999b). However, the lipid composition of vesicles and their target membranes is also critical, and lipid modifications may be required to allow the membrane fusion machinery to function.
Phospholipases hydrolyze phospholipids, the backbone of biological membranes. Phospholipase activity not only has a profound impact on the structure and stability of cellular membranes but also plays a pivotal role in regulating many critical cellular functions. Phospho lipase D (PLD) generates phosphatidic acid (PA), a multifunctional lipid that has been proposed variously to alter membrane curvature, serve as a protein attachment site, activate selected enzymes or represent the starting material for the production of additional signaling lipids, particularly in the context of membrane vesicle trafficking and cytoskeletal dynamics (Cross et al., 1996; Ktistakis et al., 1996; Honda et al., 1999; Jones et al., 1999; Liscovitch et al., 2000). There are two mammalian PLD family members. PLD1 has a low basal activity and is activated in vitro in a synergistic manner by protein kinase C-α (PKCα), ADP ribosylation factor (ARF) and Rho family members (Hammond et al., 1997), whereas PLD2 is constitutively active (Colley et al., 1997). Both PLDs require phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] as a co-factor (Liscovitch et al., 1994; Colley et al., 1997; Hammond et al., 1997). Within cells, the mechanisms controlling PLD activation by agonists involve the same regulators but are less well understood.
Studies in HL-60 cells (Stutchfield and Cockcroft, 1993) and platelets (Haslam and Coorsen, 1993) suggested that PLD might participate in regulated exocytosis in an ARF-dependent manner (Fensome et al., 1996; Jones et al., 1999). In neutrophils and RBL-2H3 basophilic leukemia cells, most PLD activity co-localizes with secretory vesicles and, upon stimulation, translocates to the plasma membrane (Morgan et al., 1997; Brown et al., 1998). Adrenal medullary chromaffin cells are neuroendocrine cells that, along with their tumor cell derivatives, PC12 cells, have been used extensively as neuronal cell models. The large dense-core peptidergic vesicles present in certain neurons are very similar to the catecholamine-containing granules found in chromaffin cells. Thus, most of the information on large dense-core granule exocytosis has been inferred from studies on chromaffin cells over many years. We recently demonstrated that in chromaffin cells, secretagogues stimulate the rapid translocation of ARF6 from secretory granules to the plasma membrane and the concomitant activation of PLD in the plasma membrane (Galas et al., 1997; Caumont et al., 1998). The activation of PLD correlated in timing and calcium dependence with the exocytotic response, and the latter was attenuated by alcohols that inhibit the production of PA by PLD (Caumont et al., 1998). Collectively, these results strongly argue but do not prove that an ARF-regulated PLD plays an important role in regulated exocytotic secretion.
Using a variety of direct means to test this hypothesis, we demonstrate here that plasma membrane-associated PLD1 acts at a late stage of the secretory pathway in chromaffin and PC12 cells. We propose that PLD1 constitutes a key factor for regulated exocytosis in neuroendocrine cells, operating subsequent to the cytoskeletal-mediated recruitment of secretory granules to the exocytotic sites.
Results
Identification and intracellular localization of phospholipase D in chromaffin cells
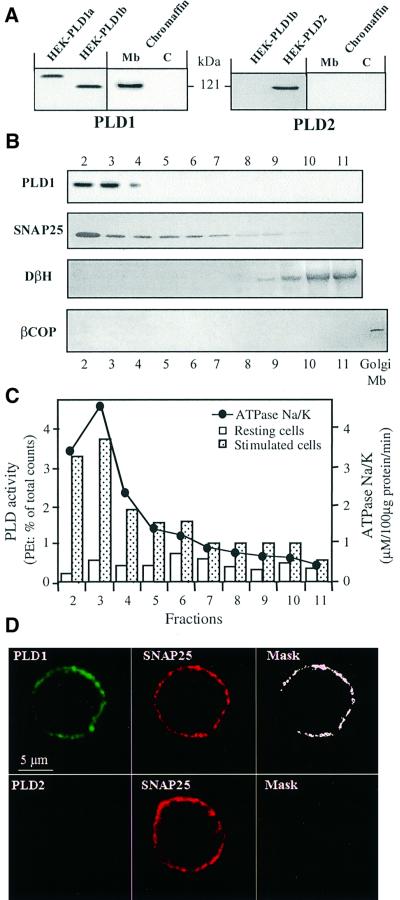
Isoform-specific antibodies were used to probe for PLD1 and PLD2 in chromaffin cell cytosol and crude membrane fractions (Figure 1A). PLD1 was detected in the membrane-bound compartment, whereas PLD2 was not found in either fraction. The PLD1 observed co-migrated with PLD1b (Figure 1A), a splice variant lacking exon 17 (Hammond et al., 1997). Analysis of fractions collected from a sucrose density gradient layered with the crude membrane preparation revealed that the strongest immunosignal for PLD1 was detected in fractions 2 and 3 (Figure 1B), which contained the plasma membranes as assessed by following Na+/K+-ATPase activity and the distribution of SNAP-25. The Golgi marker β-COP was not detected in the gradient profile, demonstrating that the PLD1 did not originate from contaminating Golgi membranes. PLD1 was also not detected in the fractions containing secretory chromaffin granules as marked by dopamine-β-hydroxylase. This fractionation pattern was not altered in chromaffin cells stimulated for 4 min with the secretagogue nicotine (10 μM; data not shown), suggesting that PLD1 does not translocate in this cell type during periods of active exocytosis. In parallel, [3H]myristic acid-labeled chromaffin cells were permeabilized with streptolysin-O (SLO) and stimulated by elevating cytosolic calcium; cells were then collected and processed for subcellular fractionation. The majority of the PLD activity elicited was found in fractions 2 and 3 (Figure 1C), indicating that the plasma membrane-associated PLD1 is activated in stimulated chromaffin cells.
Fig. 1. Chromaffin cells express PLD1b, which localizes to and is activated at the plasma membrane. (A) Cultured chromaffin cells were collected, homogenized and centrifuged at 20 000 g to separate cytosol (C) from the crude membranes (Mb). Note that light membranes, such as the Golgi and endosomes, are not pelleted under these conditions. The fractions were then subjected to protein determination, gel electrophoresis and immunodetection on nitrocellulose sheets using anti-PLD1 and anti-PLD2 antibodies. Lysates from HEK293 cells transfected with pCGN-PLD1a, pCGN-PLD1b and pCGN-PLD2 plasmids were used as positive controls. (B) Fractions 2–11 (40 µg of protein per fraction) collected from a continuous sucrose density gradient layered with the crude chromaffin membrane pellet were immunodetected as above using antibodies against PLD1, SNAP-25 (plasma membrane marker), dopamine-β-hydroxylase (chromaffin granule marker) and β-COP (Golgi membrane marker). (C) Chromaffin cells labeled with [3H]myristic acid were permeabilized with SLO and subsequently incubated in KG medium containing 1% ethanol and 100 µM GTPγS in the presence (closed bars) or absence (open bars) of 20 µM free Ca2+. Cells were then collected and processed for subcellular fractionation on a continuous sucrose density gradient. Fractions were assayed for Na+/K+-ATPase for plasma membranes. Phospholipids were extracted from each fraction and the amount of [3H]phosphatidylethanolamine formed determined by TLC. Most of the Ca2+-stimulated PLD activity is detected in the plasma membrane-enriched fractions, which similarly contained PLD1. Data are the mean values of duplicate determinations. Similar results were obtained in four independent experiments. (D) Immunofluorescence confocal micrographs of cultured chromaffin cells double-labeled with anti-PLD1 or anti-PLD2 antibodies, detected with secondary Cy2-conjugated anti-rabbit antibodies, and anti-SNAP-25 antibodies detected with Cy3-conjugated anti-mouse antibodies. The same optical section recorded sequentially in the Cy2 (PLD) and Cy3 (SNAP-25) channels is presented. Masks representing the regions of co-localization are obtained by selecting the pixels double-labeled with Cy2 and Cy3.
The intracellular distribution of PLD in cultured chromaffin cells was investigated further by immunofluorescence and confocal microscopy. Immunostaining with anti-PLD1 antibodies revealed a peripheral fluorescent pattern that was very similar to the labeling obtained with anti-SNAP-25 antibodies (Figure 1D), confirming the association of PLD1 with the plasma membrane.
A short-chain ceramide inhibits endogenous PLD and prevents catecholamine release in chromaffin cells
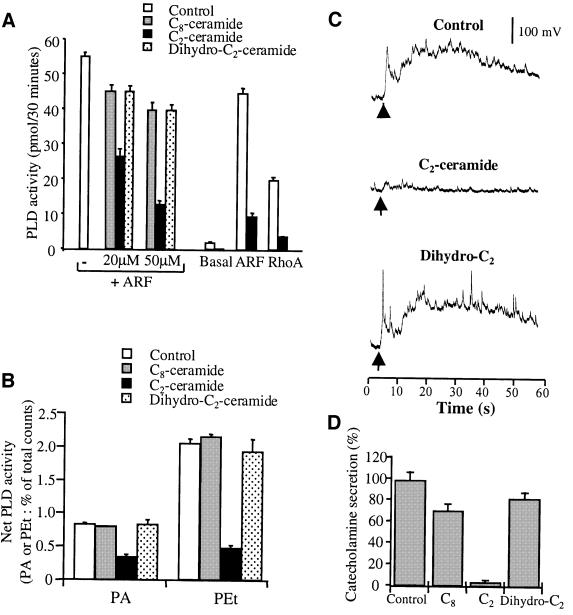
Ceramides have been reported to inhibit PLD activation in various cell types (Jones and Murray, 1995; Abousalham et al., 1997). We found that C2-ceramide, but not C8- nor dihydro-C2-ceramide, directly inhibits purified recombinant PLD1 (Figure 2A) and prevents the activation of endogenous PLD in permeabilized chromaffin cells stimulated by calcium (Figure 2B). To examine the ceramide effect on catecholamine secretion, we used single-cell amperometry (Wightman et al., 1991). Chromaffin cells pre-incubated with C2-, C8- or dihydro-C2-ceramide were stimulated by a local application of nicotine, following which exocytotic release of catecholamines was monitored by measuring oxidation currents from individual cells (Figure 2C). Nicotine evoked a rapid increase in the oxidation current, which lasted 60–90 s and consisted of a broad secretion envelope superimposed with many spikes. Incubation with C2-ceramide produced a strong inhibition of the amperometric response (Figure 2C) as quantified by integrating the area below the current curve (Figure 2D). In contrast, dihydro-C2- and C8-ceramide affected the secretory activity only very modestly. Although ceramides can affect other cellular processes, the close correlation between the ability of C2-ceramide to block PLD activation and its inhibitory effect on catecholamine secretion supports the idea that PLD plays a causal role in chromaffin cell exocytosis.
Fig. 2. Effects of ceramides on PLD activation and catecholamine secretion from stimulated chromaffin cells. (A) Purified PLD1 was assayed in vitro under basal and stimulated conditions in the presence of the indicated ceramides. ARF-stimulated PLD1 activity was assayed in the absence or presence of either C8-, C2- or dihydro-C2 ceramides (20 or 50 µM). Basal, ARF-stimulated and Rho-stimulated PLD1 were assayed in the absence or presence of 50 µM C2-ceramide. (B) Chromaffin cells labeled with [3H]myristic acid were incubated for 1 h with the indicated ceramides at 50 µM. Cells were then permeabilized with SLO and stimulated with 20 µM free Ca2+ in the presence of 1% ethanol. Phospholipids subsequently were extracted and analyzed by TLC. Data are the mean values of duplicate determinations ± SEM. (C) Chromaffin cells were incubated for 1 h in the presence of the indicated ceramides at 50 µM. Cells were then stimulated by a local application of 100 µM nicotine for 10 s (arrow) and the exocytotic release of catecholamines was estimated by electrochemical detection with a carbon fiber electrode placed adjacent to single chromaffin cells. Typical amperometric responses from nicotine-stimulated chromaffin cells pre-incubated in Locke’s solution (control) or in Locke’s solution containing either C2- or dihydro-C2-ceramide are shown. (D) Amperometric responses were integrated to obtain the total catecholamine secretion expressed in pA/s. Results are expressed relative to the secretory response obtained for the control cells. Data are the means of 30 cells/group from the same experiment ± SEM. Similar results were obtained in three independent experiments performed on different culture preparations.
PLD1 and a catalytically inactive PLD1 mutant affect secretion in neuroendocrine cells
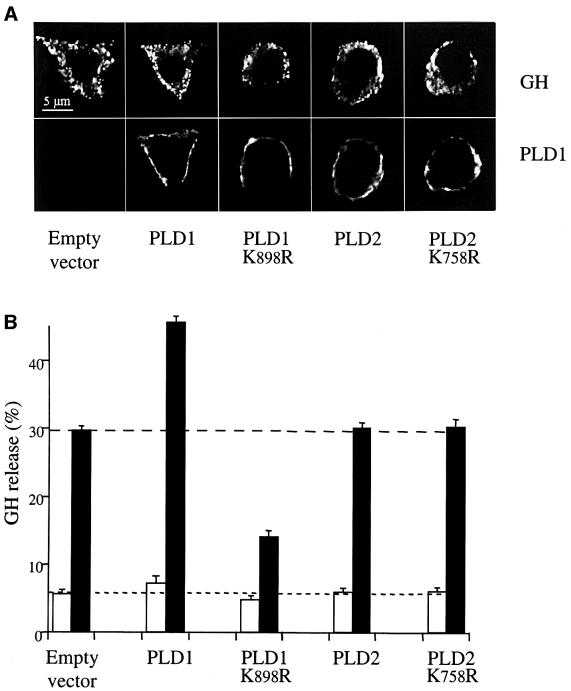
To establish whether PLD1 plays a role in exocytosis, we examined the effect of its overexpression in PC12 cells using growth hormone (GH) secretion as a reporter for exocytosis (Wick et al., 1993). GH was stored within secretory granules as observed by the punctuate fluorescent staining pattern using anti-GH antibodies (Figure 3A). Transfection with plasmids encoding wild-type or catalytically inactive PLDs did not alter total GH levels, as measured by radioimmunoassay (data not shown), or its intracellular distribution (Figure 3A), indicating that the synthesis and transport of GH through the early secretory pathway were not altered. In response to a depolarizing concentration of potassium, overexpression of PLD2 did not affect GH release (Figure 3B). In contrast, overexpression of PLD1b increased GH secretion; this increase was very reproducible and was observed in separate transfections performed on different cell preparations. Thus, PLD1, but not PLD2, promotes regulated exocytosis in PC12 cells.
Fig. 3. Overexpression of wild-type or catalytically inactive PLD1 but not PLD2 alters secretion of co-expressed GH from PC12 cells. PC12 cells were transfected with either pCGN (empty vector), pCGN-PLD1, pCGN-PLD1(K898R), pCGN-PLD2 or pCGN-PLD2(K758R) plasmids along with the plasmid encoding GH (4 µg/well of each plasmid). (A) At 48 h post-transfection, the proteins expressed were visualized by immunocytochemistry and confocal microscopy. Approximately 10–20% of cells were transfected successfully with the GH-expressing vector and, of these, >90% became co-transfected with the second plasmid. Neither wild-type nor mutated PLD1 or PLD2 proteins affected the punctuate pattern of fluorescence observed with the anti-GH antibodies, which is consistent with the storage of GH within secretory granules. Note the distinct PLD and GH labelings, revealing that the overexpressed PLDs are not associated with secretory granules. (B) Transfected cells were washed and subsequently incubated for 10 min in calcium-free Locke’s solution (open bars) or stimulated for 10 min with elevated K+ (closed bars). Extracellular fluids were then collected and GH present in solutions and in cells was estimated by radioimmunoassay. GH release is expressed as the percentage of total GH present in the cells before the 10 min incubation period. Data are given as the mean values ± SEM (n = 3). Similar results were obtained in three independent experiments performed with different cell cultures.
Next, we examined the effects of catalytically inactive PLD1(K898R) and PLD2(K758R) (Sung et al., 1997). PLD1(K898R) inhibited secretion of GH whereas PLD2(K758R) was without effect (Figure 3B). The residual K+-evoked secretory activity measured in the PLD1(K898R)-expressing cells may have been due to endogenous PLD1 that was not fully inhibited (western blotting confirmed the presence of PLD1 in PC12 cells; data not shown), because a fraction of the cells (∼10%) were not co-transfected with both plasmids and/or because PLD1(K898R) was not expressed at a high enough level to suppress it fully. Taken together, however, the findings that PLD1 increases GH exocytosis and that catalytically inactive PLD1 inhibits exocytosis support a function for PLD1 in the exocytotic pathway of large dense-core granules in PC12 cells.
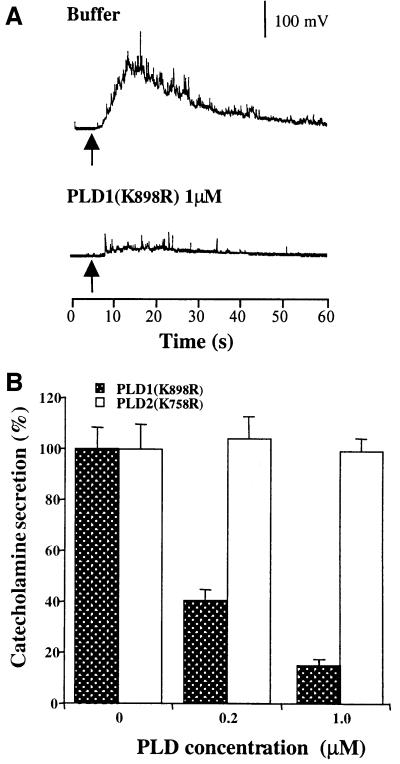
Long-term overexpression of PLD could affect various steps in the secretory pathway. To probe further the involvement of PLD1 in the final exocytotic event, we expressed and purified the inactive PLD1(K898R) and PLD2(K758R) mutant proteins and microinjected them into adrenal chromaffin cells. The cells were stimulated 10–15 min later by a local application of nicotine, following which exocytotic release of catecholamines was monitored. Microinjection of PLD2(K758R) did not modify the amperometric response to nicotine (Figure 4). In contrast, PLD1(K898R) produced a strong, dose-dependent inhibition of catecholamine secretion. Micro injection of PLD1(K898R) also resulted in a marked reduction in the number of release events (spikes) evoked over the 30 s period following nicotinic stimulation. From a series of recordings on separate cells, we found a strong reduction in spike numbers in cells injected with 1 µM PLD1(K898R) (5 ± 2 spikes/cell) as compared with control buffer-injected (35 ± 4 spikes/cell) or PLD2(K758R)-injected (32 ± 3 spikes/cell) cells.
Fig. 4. Catalytically inactive PLD1(K898R) but not catalytically inactive PLD2(K758R) inhibits nicotine-evoked catecholamine secretion from single chromaffin cells. Chromaffin cells were microinjected with buffer, PLD1(K898R) or PLD2(K758R). The concentration of recombinant proteins in the pipet was 0.2 or 1 µM. Cells were stimulated 10–15 min later by a local application of 100 µM nicotine for 5 s (arrow). Catecholamine secretion was recorded with a carbon fiber electrode. (A) Representative amperometric responses. (B) Total catecholamine secretion expressed in pA/s. Results are expressed relative to the secretory response obtained for the control cells. Data are the means of 25 cells/group from the same dish ± SEM. Similar results were obtained in four independent experiments performed on two culture preparations and with different batches of recombinant proteins.
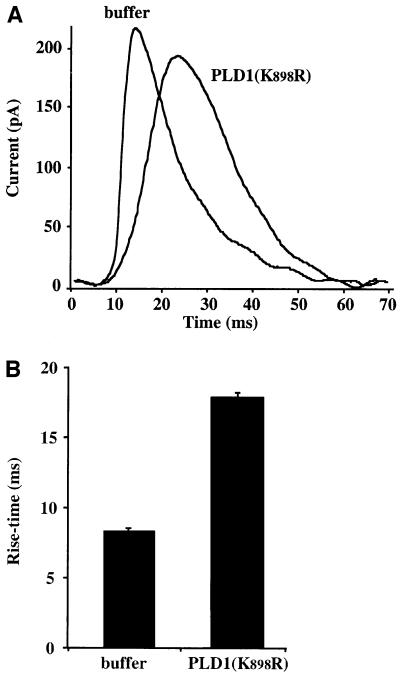
Microinjection of PLD1(K898R) not only inhibited the extent of exocytosis and number of spikes, but also affected the characteristics of the remaining spikes. As illustrated in Figure 5A, the overall shape of the residual spikes in PLD1(K898R)-injected cells was often broader than those typically observed in control non-injected or buffer-injected cells. Overlay of the spikes from buffer-injected and PLD1(K898R)-injected cells revealed an apparent increase in rise time from 8 ms for control spikes to 18 ms for spikes from PLD1(K898R)-injected cells (Figure 5B). Analysis of transient current spikes provides information about the kinetics and amplitude of release of catecholamines from single granule fusion events (Wightman et al., 1991; Chow et al., 1992; Albillos et al., 1997). If the inhibitory effect of PLD1(K898R) was caused by a blockade of granule recruitment or docking, then only a reduction in spike number and no effect on spike kinetics would be expected. The spike rise time is thought to reflect the kinetics of the initial fusion event and/or the rate of fusion pore opening (Graham and Burgoyne, 2000). The present data demonstrating that PLD1(K898R) affects the rise time of the spike are consistent with a possible post-docking role for PLD1 acting at the level of the fusion machinery or fusion pore expansion. These findings, therefore, support the idea that PLD1 plays an important role in the late steps of calcium-regulated exocytosis in neuroendocrine cells.
Fig. 5. Catalytically inactive PLD1(K898R) increases the rising phase of individual amperometric spikes. (A) Example spikes representing the average spikes in control buffer-injected chromaffin cells and PLD1(K898R)-injected cells on an expanded time base. (B) Mean values (±SEM) for the rise time of amperometric spikes from control (n = 285 spikes) and PLD1(K898R)-injected (n = 203 spikes) cells.
PtdIns(4,5)P2 targeting and direct stimulation by protein kinase C regulate PLD1 during exocytosis
In addition to members of the ARF and Rho families of GTPases, phosphoinositides and PKC also regulate the degree of PLD1 activation. Although its mode of action remains controversial, PKC is thought to be an important modulator of Ca2+-regulated exocytosis (Gillis et al., 1996; Chen et al., 1999a). We therefore investigated whether PKC is required to integrate PLD1 functionally into the exocytotic machinery, using PIM87, a mutant PLD1 allele unresponsive to direct stimulation by PKC but otherwise seemingly identical to the wild-type allele (Zhang et al., 1999). As shown in Table I, co-expression of PIM87 in PC12 cells resulted in a substantial increase in K+-evoked GH secretion. However, this increase was only half of that induced by wild-type PLD1. Thus, direct stimulation of PLD1 by PKC appears to be physiologically important in the exocytotic process, although this is likely to be only part of the mechanism through which PLD1 becomes activated in this setting.
Table I. Requirement for PKC and phosphoinositide interaction for PLD1-facilitated secretion of co-expressed GH from PC12 cells.
| Mutant phenotype | Basal release | K+-evoked secretion | |
|---|---|---|---|
| Empty vector | 6.1 ± 0.6 | 29.4 ± 0.8 | |
| PLD1 wild-type | 9.4 ± 0.8 | 45.8 ± 0.7 | |
| PIM 87 | PKC-non-responsive | 7.4 ± 0.3 | 37.1 ± 1.1 |
| PLD1 (K898R) | catalytically inactive | 3.3 ± 0.3 | 14.3 ± 0.6 |
| PLD1 (R690G/R694G) | PIP2-non-interacting | 6.9 ± 0.5 | 30.3 ± 0.5 |
| PLD1 (K898R/R690G/R694) | catalytically inactive PIP2-non-interacting | 6.7 ± 0.6 | 28.8 ± 1.4 |
PC12 cells overexpressing GH together with wild-type PLD1, PIM87, PLD1(R690G/R694G), PLD1(K898R) or PLD1(K898R/R690G/R694G) were maintained in calcium-free Locke’s solution (basal release) or stimulated for 10 min with Locke’s containing 59 mM K+ (K+-evoked secretion). Secretory activity was assayed by measuring GH released into the extracellular medium or remaining in cells. Data are given as the mean values ± SE (n = 6). Similar results were obtained in independent experiments performed with two different cell cultures.
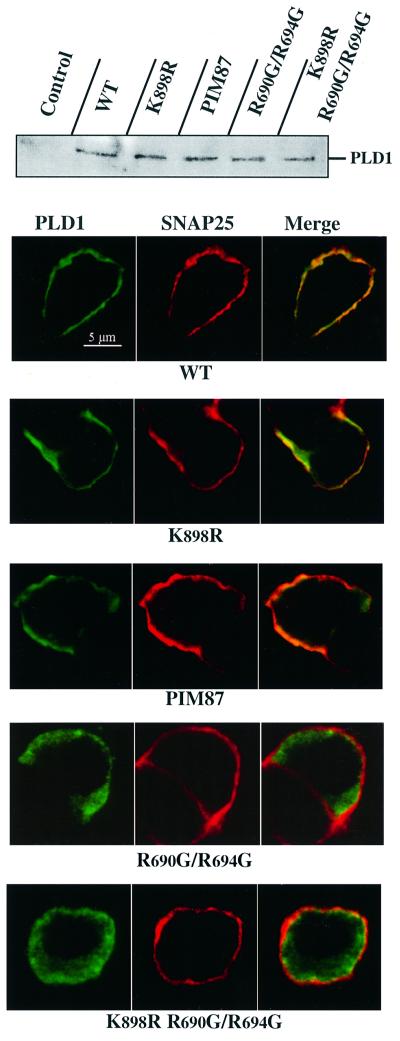
The synthesis of PtdIns(4,5)P2 is essential for priming the exocytotic apparatus for Ca2+-activated fusion (Martin et al., 1997). One role that PtdIns(4,5)P2 elevation could play is to stimulate PLD1, since PLD activity is almost completely dependent on its presence. To examine this issue, we used a PLD1 allele mutated at the motif that is critical for stimulation of PLD2 by phosphoinositides (Sciorra et al., 1999). PLD1(R690G/R694G) exhibits a very low catalytic activity (<1% of wild-type PLD1 activity; data not shown), and would accordingly be expected to behave as a dominant-negative, similar to the catalytically inactive PLD1(K898R). Surprisingly though, expression of PLD1(R690G/R694G) in PC12 cells did not significantly inhibit basal or K+-evoked GH secretion (Table I). One explanation for this would be that PLD1(R690G/R694G) was unable to interfere with the exocytotic machinery because it failed to localize properly as a result of its loss of interaction with PtdIns(4,5)P2. We therefore investigated the intracellular distribution of wild-type and mutated PLD1 proteins in the transfected PC12 cells. Wild-type PLD1, PLD1(K898R) and PIM87 co-localized with SNAP-25 in the cell periphery (Figure 6). This pattern of distribution is consistent with the plasma membrane localization of endogenous PLD1 in chromaffin cells. In contrast, PLD1(R690G/R694G) exhibited a more diffuse staining pattern, indicating that it localized predominantly to the cytosol or internal membrane compartments, and suggesting that binding to PtdIns(4,5)P2 represents the primary mechanism for the spatial and functional recruitment of PLD1 to exocytotic sites at the plasma membrane. To probe further the idea that binding to PtdIns(4,5)P2 is an essential feature of the involvement of PLD1 in exocytosis, we examined whether the inhibition of secretion caused by the catalytically inactive PLD1(K898R) might be prevented by rendering this mutant incapable of binding PtdIns(4,5)P2. Therefore, PC12 cells were transfected with the PLD1(K898R/R690G/R694G) mutant. As shown in Figure 6, this catalytically inactive PLD1 mutant mislocalized and was incapable of inhibiting high K+-evoked GH release in PC12 cells (Table I), reinforcing the idea that binding to PtdIns(4,5)P2 at the plasma membrane is essential for the functional integration of PLD1 into the exocytotic pathway.
Fig. 6. PtdIns(4,5)P2 interaction targets PLD1 to sites of regulated exocytosis in PC12 cells. PC12 cells cultured on glass coverslips were co-transfected with the GH expression plasmid in combination with wild-type PLD1, PLD1(K898R), PIM87, PLD1(R690G/R694G) or PLD1(K898R/R690G/R694G). At 48 h post-transfection, the proteins expressed were detected by western blots and their intracellular distribution visualized by immunocytochemistry. Confocal fluorescent images were taken through the center of the nucleus. Images obtained in the Cy2 (green; PLD1) and Cy3 (red; SNAP-25) channels were recorded simultaneously in the same optical section by a double exposure procedure. The yellow–orange staining indicates areas of co-localization. Other than PLD1(R690G/R694G) and PLD1(K898R/R690G/R694G), the wild-type and mutated PLD1 proteins co-localized with SNAP-25 at the plasma membrane.
PLD1 does not mediate the actin reorganization required for exocytosis
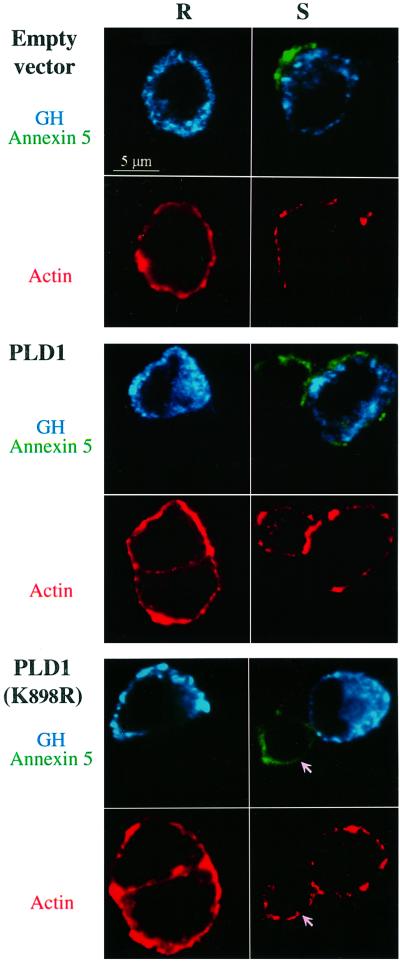
In neuroendocrine cells, actin filament rearrangements are a prerequisite for exocytosis, enabling docking of secretory granules with the plasma membrane. Since one of the proposed functions of PLD is to mediate reorganization of the actin cytoskeleton (Cross et al., 1996; Colley et al., 1997; Honda et al., 1999), we investigated whether PLD1 overexpression affected this event. PC12 cells co-transfected with GH and either wild-type PLD1 or the inactive PLD1(K898R) were stimulated by K+ in the presence of annexin 5. Annexin 5 specifically binds to phosphatidylserine, which is concentrated on the inner face of secretory granule membranes and becomes exposed on the cell surface during exocytosis. Thus, secretory activity can be evaluated by the appearance of fluorescent annexin 5 patches at the cell surface (Demo et al., 1998). Following stimulation, PC12 cells were fixed, processed for immunofluorescence with anti-GH antibodies and stained with rhodamine–phalloidin to visualize the actin filament network.
As illustrated in Figure 7, unstimulated (resting) PC12 cells showed no fluorescent annexin 5 patches, confirming that the annexin 5 binding assay represents a valid marker for secretagogue-evoked exocytotic activity. Stimulation with elevated K+ triggered the appearance of a patchy pattern of surface staining corresponding to annexin 5 binding, in control cells and in cells overexpressing PLD1, but not in cells expressing the inactive PLD1(K898R) mutant. This observation is in line with the finding that PLD1(K898R) inhibits GH secretion from PC12 cells and catecholamine secretion from chromaffin cells. In resting cells, rhodamine–phalloidin fluorescence was most intense at the cell periphery, forming a continuous cortical ring, in agreement with the fact that in secretory cells the majority of actin filaments are concentrated in the subplasmalemmal region (Figure 7). Stimulation with elevated K+ reduced the binding of rhodamine–phalloidin in the cell periphery, revealing the disassembly of cortical actin generally observed in secretagogue-activated cells. Expression of PLD1 or the PLD1(K898R) mutant had no apparent effect on the distribution of actin filaments in resting cells, nor did it interfere with the actin reorganization in stimulated cells (Figure 7). Thus, the function of PLD1 in the exocytotic pathway does not appear to be related to the reorganization of the actin cytoskeleton required for the recruitment of secretory granules to the plasma membrane, but rather, potentially, to the subsequent docking and/or fusion events of the secretory granules with the plasma membrane.
Fig. 7. Overexpression of catalytically inactive PLD1(K898R) blocks vesicular fusion but not the actin cytoskeletal reorganization required for regulated exocytosis. PC12 cells were transfected with either pCGN (empty vector), pCGN-PLD1 or pCGN-PLD1(K898R) in combination with the GH expression plasmid (4 µg/well of each plasmid). At 48 h post-transfection, cells were washed and then incubated for 10 min in either Locke’s solution (resting cells) or elevated K+ (stimulated cells) in the presence of Alexa 488-conjugated annexin 5 to reveal the exocytotic activity (green). Cells subsequently were fixed and stained with rabbit anti-GH and secondary Cy5-labeled anti-rabbit antibodies to identify transfected cells (blue), and with rhodamine-conjugated phalloidin to visualize actin filaments (red). Stimulation with high K+ triggers a partial disruption of the cortical actin ring in control, PLD1- and PLD1(K898R)-expressing PC12 cells. Exocytotic patches at the cell surface were observed only in control and PLD1-expressing cells. Overexpression of the inactive PLD1(K898R) inhibited the exocytotic response without affecting the disassembly of the subplasmalemmal actin. Arrows point to a non-transfected cell undergoing actin depolymerization and displaying exocytotic patches in response to elevated K+, while the adjacent transfected cell expressing GH and PLD1(K898R) exhibits no exocytotic patches. Similar patterns of inhibition mediated by PLD1(K898R) but not wild-type PLD1 were observed in three separate experiments.
Discussion
Dramatic advances have been made recently in our understanding of the protein machinery responsible for the formation, targeting and fusion of vesicles along the secretory pathway. Since ARF proteins have been implicated as ubiquitous regulators in membrane trafficking, we investigated their possible function in calcium-regulated exocytosis. Using adrenal chromaffin cells, which have proved to be a good model for the study of neuroendocrine secretion, we found that ARF6 is associated specifically with the membranes of secretory chromaffin granules (Galas et al., 1997). Interestingly, secretagogue-evoked stimulation triggered the rapid translocation of ARF6 from secretory chromaffin granules to the plasma membrane and the concomitant activation of PLD in the plasma membrane, and both the PLD activation and catecholamine secretion were inhibited specifically by an ARF6 N-terminal synthetic peptide (Caumont et al., 1998). We accordingly proposed that ARF6 participates in the exocytotic reaction in chromaffin cells, most probably through the activation of a plasma membrane-bound PLD. In this report, we identify the activity as the classic ARF-responsive PLD1b and establish a causal relationship between activation of PLD1 and elicitation of exocytosis.
PLD hydrolyzes phosphatidylcholine to generate PA and choline. PA has been proposed to affect membrane delivery and trafficking at various stages of the secretory pathway. Inhibition of PA production by alcohol has been reported to inhibit transport from endoplasmic reticulum to the Golgi apparatus (Bi et al., 1997) or the formation of Golgi-coated vesicles (Ktistakis et al., 1996). Further more, recombinant PLD has been reported to stimulate the release of nascent secretory vesicles from the trans-Golgi network (Chen et al., 1997). A role for PLD in regulated exocytotic secretion was proposed previously (Haslam and Coorsen, 1993; Fensome et al., 1996; Morgan et al., 1997; Brown et al., 1998), although it was contested by a report stating that addition of bacterial PLD to permeabilized and cytosol-depleted chromaffin and PC12 cells did not restore exocytosis (Glenn et al., 1998). It is now appreciated, however, that the exocytotic machinery in permeabilized and cytosol-depleted cells is affected by the leakage of multiple essential components (Sarafian et al., 1991; Hay et al., 1995). In this study, we demonstrated a causal role for ARF-regulated PLD1 in neuroendocrine cell exocytosis by establishing that (i) overexpression of PLD1 enhanced exocytosis whereas overexpression of a dominant-negative PLD1 inhibited regulated exocytosis by PC12 cells, and (ii) microinjection of a dominant-negative PLD1 blocked exocytosis by chromaffin cells. Studies in PC12 cells were conducted using a transient transfection assay involving GH, which has been described previously (Wick et al., 1993; Chung et al., 1995). GH is targeted to dense-core granules in this setting, and the ensuing secretagogue-evoked GH release shows all the characteristics expected for dense-core granule exocytosis. To our knowledge, this is the first direct demonstration of a functional role for PLD1 in dense-core granule exocytosis and, in fact, the first demonstration of a function for PLD1 in any vesicular trafficking pathway using molecular tools in living cells.
One potential mechanism of action for PLD1 in exocytosis may involve the ability of PA to regulate phosphatidylinositol 4-phosphate 5-kinase, which synthesizes PtdIns(4,5)P2 (Honda et al., 1999). This unique lipid is essential in many membrane trafficking events (De Camilli et al., 1996), including exocytosis in chromaffin and PC12 cells (Hay et al., 1995; Wiedemann et al., 1996). Indeed, the recruitment of PLD1 to the exocytotic machinery may be directly sensitive to local variations of PtdIns(4,5)P2, as suggested by the lack of effect of the phosphoinositide-unresponsive PLD1 mutant on GH secretion in transfected PC12 cells. PtdIns(4,5)P2 also plays a pivotal role in actin cytoskeleton dynamics. In chromaffin cells, PtdIns(4,5)P2 modulates the activity of actin-binding proteins concentrated in the subplasmalemmal region and is involved in the actin filament rearrangements required for exocytosis (Zhang et al., 1996). However, we show here that neither PLD1 nor PLD1 (K898R) affected the secretagogue-induced reorganization of cortical actin, although some fine modifications in the actin cytoskeleton cannot be completely excluded. This observation is in line with a previous study which indicated that overexpression of PLD1 did not modify the actin cytoskeleton in fibroblasts (Colley et al., 1997). Thus, the strong inhibition of secretion induced by the catalytically inactive PLD1(K898R) cannot be attributed to a stabilization of the cortical actin barrier that would have trapped secretory granules and prevented docking at the plasma membrane.
The docking and controlled fusion of a secretory granule with the plasma membrane require multiple molecular steps, and numerous proteins have been implicated in this process, although it has often remained challenging to identify the precise site or step at which a particular protein is operating. This report demonstrates the functional relevance of PLD1 in the process of calcium-regulated exocytosis. High time resolution assays for exocytosis (Neher, 1998; Ashery et al., 2000) are now required to provide any detailed insight as to how and when PLD1 exerts its function in the exocytotic pathway. Based on our amperometric and cytochemical data, we would like to propose that PLD1’s role lies subsequent to the cytoskeletal-mediated recruitment of secretory granules to the plasma membrane. A tempting speculation for PLD1’s function relates to changes in the lipid bilayer properties and configurations created by local elevations of PA. Current models for exocytosis postulate that activation of intracellular messengers causes a macromolecular protein scaffold (SNAREs) to draw the plasma membrane into a highly curved dimple in close proximity to the secretory granule membrane (Monck and Fernandez, 1994). Fusion of the two membranes then requires the temporal formation of non-bilayer intermediates resulting in hemifusion, followed by membrane breakdown and formation of a pore (Chernomordik et al., 1995). For most lipids found in biological membranes, the lamella bilayer is the most energetically favorable structure. However, cone-shaped lipids, such as PA, promote negative curvature (Monck and Fernandez, 1994) and the formation of hemifusion intermediates (Chernomordik et al., 1995). Thus, the predicted effect of a local elevation of PA due to the activation of PLD at exocytotic sites would be to promote membrane bending, particularly in the presence of calcium, thereby facilitating hemifusion and subsequent formation of the exocytotic fusion pore. Although additional experimental evidence is now required to prove or refute this possibility, it is of interest to note that a similar model, based on lipid-induced changes in membrane curvature, has been proposed recently for a membrane fission event in the Golgi (Weigert et al., 1999) and in the endophilin I-mediated recycling of synaptic vesicles at the plasma membrane (Schmidt et al., 1999).
Materials and methods
Electrochemical measurement of catecholamine secretion from single chromaffin cells
Chromaffin cells were isolated from fresh bovine adrenal glands and maintained in primary culture essentially as described previously (Galas et al., 1997; Caumont et al., 1998). Cells were cultured on 35 mm Costar plates (Cambridge, MA) at a density of 7.5 × 105 cells/plate. Microinjection of PLDs in 10 mM PIPES, 50 mM K-glutamate pH 7.4 was performed as described earlier (Chasserot-Golaz et al., 1996). The calculated injected volume represented 50–100 fl. The (GluGlu-tagged) PLD proteins were prepared as described previously for wild-type PLD2, except that proteins were purified using monoclonal anti-GluGlu affinity column chromatography and eluted in injection buffer using GluGlu peptide (Colley et al., 1997; Hammond et al., 1997; Sciorra et al., 1999). GluGlu peptide was removed by several rounds of dilution and concentration in injection buffer using filter centrifugation. Electrochemical measurements of catecholamine secretion using carbon fiber electrodes (Chasserot-Golaz et al., 1996) were performed 15 min after microinjection. Catecholamine secretion was evoked by applying nicotine (100 µM) in ascorbate-free Locke’s solution for 5 s to single cells by means of a glass micropipet (Femtotips, Eppendorf). The amplitude of secretion was quantified by measuring the area below the current curve using the MacLab system.
Assay for phospholipase D activity
PLD activity was measured in SLO-permeabilized chromaffin cells using the transphosphatidylation assay exactly as described previously (Caumont et al., 1998). For in vitro assays, Glu-tagged PLD1 was expressed in Sf9 cells and purified by immunoaffinity chromatography. Each assay contained ∼20 ng of PLD protein in a final volume of 100 µl. PLD assays were performed as described previously (Hammond et al., 1997) except that the indicated concentrations of ceramides (Biomol Inc.) were included in the substrate-containing liposomes. PLD activity was determined by measuring initial rates of substrate hydrolysis (<15%). The data are means ± SD of triplicate determinations.
Subcellular fractionation of chromaffin cells
Cultured chromaffin cells were homogenized in 0.32 M sucrose, 10 mM Tris pH 7.4 and then centrifuged at 800 g for 15 min. Following centrifugation at 20 000 g for 20 min, the pellet containing the crude membrane fraction was resuspended in 0.32 M sucrose (10 mM Tris pH 7.4), layered on a continuous sucrose density gradient (1–2.2 M sucrose, 10 mM Tris pH 7.4) and centrifuged for 90 min at 100 000 g. Twelve 1 ml fractions were collected from the top to the bottom and analyzed for protein content by the Bradford procedure. Na+/K+-ATPase in the fractions of the gradient was estimated as described previously (Caumont et al., 1998). Golgi membranes were prepared from cultured chromaffin cells as described (Galas et al., 1997).
Transfection of PC12 cells and assay of growth hormone release
PC12 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with glucose (4500 mg/l) and containing 30 mM NaHCO3, 5% fetal bovine serum, 10% horse serum and 100 U/ml penicillin/streptomycin. Mammalian expression vectors (pCGN) with DNA encoding wild-type PLD1 (Hammond et al., 1997), wild-type PLD2 (Colley et al., 1997), the catalytically inactive point mutants PLD1(K898R) or PLD2(K758R) (Sung et al., 1997), the phosphoinositide-unresponsive mutant PLD1(R690G/R694G) (Sciorra et al., 1999) or PKC-insensitive PLD1 mutant PIM87 (Zhang et al., 1999) were introduced into PC12 cells (6-well dishes, 80% confluent) together with the GH plasmid pXGH5 (Wick et al., 1993), using GenePorter (Gene Therapy Systems) according to the manufacturer’s instruction. Expression of GH was assessed after 48 h by immunocytochemistry (see below). Expression of PLD1 and PLD2 proteins was verified after 48 h by western blot analysis. Transfection efficiency was estimated by counting the number of GH-positive cells among 500 cells in randomly selected areas of the coverslips.
GH release experiments were performed 48 h after transfection. PC12 cells were washed twice with Locke’s solution and then incubated for 10 min with calcium-free Locke’s solution (basal release) or stimulated with an elevated K+ solution (Locke’s containing 59 mM KCl and 85 mM NaCl). The supernatant was collected and the cells were harvested by scraping in 10 mM phosphate-buffered saline (PBS). The amounts of GH secreted into the medium and retained in the cells were measured using a radioimmunoassay kit (Nichols Institute, San Juan, Capistrano, CA).
Immunoblotting, immunofluorescence and confocal microscopy
One-dimensional SDS–gel electrophoresis was performed on an 8% acrylamide gel in Tris–glycine buffer. The proteins were transferred to nitrocellulose sheets at a constant current of 120 mA for 1 h. Blots were developed using secondary antibodies coupled to horseradish peroxidase (Amersham, Les Ulis, France) and the immunoreactive bands detected using the ECL system (Amersham, Les Ulis, France).
For double-labeling immunocytochemistry (Chasserot-Golaz et al., 1996), fixed chromaffin cells were incubated for 2 h with the primary antibodies: rabbit anti-PLD1 (1:50) or anti-PLD2 (1:50) and mouse monoclonal anti-SNAP25 (1:200) or anti-GH (1:200). Cells were then washed and incubated for 1 h with cyanine 2 (Cy2)-labeled anti-rabbit and Cy3-labeled anti-mouse antibodies (1:1000; Amersham, Les Ulis, France). To visualize exocytosing granules, PC12 cells were maintained in Locke’s solution or stimulated for 10 min with elevated K+ in the presence of 50 µg of Alexa 488-conjugated annexin 5 (Molecular Probes) (Demo et al., 1998). Cells were then fixed and incubated with rabbit polyclonal anti-human GH antibodies (1:200) detected with Cy5-labeled anti-rabbit secondary antibodies (1:1000; Amersham, Les Ulis, France). Actin filaments were stained by incubation with rhodamine (TRITC)-conjugated phalloidin (Sigma) at a concentration of 0.2 µg/ml in PBS for 15 min. Stained cells were visualized using the Zeiss laser scanning microscope (Chasserot-Golaz et al., 1996).
Antibodies and cell-permeable ceramides
The following antibodies were used: polyclonal anti-PLD1 and anti-PLD2 antibodies raised in rabbit against the N-terminal domains of PLD1 and PLD2 (QCB, Biosource International, France); rabbit polyclonal anti-human GH antibodies (kindly provided by Dr A.F.Parlow and the NIDDK’s National Hormone and Pituitary Program, Torrance, CA); monoclonal anti-human GH antibodies (Nichols Institute, San Juan, Capistrano, CA); monoclonal anti-SNAP-25 antibodies (Sternberger Monoclonals Inc., Lutherville, MD); rabbit polyclonal anti-dopamine-β-hydroxylase antibodies (Sontag et al., 1988); and rabbit polyclonal anti-β-COP antibodies (Sigma). d-erythro-C2-ceramide, d-erythro-C8-ceramide and d-erythro-dihydro-C2-ceramide (N-acetydihydrosphingosine) were obtained from Biomol (Plymouth Meeting, PA) and resuspended in ethanol to prepare a stock solution of 5 mg/ml. In all experiments, control cells were incubated in the presence of 0.1 or 0.3% ethanol, which corresponded to 20 and 50 µM ceramide, respectively.
Acknowledgments
Acknowledgements
We wish to thank Dr A.F.Parlow and the NIDDK’s National Hormone and Pituitary Program for generously providing polyclonal anti-GH antibodies, and gratefully acknowledge François Gonon (CNRS-UMR 5541, Bordeaux, France) for help and advice in amperometry and generously providing us with carbon fiber electrodes. A.-S.C. was supported by a grant from the Ligue Nationale contre le Cancer and G.D. is a fellow of the American Heart Association. This work was supported by Association de la Recherche sur le Cancer ARC Grant 5802 to M.-F.B. and by grants from the NIH to A.J.M. and M.A.F.
References
- Abousalham A., Liossis,C., O’Brien,L. and Brindley,D.N. (1997) Cell-permeable ceramides prevent the activation of phospholipase D by ADP-ribosylation factor and RhoA. J. Biol. Chem., 272, 1069–1075. [DOI] [PubMed] [Google Scholar]
- Albillos A., Dernick,G., Horstmann,H., Almers,W., Alvarez de Toledo, G. and Lindau,M. (1997) The exocytotic event in chromaffin cells revealed by patch amperometry. Nature, 389, 509–512. [DOI] [PubMed] [Google Scholar]
- Ashery U., Varoqueaux,F., Voets,T., Betz,A., Thakur,P., Koch,H., Neher,E., Brose,N. and Rettig,J. (2000) Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. EMBO J., 19, 3586–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi K., Roth,M.G. and Ktistakis,N.T. (1997) Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr. Biol., 7, 301–307. [DOI] [PubMed] [Google Scholar]
- Brown F.D., Thompson,N., Saqib,K., Clark,J.M., Powner,D., Thompson,N.T., Solari,R. and Wakelam,M.J.O. (1998) Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr. Biol., 8, 835–838. [DOI] [PubMed] [Google Scholar]
- Caumont A.S., Galas,M.C., Vitale,N., Aunis,D. and Bader,M.F. (1998) Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J. Biol. Chem., 273, 1373–1379. [DOI] [PubMed] [Google Scholar]
- Chasserot-Golaz S. et al. (1996) Annexin II in exocytosis: catecholamine secretion requires the translocation of p36 to the subplasmalemmal region in chromaffin cells. J. Cell Biol., 133, 1217–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.A., Duvvuri,V., Schulman,H. and Scheller,R.H. (1999a) Calmodulin and protein kinase C increase Ca2+-stimulated secretion by modulating membrane-attached exocytotic machinery. J. Biol. Chem., 274, 26469–26476. [DOI] [PubMed] [Google Scholar]
- Chen Y.A., Scales,S.J., Patel,S.M., Doung,Y.C. and Scheller,R.H. (1999b) SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell, 97, 165–174. [DOI] [PubMed] [Google Scholar]
- Chen Y.G., Siddhanta,A., Austin,C.D., Hammond,S.M., Sung,T.C., Frohman,M.A., Morris,A.J. and Shields,D. (1997) Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J. Cell Biol., 138, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L.V., Chanturiya,A., Green,J. and Zimmerberg,J. (1995) The hemifusion intermediate and its conversion to complete fusion: regulation by membrane composition. Biophys. J., 69, 922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R.H., von Ruden,L. and Neher,E. (1992) Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature, 356, 60–63. [DOI] [PubMed] [Google Scholar]
- Chung S.H., Takai,Y. and Holz,R.W. (1995) Evidence that the Rab3a-binding protein, rabphilin3a, enhances regulated secretion. Studies in adrenal chromaffin cells. J. Biol. Chem., 270, 16714–16718. [DOI] [PubMed] [Google Scholar]
- Colley W.C., Sung,T., Roll,R., Jenco,J., Hammond,S.M., Altshuller,Y., Bar-Sagi,D., Morris,A.J. and Frohman,M.A. (1997) Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol., 7, 191–201. [DOI] [PubMed] [Google Scholar]
- Cross M.J., Roberts,S., Ridley,A.J., Hodgkin,M.N., Stewart,A., Claesson-Welsh,L. and Wakelam,M.J.O. (1996) Stimulation of actin stress fibre formation mediated by activation of phospholipase D. Curr. Biol., 6, 588–597. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Scott,D.E., McPherson,P.S. and Novick,P. (1996) Phosphoinositides as regulators in membrane traffic. Science, 271, 1533–1539. [DOI] [PubMed] [Google Scholar]
- Demo S.D. et al. (1998) Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-5 binding assay. Cytometry, 36, 340–348. [DOI] [PubMed] [Google Scholar]
- Fensome A., Cunningham,E., Prosser,S., Tan,S.K., Swigart,P., Thomas,G., Hsuan,J. and Cockcroft S. (1996) ARF and PITP restore GTPγS-stimulated protein secretion from cytosol-depleted HL60 cells by promoting PIP2 synthesis. Curr. Biol., 6, 730–738. [DOI] [PubMed] [Google Scholar]
- Galas M.C., Helms,J.B., Vitale,N., Thierse,D., Aunis,D. and Bader,M.F. (1997) Regulated exocytosis in chromaffin cells. A potential role for a secretory granule-associated ARF6 protein. J. Biol. Chem., 272, 2788–2793. [DOI] [PubMed] [Google Scholar]
- Gillis K.D., Mossner,R. and Neher,E. (1996) Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron, 16, 1209–1220. [DOI] [PubMed] [Google Scholar]
- Glenn D.E., Thomas,G.M.H., O’Sullivan,A.J. and Burgoyne,R.D. (1998) Examination of the role of ADP-ribosylation factor and phospholipase D activation in regulated exocytosis in chromaffin and PC12 cells. J. Neurochem., 71, 2023–2033. [DOI] [PubMed] [Google Scholar]
- Graham M.E. and Burgoyne,R.D. (2000) Comparison of cysteine string protein (Csp) and mutant α-SNAP overexpression reveals a role for Csp in late steps of membrane fusion in dense-core granule exocytosis in adrenal chromaffin cells. J. Neurosci., 15, 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S.M. et al. (1997) Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor and Rho family monomeric GTP-binding proteins and protein kinase C-α. J. Biol. Chem., 272, 3860–3868. [DOI] [PubMed] [Google Scholar]
- Hanson P.I., Heuser,J.E. and Jahn,R. (1997) Neurotransmitter release: four years of SNARE complexes. Curr. Biol., 7, 310–315. [DOI] [PubMed] [Google Scholar]
- Haslam R.J. and Coorssen,J.R. (1993) Evidence that activation of phospholipase D can mediate secretion from permeabilized platelets. Adv. Exp. Med. Biol., 344, 149–164. [DOI] [PubMed] [Google Scholar]
- Hay J.C., Fisette,P.L., Jenkins,G.H., Anderson,R.A. and Martin,T.F.J. (1995) ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature, 374, 172–177. [DOI] [PubMed] [Google Scholar]
- Honda A. et al. (1999) Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell, 99, 521–532. [DOI] [PubMed] [Google Scholar]
- Jones D., Morgan,C. and Cockcroft,S. (1999) Phospholipase D and membrane traffic. Potential roles in regulated exocytosis, membrane delivery and vesicle budding. Biochim. Biophys. Acta, 1439, 229–244. [DOI] [PubMed] [Google Scholar]
- Jones M.J. and Murray,A.W. (1995) Evidence that ceramide selectively inhibits protein kinase C-translocation and modulates bradykinin activation of phospholipase D. J. Biol. Chem., 270, 5007–5013. [DOI] [PubMed] [Google Scholar]
- Ktistakis N.T., Brown,H.A., Waters,M.G., Sternweis,P.C. and Roth,M.G. (1996) Evidence that phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol., 134, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M., Chalifa,V., Pertile,P., Chen,C.S. and Cantley,L.C. (1994) Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J. Biol. Chem., 269, 21403–21406. [PubMed] [Google Scholar]
- Liscovitch M., Czarny,M., Fiucci,G. and Tang,X. (2000) Phospho lipase D: molecular and cell biology of a novel gene family. Biochem. J., 345, 401–415. [PMC free article] [PubMed] [Google Scholar]
- Martin T.F.J., Loyet,K.M., Barry,V.A. and Kowalchyk,J.A. (1997) The role of PtdIns(4,5)P2 in exocytotic membrane fusion. Biochem. Soc. Trans, 25, 1137–1141. [DOI] [PubMed] [Google Scholar]
- Monck J.R. and Fernandez,J.M. (1994) The exocytotic fusion pore and neurotransmitter release. Neuron, 12, 707–716. [DOI] [PubMed] [Google Scholar]
- Morgan C.P., Sengelov,H., Whatmore,J., Borregaard,N. and Cockcroft,S. (1997) ADP-ribosylation-factor-regulated phospholipase D activity localizes to secretory vesicles and mobilizes to the plasma membrane following N-formylmethionyl-leucyl-phenylalanine stimulation of human neutrophils. Biochem. J., 325, 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. (1998) Vesicle pools and calcium microdomains: new tools for understanding their roles in neurotransmitter release. Neuron, 20, 389–399. [DOI] [PubMed] [Google Scholar]
- Sarafian T., Pradel,L.A., Henry,J.P., Aunis,D. and Bader,M.F. (1991) The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J. Cell Biol., 114, 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Wolde,M., Thiele,C., Fest,C., Kratzin,H., Podtelejnikov,A.V., Witke,W., Huttner,W.B. and Söling,H.D. (1999) Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature, 401, 133–141. [DOI] [PubMed] [Google Scholar]
- Sciorra V.A, Rudge,S. Frohman,M.A, Engebrecht,J. and Morris,A.J. (1999) Identification of a non-PH domain PIP2-binding site in mammalian and yeast PLDs. EMBO J., 18, 5911–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag J.M., Aunis,D. and Bader,M.F. (1988) Peripheral actin filaments control calcium-mediated catecholamine release from streptolysin-O permeabilized chromaffin cells. Eur. J. Cell Biol., 46, 316–326. [PubMed] [Google Scholar]
- Stutchfield J. and Cockroft,S. (1993) Correlation between secretion and phospholipase D activation in differentiated HL60 cells. Biochem. J., 293, 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung T.C. et al. (1997) Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J., 16, 4519–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman,B.V., McNew,J.A., Westermann,B., Gmachi,M., Parlati,F., Sollner,T.H. and Rothman,J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Weigert R. et al. (1999) CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature, 402, 429–433. [DOI] [PubMed] [Google Scholar]
- Wick P.F., Senter,R.A., Parsel,L.A., Uhler,M.D. and Holz,R.W. (1993) Transient transfection studies of secretion in bovine chromaffin cells and PC12 cells. Generation of kainate-sensitive chromaffin cells. J. Biol. Chem., 268, 10983–10989. [PubMed] [Google Scholar]
- Wiedemann C., Schafer,T. and Burger,M.M. (1996) Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J., 15, 2094–2101. [PMC free article] [PubMed] [Google Scholar]
- Wightman R.J., Jankowsky,J.A., Kennedy,R.T., Kawagoe,K.T., Schroeder,T.J., Leszczyszyn,D.J., Near,J.A., Diliberto,E.J. and Viveros,O.H. (1991) Temporally resolved catcholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Natl Acad. Sci. USA, 88, 10754–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Marcu,M.G., Nau-Staudt,K. and Trifaro,J.M. (1996) Recombinant scinderin enhances exocytosis, an effect blocked by two scinderin-derived actin-binding peptides and PIP2. Neuron, 17, 287–296. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Altshuller,Y.M., Hammond,S.M., Morris,A.J. and Frohman, M.A. (1999) Loss of receptor regulation by a phospholipase D1 mutant unresponsive to protein kinase C. EMBO J., 18, 6339–6348. [DOI] [PMC free article] [PubMed] [Google Scholar]